Approach for Self-Calibrating CO2 Measurements with Linear Membrane-Based Gas Sensors
Abstract
:1. Introduction
2. Theoretical Description
3. Experimental Proof
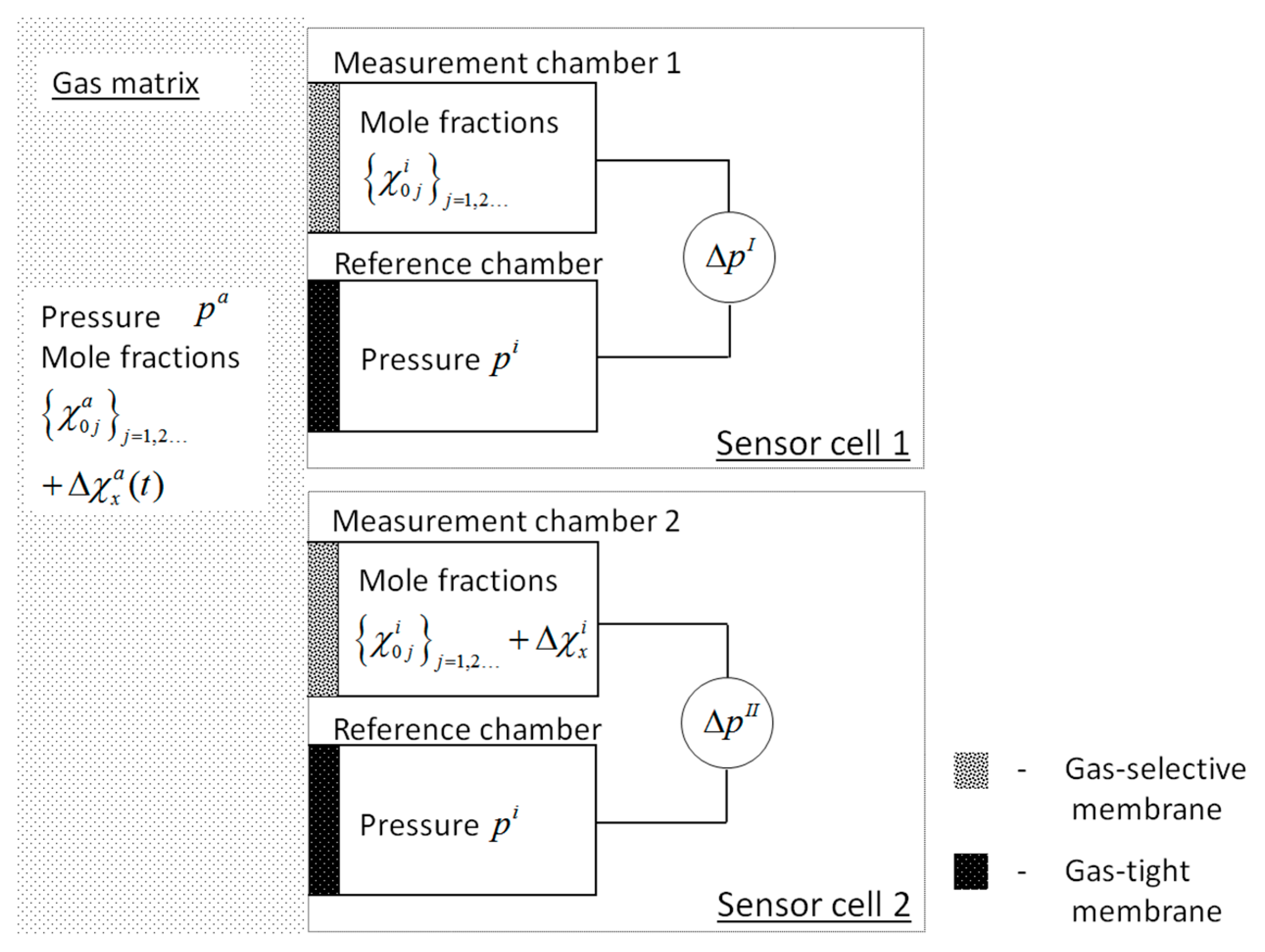
3.1. Setup and Experimental Realization
3.2. Mixed Gas Experiments
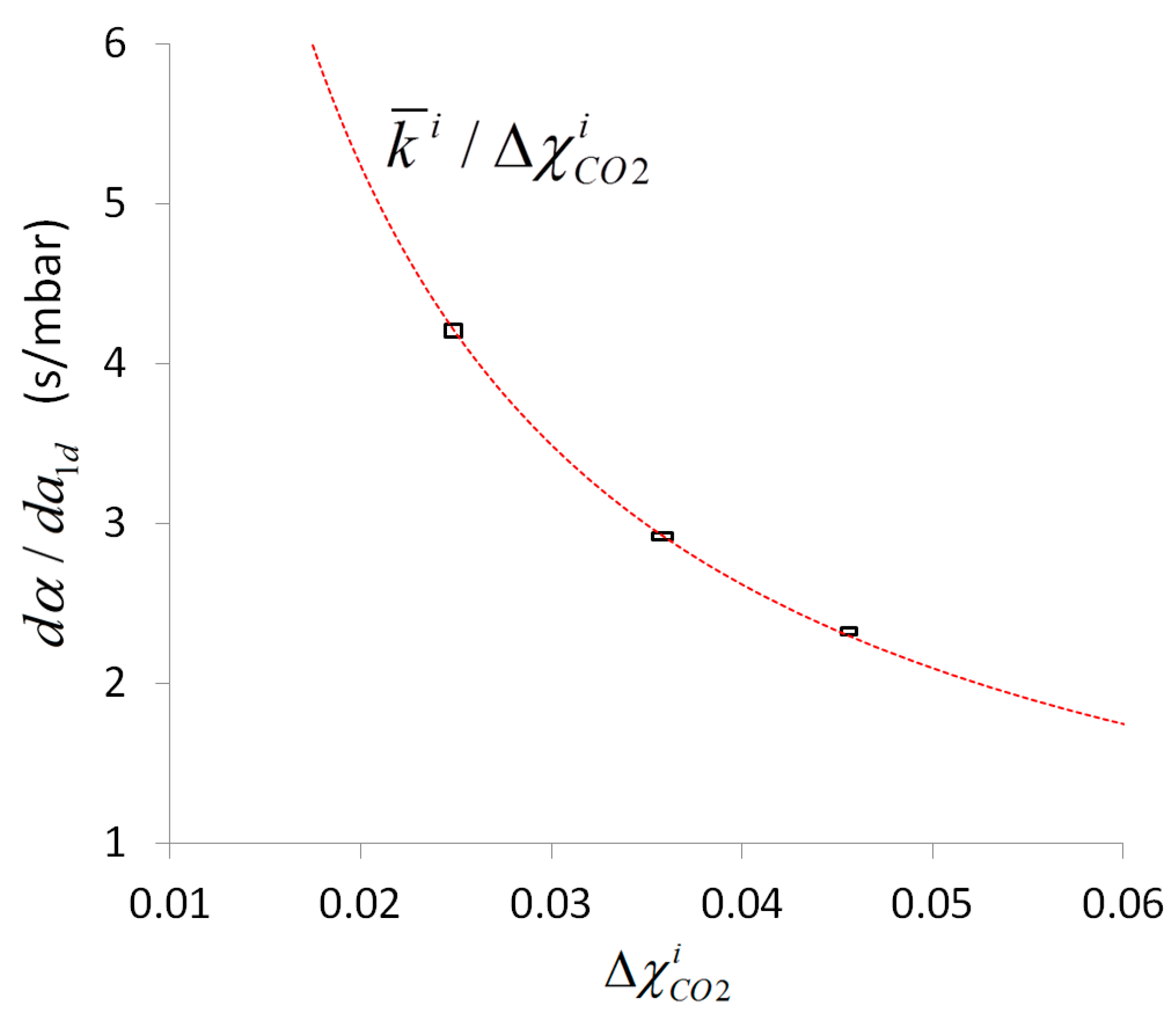
4. Results and Discussion
4.1. Normalization of Sensor Response
4.2. Preparation of Datasets
4.3. Offset Determination
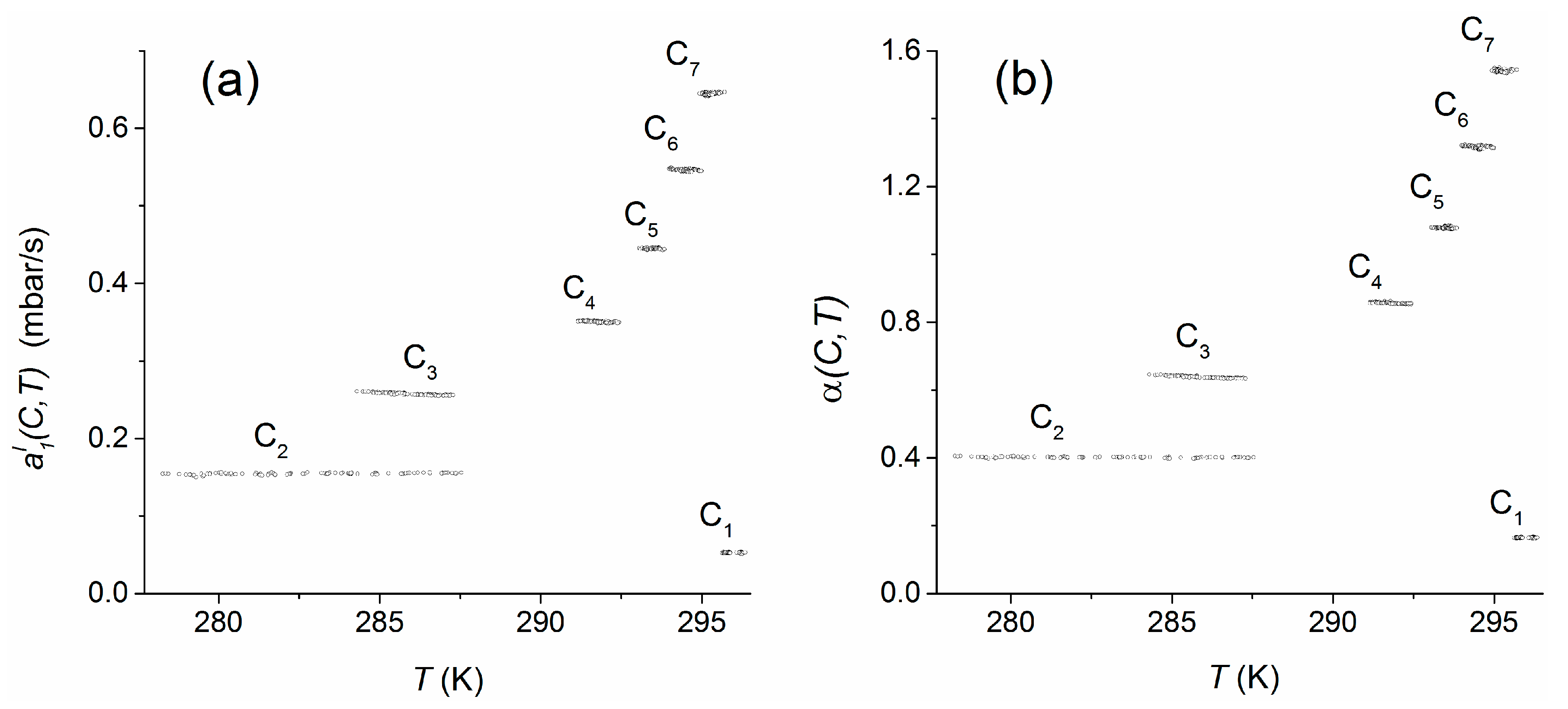
4.4. Temperature Dependency
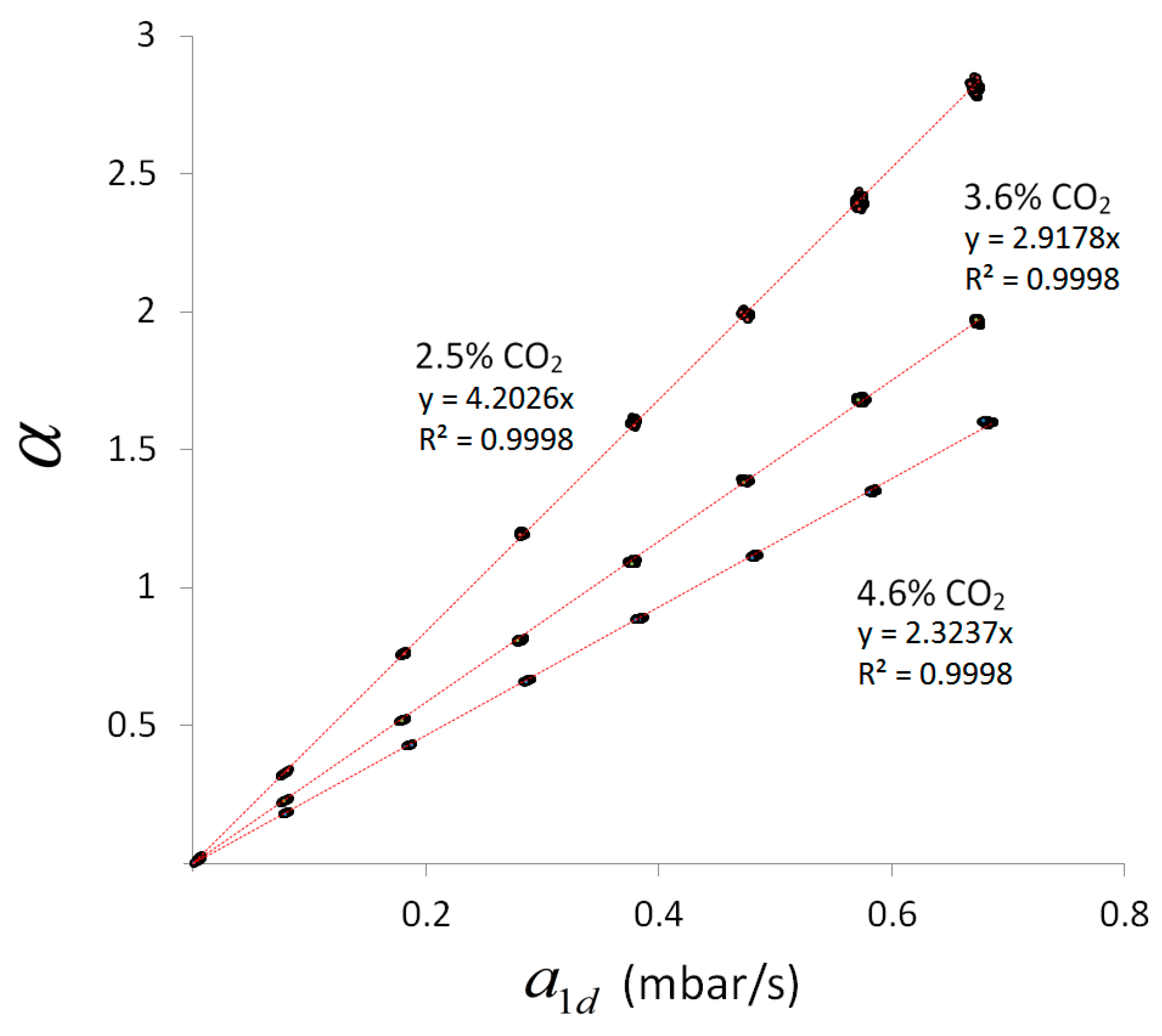
4.5. Scaling Behavior of Combined Line-Sensor Response
4.6. Impact of Fluctuations of Internal Standard Concentration
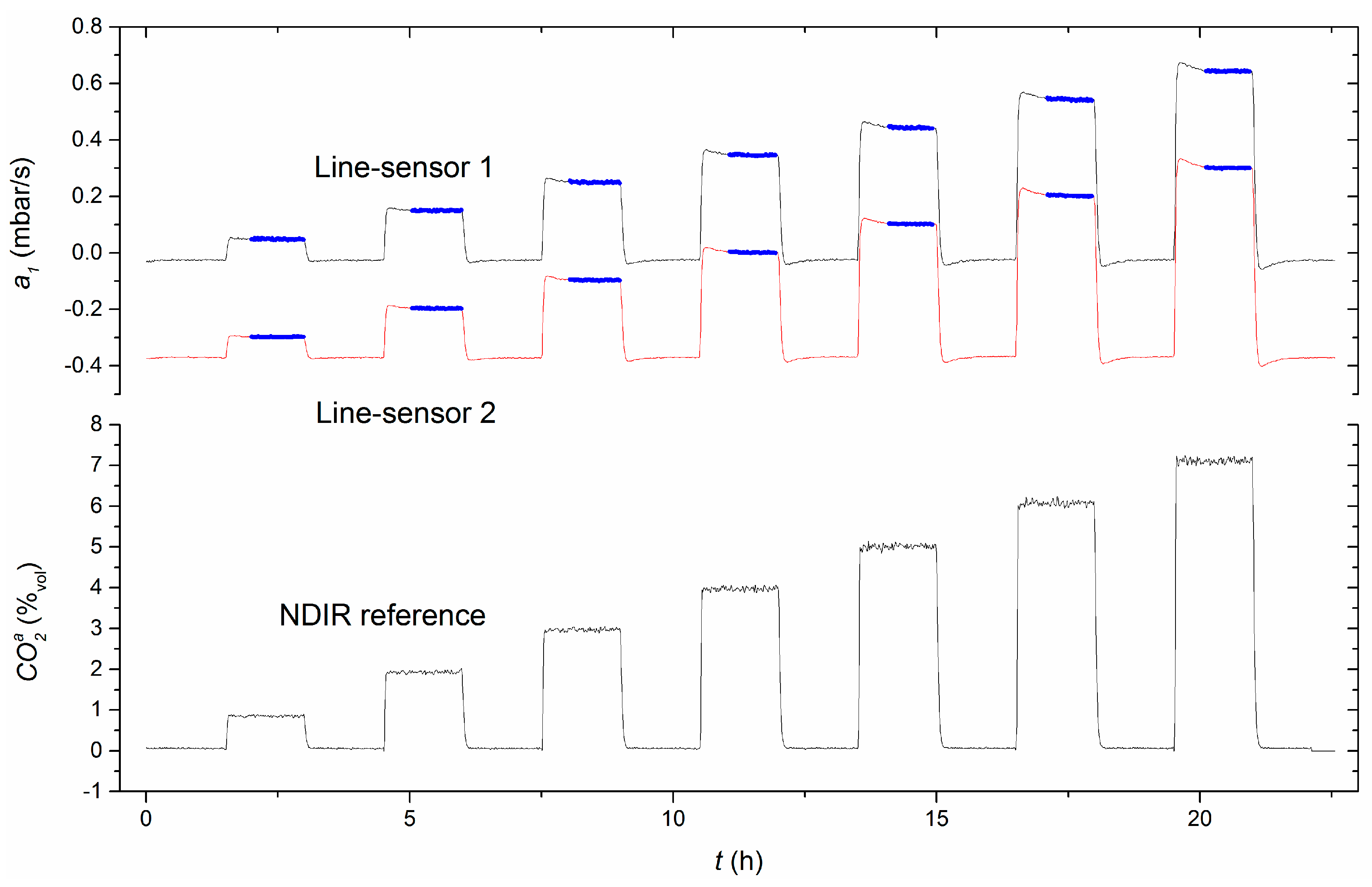
4.7. Measurement Comparison of Combined Line-Sensor and NDIR Reference
5. Summary and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Appendix A
