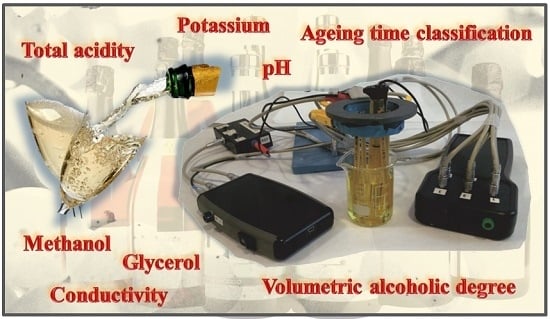
Portable Electronic Tongue Based on Microsensors for the Analysis of Cava Wines
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Solutions
2.2. Cava Wine Samples
2.3. Sensors and Devices Used
2.4. Measurement Equipment
2.5. Characterization of Sensors and Electronics
2.6. Electronic Tongue Measurement Procedure
2.7. Data Treatment and Analysis
3. Results and Discussion
3.1. Characterization of Sensors and Electronics
3.2. Classification of the Cava Wine Samples
3.3. Quantification of Legal Parameters
3.4. Quantification of Other Parameters
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Amodio, M.; Brattoli, M.; Dambruoso, P.; de Gennaro, L.; de Gennaro, G.; Loiotile, A.D.; Trizio, L. Odour Impact Assessment by a Multiparametric System (Electronic Noses/CH4-NMHC Analyser). In Nose 2012: 3rd International Conference on Environmental Odour Monitoring and Control; DelRosso, R., Pierucci, S., Klemes, J.J., Eds.; Aidic Servizi Srl: Milano, Italy, 2012; Volume 30, pp. 199–204. [Google Scholar]
- Gutierrez, M.; Llobera, A.; Ipatov, A.; Vila-Planas, J.; Minguez, S.; Demming, S.; Buttgenbach, S.; Capdevila, F.; Domingo, C.; Jimenez-Jorquera, C. Application of an E-Tongue to the Analysis of Monovarietal and Blends of White Wines. Sensors 2011, 11, 4840–4857. [Google Scholar] [CrossRef] [PubMed]
- Totu, E.E.; Manuc, D. Multisensor for Clinical Analysis with Impact on Public Health Evaluation. Rev. Chim. 2008, 59, 947–951. [Google Scholar]
- Praveena, S.M.; Aris, A.Z. A baseline study of tropical coastal water quality in Port Dickson, Strait of Malacca, Malaysia. Mar. Pollut. Bull. 2013, 67, 196–199. [Google Scholar] [CrossRef] [PubMed]
- Guth, U.; Vonau, W.; Zosel, J. Recent developments in electrochemical sensor application and technology—A review. Meas. Sci. Technol. 2009, 20, 042002. [Google Scholar] [CrossRef]
- Holmberg, M.; Eriksson, M.; Krantz-Rülcker, C.; Artursson, T.; Winquist, F.; Lloyd-Spetz, A.; Lundström, I. 2nd Workshop of the Second Network on Artificial Olfactory Sensing (NOSE II). Sens. Actuators B Chem. 2004, 101, 213–223. [Google Scholar] [CrossRef]
- Vlasov, Y.; Legin, A.; Rudnitskaya, A.; Di Natale, C.; D’Amico, A. Nonspecific sensor arrays (“electronic tongue”) for chemical analysis of liquids (IUPAC Technical Report). Pure Appl. Chem. 2005, 77, 1965–1983. [Google Scholar] [CrossRef]
- Rothe, J.; Frey, O.; Stettler, A.; Chen, Y.H.; Hierlemann, A. Fully Integrated CMOS Microsystem for Electrochemical Measurements on 32 × 32 Working Electrodes at 90 Frames Per Second. Anal. Chem. 2014, 86, 6425–6432. [Google Scholar] [CrossRef] [PubMed]
- Codinachs, L.M.I.; Baldi, A.; Merlos, A.; Abramova, N.; Ipatov, A.; Jimenez-Jorquera, C.; Bratov, A. Integrated multisensor for FIA-based electronic tongue applications. IEEE Sens. J. 2008, 8, 608–615. [Google Scholar] [CrossRef]
- Baldwin, E.A.; Bai, J.H.; Plotto, A.; Dea, S. Electronic Noses and Tongues: Applications for the Food and Pharmaceutical Industries. Sensors 2011, 11, 4744–4766. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Méndez, M.L. Electronic Noses and Tongues in Food Science; Elsevier Inc.: Oxford, UK, 2016; pp. 151–273. [Google Scholar]
- Zeravik, J.; Hlavacek, A.; Lacina, K.; Skladal, P. State of the Art in the Field of Electronic and Bioelectronic Tongues-Towards the Analysis of Wines. Electroanalysis 2009, 21, 2509–2520. [Google Scholar] [CrossRef]