| No. of Exp. | Time Lag (s) | Internal Standard Concentrations (%vol) | Test-Column Concentrations (%vol) | |||
|---|---|---|---|---|---|---|
| Name | ||||||
| 1 | 805 | 2.523 | 0.026 | C0 | 0.059 | 0.009 |
| C1 | 0.859 | 0.019 | ||||
| C2 | 1.968 | 0.023 | ||||
| C3 | 3.045 | 0.029 | ||||
| C4 | 4.027 | 0.032 | ||||
| C5 | 5.025 | 0.037 | ||||
| C6 | 6.045 | 0.042 | ||||
| C7 | 7.096 | 0.050 | ||||
| 2 | 630 | 3.618 | 0.036 | C0 | 0.061 | 0.008 |
| C1 | 0.855 | 0.020 | ||||
| C2 | 1.929 | 0.027 | ||||
| C3 | 2.975 | 0.033 | ||||
| C4 | 3.970 | 0.043 | ||||
| C5 | 5.021 | 0.044 | ||||
| C6 | 6.077 | 0.056 | ||||
| C7 | 7.109 | 0.053 | ||||
| 3 | 767 | 4.597 | 0.024 | C0 | 0.065 | 0.006 |
| C1 | 0.882 | 0.012 | ||||
| C2 | 2.024 | 0.019 | ||||
| C3 | 3.064 | 0.017 | ||||
| C4 | 4.064 | 0.024 | ||||
| C5 | 5.097 | 0.030 | ||||
| C6 | 6.159 | 0.031 | ||||
| C7 | 7.420 | 0.042 | ||||
| 4 | 595 | 4.598 | 0.047 | C0 | 0.051 | 0.008 |
| C1 | 0.845 | 0.017 | ||||
| C2 | 2.045 | 0.039 | ||||
| C3 | 3.060 | 0.038 | ||||
| C4 | 4.004 | 0.040 | ||||
| C5 | 4.976 | 0.053 | ||||
| C6 | 6.011 | 0.052 | ||||
| C7 | 7.031 | 0.045 | ||||
References
- Azad, A.M.; Akbar, S.A.; Mhaisalkar, S.G.; Birkefeld, L.D.; Goto, K.S. Solid-state gas sensors: A review. J. Electrochem. Soc. 1992, 139, 3690–3704. [Google Scholar] [CrossRef]
- Di Natale, C.; Paolesse, R.; Martinelli, E.; Capuano, R. Solid-state gas sensors for breath analysis: A review. Anal. Chim. Acta 2014, 824, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cheng, S.; Liu, H.; Hu, S.; Zhang, D.; Ning, H. A Survey on Gas Sensing Technology. Sensors 2012, 12, 9635–9665. [Google Scholar] [CrossRef] [PubMed]