- Buxaderas, S.; López-Tamames, E. Chapter 1-Sparkling Wines: Features and Trends from Tradition. In Advances in Food and Nutrition Research; Jeyakumar, H., Ed.; Academic Press: Waltham, MA, USA, 2012; Volume 66, pp. 1–45. [Google Scholar]
- Pozo-Bayón, M.Á.; Martínez-Rodríguez, A.; Pueyo, E.; Moreno-Arribas, M.V. Chemical and biochemical features involved in sparkling wine production: From a traditional to an improved winemaking technology. Trends Food Sci. Technol. 2009, 20, 289–299. [Google Scholar] [CrossRef]
- Ceto, X.; Gutierrez, J.M.; Moreno-Baron, L.; Alegret, S.; del Valle, M. Voltammetric Electronic Tongue in the Analysis of Cava Wines. Electroanalysis 2011, 23, 72–78. [Google Scholar] [CrossRef]
- Ceto, X.; Capdevila, J.; Puig-Pujol, A.; del Valle, M. Cava Wine Authentication Employing a Voltammetric Electronic Tongue. Electroanalysis 2014, 26, 1504–1512. [Google Scholar] [CrossRef]
- Ceto, X.; Capdevila, J.; Minguez, S.; del Valle, M. Voltammetric BioElectronic Tongue for the analysis of phenolic compounds in rose cava wines. Food Res. Int. 2014, 55, 455–461. [Google Scholar] [CrossRef]
- Ouyang, Q.; Zhao, J.W.; Chen, Q.S. Classification of rice wine according to different marked ages using a portable multi-electrode electronic tongue coupled with multivariate analysis. Food Res. Int. 2013, 51, 633–640. [Google Scholar] [CrossRef]
- Garcia-Breijo, E.; Atkinson, J.; Gil-Sanchez, L.; Masot, R.; Ibanez, J.; Garrigues, J.; Glanc, M.; Laguarda-Miro, N.; Olguin, C. A comparison study of pattern recognition algorithms implemented on a microcontroller for use in an electronic tongue for monitoring drinking waters. Sens. Actuator A Phys. 2011, 172, 570–582. [Google Scholar] [CrossRef]
- Blanco, C.A.; de la Fuente, R.; Caballero, I.; Rodriguez-Mendez, M.L. Beer discrimination using a portable electronic tongue based on screen-printed electrodes. J. Food Eng. 2015, 157, 57–62. [Google Scholar] [CrossRef]
- Tahara, Y.; Nakashi, K.; Ji, K.; Ikeda, A.; Toko, K. Development of a Portable Taste Sensor with a Lipid/Polymer Membrane. Sensors 2013, 13, 1076–1084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cruz, A.F.D.; Norena, N.; Kaushik, A.; Bhansali, S. A low-cost miniaturized potentiostat for point-of-care diagnosis. Biosens. Bioelectron. 2014, 62, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Gimenez-Gomez, P.; Escude-Pujol, R.; Jimenez-Jorquera, C.; Gutierrez-Capitan, M. Multisensor Portable Meter for Environmental Applications. IEEE Sens. J. 2015, 15, 6517–6523. [Google Scholar] [CrossRef]
- Gutierrez, M.; Llobera, A.; Vila-Planas, J.; Capdevila, F.; Demming, S.; Buttgenbach, S.; Minguez, S.; Jimenez-Jorquera, C. Hybrid electronic tongue based on optical and electrochemical microsensors for quality control of wine. Analyst 2010, 135, 1718–1725. [Google Scholar] [CrossRef] [PubMed]
- Ministerio de Agricultura, Pesca y Alimentación. Ministerio de Agricultura, Pesca y Alimentación. ORDEN de 14 de Noviembre de 1991 por la que se Aprueba el Reglamento de la Denominación “Cava” y de su Consejo Regulador. In BOE-A-1991-28079; Secretaría General Técnica: Madrid, Spain, 1991; p. 37587. [Google Scholar]
- Community Methods for the Analysis of Wines. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1477386665745&uri=CELEX:31990R2676 (accesed on 25 August 2016).
- Compedium of International Methods of Wine and Must Analysis. Available online: http://www.oiv.int/public/medias/2624/compendium-2016-en-vol1.pdf (accesed on 25 August 2016).
- Jiménez, C.; Bratov, A.; Abramova, N.; Baldi, A. ISFET based sensors: fundamentals and applications. In Encyclopedia of Sensors; Grimes, C.A., Dickey, E.C., Pishko, M.V., Eds.; American Scientific Publishers: New York, NY, USA, 2005; Volume X. [Google Scholar]
- Artigas, J.; Beltran, A.; Jimenez, C.; Baldi, A.; Mas, R.; Dominguez, C.; Alonso, J. Application of ion sensitive field effect transistor based sensors to soil analysis. Comput. Electron. Agric. 2001, 31, 281–293. [Google Scholar] [CrossRef]
- Bratov, A.; Abramova, N.; Dominguez, C. Investigation of chloride sensitive ISFETs with different membrane compositions suitable for medical applications. Anal. Chim. Acta 2004, 514, 99–106. [Google Scholar] [CrossRef]
- Makarychev-Mikhailov, S.; Goryacheva, O.; Mortensen, J.; Legin, A.; Levitchev, S.; Vlasov, Y. Carbonate sensors based on 4-hexyltrifluoroacetophenone modified by acceptor substituents in phenyl ring. Electroanalysis 2003, 15, 1291–1296. [Google Scholar] [CrossRef]
- Beltran, A.; Artigas, J.; Jimenez, C.; Mas, R.; Bartroli, J.; Alonso, J. Development of durable nitrate-selective membranes for all-solid state ISE and ISFET sensors based on photocurable compositions. Electroanalysis 2002, 14, 213–220. [Google Scholar] [CrossRef]
- Orozco, J.; Baldi, A.; Baena, R.; Cadarso, A.; Bratov, A.; Jimenez, C. Portable system based on microsensors for environmental monitoring applications. Meas. Sci. Technol. 2007, 18, 935–940. [Google Scholar] [CrossRef]
- Buck, R.P.; Lindner, E. Recommendations for nomenclature of ion-selective electrodes (IUPAC recommendations 1994). Pure Appl. Chem. 1994, 66, 2527–2536. [Google Scholar] [CrossRef]
- Regulatory Board of Cava. Pliego de Condiciones Denominación de Origen Protegida “Cava”. Available online: http://www.docava.es/wp-content/uploads/2015/05/PLIEGO-DOP-CAVA.pdf (accesed on 25 August 2016).

| Equipment | Sensors | Variables |
|---|---|---|
| Multi-ISFET meter | Six ISFETs | pH, Na+, K+, Ca2+, Cl−, and CO32− |
| Multisensor meter | Two four-electrode sensors | Conductivity and ORP |
| Two gold electrodes | Current at +1.01 V and +1.31 V |
| Parameter | Sensitivity (mV/dec) 1 | Linear Range (M) | R2 | Limit of Detection (M) |
|---|---|---|---|---|
| pH | 54.2 (0.5) | pH 1.56–11.42 | 0.9998 (n = 10) | - |
| Na+ | 54.0 (0.4) | 2.1 × 10−5–2.2 × 10−2 | 0.9994 (n = 6) | 5.9 × 10−6 |
| K+ | 57.0 (0.8) | 2.0 × 10−5–2.1 × 10−2 | 0.9996 (n = 7) | 1.8 × 10−6 |