- Lakkis, S.; Younes, R.; Alayli, Y.; Sawan, M. Review of recent trends in gas sensing technologies and their miniaturization potential. Sens. Rev. 2014, 34, 24–35. [Google Scholar] [CrossRef]
- Werle, P. A review of recent advances in semiconductor laser based gas monitors. Spectrochim. Acta A Mol. Biomol. Spectrosc. 1998, 54, 197–236. [Google Scholar] [CrossRef]
- Xu, X.; Wang, J.; Long, Y. Zeolite-based materials for gas sensors. Sensors 2006, 6, 1751–1764. [Google Scholar] [CrossRef]
- Jacobson, S. New Developments in Ultrasonic Gas Analysis and Flowmetering; IEEE: Beijing, China, 2008; pp. 508–516. [Google Scholar]
- De Graaf, G.; Bakker, F.; Wolffenbuttel, R.F. Sensor platform for gas composition measurement. Procedia Eng. 2011, 25, 1157–1160. [Google Scholar] [CrossRef]
- Arshak, K.; Moore, E.; Lyons, G.M.; Harris, J.; Clifford, S. A review of gas sensors employed in electronic nose applications. Sens. Rev. 2004, 24, 181–198. [Google Scholar] [CrossRef]
- Abbas, M.N.; Moustafa, G.A.; Mitrovics, J.; Gopel, W. Multicomponent gas analysis of a mixture of chloroform, octane and toluene using a piezoelectric quartz crystal sensor array. Anal. Chim. Acta 1999, 393, 67–76. [Google Scholar] [CrossRef]
- Llobet, E.; Ionescu, R.; Al-Khalifa, S.; Brezmes, J.; Vilanova, X.; Correig, X.; Barsan, N.; Gardner, J.W. Multicomponent gas mixture analysis using a single tin oxide sensor and dynamic pattern recognition. IEEE Sens. J. 2001, 1, 207–213. [Google Scholar] [CrossRef]
- Wang, L. Thick film CO2 sensors based on Nasicon solid electrolyte. Solid State Ion. 2003, 158, 309–315. [Google Scholar] [CrossRef]
- Diagne, E.H.A.; Lumbreras, M. Elaboration and characterization of tin oxide-lanthanum oxide mixed layers prepared by the electrostatic spray pyrolysis technique. Sens. Actuators B Chem. 2001, 78, 98–105. [Google Scholar] [CrossRef]
- Oho, T.; Tonosaki, T.; Isomura, K.; Ogura, K. A CO2 sensor operating under high humidity. J. Electroanal. Chem. 2002, 522, 173–178. [Google Scholar] [CrossRef]
- Frodl, R.; Tille, T. A High-Precision NDIR CO2 Gas Sensor for Automotive Applications. IEEE Sens. J. 2006, 6, 1697–1705. [Google Scholar] [CrossRef]
- Kwon, J.; Ahn, G.; Kim, G.; Kim, J.C.; Kim, H. A Study on NDIR-based CO2 Sensor to apply Remote Air Quality Monitoring System. In Proceedings of the 2009 ICROS-SICE International Joint Conference, Fukuoka, Japan, 18–21 August 2009; pp. 1683–1687.