| Ca2+ | 28.6 (0.6) | 5.9 × 10−7–1.9 × 10−2 | 0.9998 (n = 9) | 1.9 × 10−7 |
| Cl− | −59 (1) | 2.0 × 10−4–2.1 × 10−2 | 0.9998 (n = 4) | 2.8 × 10−5 |
| CO32− | −58 (2) | 2.1 × 10−4–2.2 × 10−2 | 0.9993 (n = 4) | 3.0 × 10−5 |
| Classes | Prediction | Sensitivity (%) | Specificity (%) | |||
|---|---|---|---|---|---|---|
| Young | Reserva | Gran Reserva | Rosé | |||
| Young | 16 | 2 | 0 | 2 | 80 | 97 |
| Reserva | 0 | 24 | 1 | 0 | 96 | 91 |
| Gran Reserva | 0 | 3 | 13 | 0 | 81 | 98 |
| Rosé | 2 | 0 | 0 | 15 | 88 | 97 |
| Sample 1 | Total Acidity (g/L) | pH | VAD (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Standard Method | Electronic Tongue | Relative Error (%) | Standard Method | Electronic Tongue | Relative Error (%) | Standard Method | Electronic Tongue | Relative Error (%) | |
| Y 3336 | 6.5 | 5.9 | 8.5 | 3.04 | 3.23 | 6.1 | 11.75 | 11.97 | 1.8 |
| Y 3709 | 6.1 | 6.0 | 1.3 | 3.01 | 3.17 | 5.4 | 12.15 | 11.97 | 1.5 |
| Y 4956 | 6.5 | 6.1 | 6.4 | 2.94 | 2.95 | 0.3 | 11.85 | 11.87 | 0.2 |
| Y 5219 | 6.7 | 6.2 | 8.1 | 3.03 | 3.07 | 1.3 | 11.55 | 11.82 | 2.4 |
| R 2719 | 5.5 | 5.8 | 5.0 | 3.31 | 3.35 | 1.2 | 13.00 | 12.06 | 7.2 |
| R 2929 | 6.1 | 6.1 | 0.4 | 3.07 | 3.08 | 0.4 | 12.30 | 11.86 | 3.5 |
| R 3727 | 6.1 | 6.2 | 0.9 | 2.96 | 3.04 | 2.8 | 11.75 | 11.90 | 1.3 |
| R 5241 | 5.8 | 6.4 | 11.0 | 3.43 | 3.44 | 0.4 | 12.05 | 11.76 | 2.4 |
| R 5608 | 6.2 | 6.1 | 2.4 | 2.94 | 3.15 | 7.3 | 12.15 | 11.92 | 1.9 |
| R 5962 | 5.6 | 6.1 | 9.0 | 3.08 | 3.08 | 0.1 | 12.10 | 11.89 | 1.7 |
| GR 2720 | 5.5 | 5.7 | 4.4 | 3.15 | 3.05 | 3.2 | 12.85 | 12.05 | 6.2 |
| GR 3182 | 5.5 | 6.1 | 11.6 | 3.02 | 3.00 | 0.6 | 12.15 | 11.91 | 2.0 |
| GR 4183 | 5.5 | 5.6 | 1.3 | 3.02 | 3.04 | 0.7 | 12.40 | 12.07 | 2.7 |
| GR 5220 | 6.5 | 7.0 | 7.4 | 2.93 | 3.10 | 5.9 | 11.75 | 12.01 | 2.2 |
| Ro 2978 | 5.5 | 5.9 | 7.8 | 3.35 | 3.19 | 4.9 | 12.20 | 12.15 | 0.4 |
| Ro 3103 | 6.2 | 6.7 | 7.8 | 3.01 | 3.04 | 1.0 | 12.20 | 12.11 | 0.7 |
| Ro 4814 | 5.8 | 6.0 | 3.2 | 3.00 | 3.10 | 3.4 | 12.30 | 11.96 | 2.8 |
| Ro 5017 | 5.5 | 6.1 | 11.3 | 3.02 | 3.05 | 1.1 | 12.05 | 11.99 | 0.5 |
| Mean Relative Error | 6.0 | Mean Relative Error | 2.6 | Mean Relative Error | 2.3 | ||||
| Sample 1 | Potassium (mg/L) | Conductivity (mS/cm) | Glycerol (g/L) | Methanol (mg/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Standard Method | Electronic Tongue | Relative Error (%) | Standard Method | Electronic Tongue | Relative Error (%) | Standard Method | Electronic Tongue | Relative Error (%) | Standard Method | Electronic Tongue | Relative Error (%) | |
| Y 3336 | 355 | 360 | 1.3 | 1.34 | 1.33 | 0.9 | 5 | 5.5 | 9.7 | 29 | 30 | 3.8 |