- Chu, C.-S.; Lo, Y.-L. Fiber-optic carbon dioxide sensor based on fluorinated xerogels doped with HPTS. Sens. Actuators B Chem. 2008, 129, 120–125. [Google Scholar] [CrossRef]
- Chu, C.-S.; Lo, Y.-L.; Sung, T.-W. Review on recent developments of fluorescent oxygen and carbon dioxide optical fiber sensors. Photonic Sens. 2011, 1, 234–250. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Wang, Y.-S.; Sun, Y.; Xu, Z.-J.; Liu, G.-R. An improved gas chromatography for rapid measurement of CO2, CH4 and N2O. J. Environ. Sci. China 2006, 18, 162–169. [Google Scholar] [PubMed]
- Van der Laan, S.; Neubert, R.E.M.; Meijer, H.A.J. A single gas chromatograph for accurate atmospheric mixing ratio measurements of CO2, CH4, N2O, SF6 and CO. Atmos. Meas. Tech. 2009, 2, 549–559. [Google Scholar] [CrossRef]
- Loftfield, N.; Flessa, H.; Augustin, J.; Beese, F. Automated Gas Chromatographic System for Rapid Analysis of the Atmospheric Trace Gases Methane, Carbon Dioxide, and Nitrous Oxide. J. Environ. Qual. 1997, 26, 560. [Google Scholar] [CrossRef]
- Wang, Y.S.; Wang, Y.H. Quick measurement of CH4, CO2 and N2O emissions from a short-plant ecosystem. Adv. Atmos. Sci. 2003, 20, 842–844. [Google Scholar]
- Seethapathy, S.; Górecki, T. Applications of polydimethylsiloxane in analytical chemistry: A review. Anal. Chim. Acta 2012, 750, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Namieśnik, J.; Zygmunt, B. Selected concentration techniques for gas chromatographic analysis of environmental samples. Chromatographia 2002, 56, S9–S18. [Google Scholar] [CrossRef]
- DeSutter, T.M.; Sauer, T.J.; Parkin, T.B. Porous tubing for use in monitoring soil CO2 concentrations. Soil Biol. Biochem. 2006, 38, 2676–2681. [Google Scholar] [CrossRef]
- Schloemer, S.; Furche, M.; Dumke, I.; Poggenburg, J.; Bahr, A.; Seeger, C.; Vidal, A.; Faber, E. A review of continuous soil gas monitoring related to CCS—Technical advances and lessons learned. Appl. Geochem. 2013, 30, 148–160. [Google Scholar] [CrossRef]
- Lazik, D.; Geistlinger, H. A new method for membrane-based gas measurements. Sens. Actuators Phys. 2005, 117, 241–251. [Google Scholar] [CrossRef]
- Lazik, D.; Ebert, S.; Leuthold, M.; Hagenau, J.; Geistlinger, H. Membrane Based Measurement Technology for in situ Monitoring of Gases in Soil. Sensors 2009, 9, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Comparability Study on Permeability Coefficients of Flexible Tubing. Available online: www.liquid-scan.de (accessed on 1 September 2014).
- Lazik, D.; Ebert, S.; Neumann, P.P.; Bartholmai, M. Characteristic length measurement of a subsurface gas anomaly—A monitoring approach for heterogeneous flow path distributions. Int. J. Greenh. Gas Control 2016, 47, 330–341. [Google Scholar] [CrossRef]
- Lazik, D.; Ebert, S. Improved membrane-based sensor network for reliable gas monitoring in the subsurface. Sensors 2012, 12, 17058–17073. [Google Scholar] [CrossRef] [PubMed]
- Lazik, D.; Ebert, S. First field test of linear gas sensor net for planar detection of CO2 leakages in the unsaturated zone. Int. J. Greenh. Gas Control 2013, 17, 161–169. [Google Scholar] [CrossRef]
- Robb, W. L. Thin silicone membranes—Their permeation properties and some applications. Ann. N. Y. Acad. Sci. 1968, 146, 119–137. [Google Scholar] [CrossRef] [PubMed]
- Merkel, T.C.; Bondar, V.I.; Nagai, K.; Freeman, B.D.; Pinnau, I. Gas sorption, diffusion, and permeation in poly (dimethylsiloxane). J. Polym. Sci. B Polym. Phys. 2000, 38, 415–434. [Google Scholar] [CrossRef]
- Barrer, R.M.; Rideal, E.K. Permeation, diffusion and solution of gases in organic polymers. Trans. Faraday Soc. 1939, 35, 628–643. [Google Scholar] [CrossRef]
- Barrie, J.A.; Levine, J.D.; Michaels, A.S.; Wong, P. Diffusion and solution of gases in composite rubber membranes. Trans. Faraday Soc. 1963, 59, 869–878. [Google Scholar] [CrossRef]



| Type of CO2 Sensor | Detection/Measurement Range | Operating Temperature (°C) | Remarks | Reference |
|---|---|---|---|---|
| Solid electrolyte (Nasicon with Na + Ba—based mixed carbonate electrodes) | 6 ppm–100%vol | ~600 | High chemical stability, fast response, improved performance against moisture | [12] |
| Metal oxide (LaOCl and SnO2) | 400–2000 ppm | 350–550 | Low cost, high sensitivity, long-term stability; limited accuracy | [13] |
| Polymer-based | 300 ppm–1.5%vol | Room temperature | Operational for room temperature and high humidity atmospheres | [14] |
| Non-dispersive infrared (NDIR) | <10%vol | - | Low cost, wide measurement range, accuracy: ±30 ppm/5% typically (may vary with range), characteristic curve producible but may suffer from thermal drift, light scattering effects etc. | [15,16] |
| Fluorescence based fiber optic | <100%vol | - | Chemically inert, sensitivity depends on various support matrices, low cross-sensitive to other gas components | [17,18] |
| Gas chromatography (atmospheric trace gases, air quality) | 50 ppm–100%vol | Standard temperature & Pressure (STP) conditions | Static field/laboratory analytical method, high cost, high sensitivity and selectivity, miniaturization potential yet to be explored. Precision: ±0.06 ppm to ±1.29 ppm | [19,20,21,22] |
| Exp. No. | Test Gas | Purge Gas | ||
|---|---|---|---|---|
| Conditions | T (K) | Internal Standard (%vol) | T (K) | |
| 1 | up to 7%vol Q = 1.5 L/min | 290.5–294.8 | ≈2.5 | 291.8–295.7 |
| 2 | 292.3–295.6 | ≈3.6 | 293.3–296.4 | |
| 3 | 282.1–296.2 | ≈4.6 | 283.8–296.9 | |
| 4 | 278.0–296.3 | ≈4.6 | 277.0–297.3 | |
| Exp. No. | (%vol) | (s/mbar) | (s/mbar) |
|---|---|---|---|
| 1 | 2.485 ± 0.026 | 4.1270 ± 0.0134 | 0.1047 ± 0.0013 |
| 2 | 3.582 ± 0.036 | 2.9178 ± 0.0079 | 0.1040 ± 0.0013 |
| 3 | 4.561 ± 0.024 | 2.3237 ± 0.0067 | 0.1055 ± 0.0009 |
| Exp. No. | (mbar/s) | ||
|---|---|---|---|
| 1 | 1.0016 ± 0.0003 | 0.2369 ± 0.0001 | 0.0020 |
| 2 | 0.9924 ± 0.0004 | 0.3440 ± 0.0001 | 0.0023 |
| 3 | 0.9921 ± 0.0004 | 0.4313 ± 0.0001 | 0.0023 |
| Exp. No. | (%vol) | |||
|---|---|---|---|---|
| 1 | 0.9922 ± 0.0007 | −0.0225 ± 0.0028 | 0.9996 | 0.043 |
| 2 | 0.9969 ± 0.0007 | −0.0566 ± 0.0029 | 0.9996 | 0.045 |
| 3 | 0.9999 ± 0.0007 | −0.0335 ± 0.0028 | 0.9997 | 0.043 |
| Exp. No. | (%vol) | |||
|---|---|---|---|---|
| 1 | 0.9921 ± 0.0007 | −0.0295 ± 0.0028 | 0.9996 | 0.043 |
| 2 | 0.9970 ± 0.0007 | −0.0402 ± 0.0029 | 0.9996 | 0.045 |
| 3 | 0.9999 ± 0.0007 | −0.0283 ± 0.0028 | 0.9997 | 0.043 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lazik, D.; Sood, P. Approach for Self-Calibrating CO2 Measurements with Linear Membrane-Based Gas Sensors. Sensors 2016, 16, 1930. https://doi.org/10.3390/s16111930
Lazik D, Sood P. Approach for Self-Calibrating CO2 Measurements with Linear Membrane-Based Gas Sensors. Sensors. 2016; 16(11):1930. https://doi.org/10.3390/s16111930
Chicago/Turabian StyleLazik, Detlef, and Pramit Sood. 2016. "Approach for Self-Calibrating CO2 Measurements with Linear Membrane-Based Gas Sensors" Sensors 16, no. 11: 1930. https://doi.org/10.3390/s16111930
APA StyleLazik, D., & Sood, P. (2016). Approach for Self-Calibrating CO2 Measurements with Linear Membrane-Based Gas Sensors. Sensors, 16(11), 1930. https://doi.org/10.3390/s16111930