| Y 3709 | 433 | 423 | 2.4 | 1.42 | 1.32 | 6.9 | 4.9 | 5.4 | 10.0 | 29 | 28 | 2.3 |
| Y 4956 | 326 | 328 | 0.6 | 1.29 | 1.26 | 2.1 | - | - | - | 30 | 30 | 1.2 |
| Y 5219 | 332 | 332 | 0.0 | 1.38 | 1.32 | 4.3 | 5.3 | 5.2 | 1.7 | 29 | 30 | 5.0 |
| R 2719 | 550 | 487 | 11.4 | 1.79 | 1.66 | 7.5 | 6.1 | 6.2 | 2.2 | 31 | 34 | 10.6 |
| R 2929 | 360 | 395 | 9.7 | 1.29 | 1.28 | 0.9 | 5.1 | 5.7 | 12.2 | 30 | 32 | 5.7 |
| R 3727 | 300 | 332 | 10.7 | 1.21 | 1.21 | 0.3 | 5.6 | 5.4 | 2.8 | 30 | 30 | 0.0 |
| R 5241 | 372 | 315 | 15.4 | 1.19 | 1.13 | 5.3 | 5.1 | 5.6 | 9.5 | 30 | 30 | 1.3 |
| R 5608 | 379 | 418 | 10.3 | 1.31 | 1.31 | 0.1 | 5.8 | 5.8 | 0.7 | 29 | 31 | 6.1 |
| R 5962 | 320 | 334 | 4.4 | 1.2 | 1.19 | 0.5 | 5.5 | 5.7 | 2.9 | 28 | 30 | 7.5 |
| GR 2720 | 544 | 518 | 4.7 | 1.73 | 1.70 | 1.6 | 6.9 | 6.3 | 8.1 | 31 | 32 | 3.0 |
| GR 3182 | 321 | 327 | 1.9 | 1.14 | 1.16 | 2.0 | 5.1 | 5.4 | 6.1 | 30 | 30 | 1.5 |
| GR 4183 | 391 | 408 | 4.2 | 1.42 | 1.41 | 0.6 | 5.5 | 5.4 | 2.5 | 28 | 31 | 10.8 |
| GR 5220 | 300 | 301 | 0.2 | 1.33 | 1.29 | 2.9 | 5.1 | 5.3 | 4.4 | 29 | 28 | 2.3 |
| Ro 2978 | 430 | 452 | 5.1 | 1.29 | 1.41 | 9.1 | 5.4 | 5.9 | 8.5 | 38 | 33 | 13.4 |
| Ro 3103 | 367 | 346 | 5.6 | 1.44 | 1.31 | 9.4 | 5.4 | 5.7 | 4.9 | 38 | 36 | 5.5 |
| Ro 4814 | 459 | 398 | 13.4 | 1.39 | 1.33 | 4.0 | 5.5 | 5.8 | 5.8 | 36 | 32 | 12.0 |
| Ro 5017 | 318 | 306 | 3.6 | 1.22 | 1.20 | 1.7 | 5.4 | 5.7 | 6.2 | 27 | 30 | 9.7 |
| Mean Relative Error | 5.8 | Mean Relative Error | 3.3 | Mean Relative Error | 5.8 | Mean Relative Error | 5.6 | |||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giménez-Gómez, P.; Escudé-Pujol, R.; Capdevila, F.; Puig-Pujol, A.; Jiménez-Jorquera, C.; Gutiérrez-Capitán, M. Portable Electronic Tongue Based on Microsensors for the Analysis of Cava Wines. Sensors 2016, 16, 1796. https://doi.org/10.3390/s16111796
Giménez-Gómez P, Escudé-Pujol R, Capdevila F, Puig-Pujol A, Jiménez-Jorquera C, Gutiérrez-Capitán M. Portable Electronic Tongue Based on Microsensors for the Analysis of Cava Wines. Sensors. 2016; 16(11):1796. https://doi.org/10.3390/s16111796
Chicago/Turabian StyleGiménez-Gómez, Pablo, Roger Escudé-Pujol, Fina Capdevila, Anna Puig-Pujol, Cecilia Jiménez-Jorquera, and Manuel Gutiérrez-Capitán. 2016. "Portable Electronic Tongue Based on Microsensors for the Analysis of Cava Wines" Sensors 16, no. 11: 1796. https://doi.org/10.3390/s16111796
APA StyleGiménez-Gómez, P., Escudé-Pujol, R., Capdevila, F., Puig-Pujol, A., Jiménez-Jorquera, C., & Gutiérrez-Capitán, M. (2016). Portable Electronic Tongue Based on Microsensors for the Analysis of Cava Wines. Sensors, 16(11), 1796. https://doi.org/10.3390/s16111796