Elimination of Drifts in Long-Duration Monitoring for Apnea-Hypopnea of Human Respiration
Abstract
:1. Introduction
2. Mechanism Analysis for Humidity Drift
2.1. Hot-Film Airflow Sensor
2.2. Simulation of Humidity Drift for Hot-Film Flow Sensor
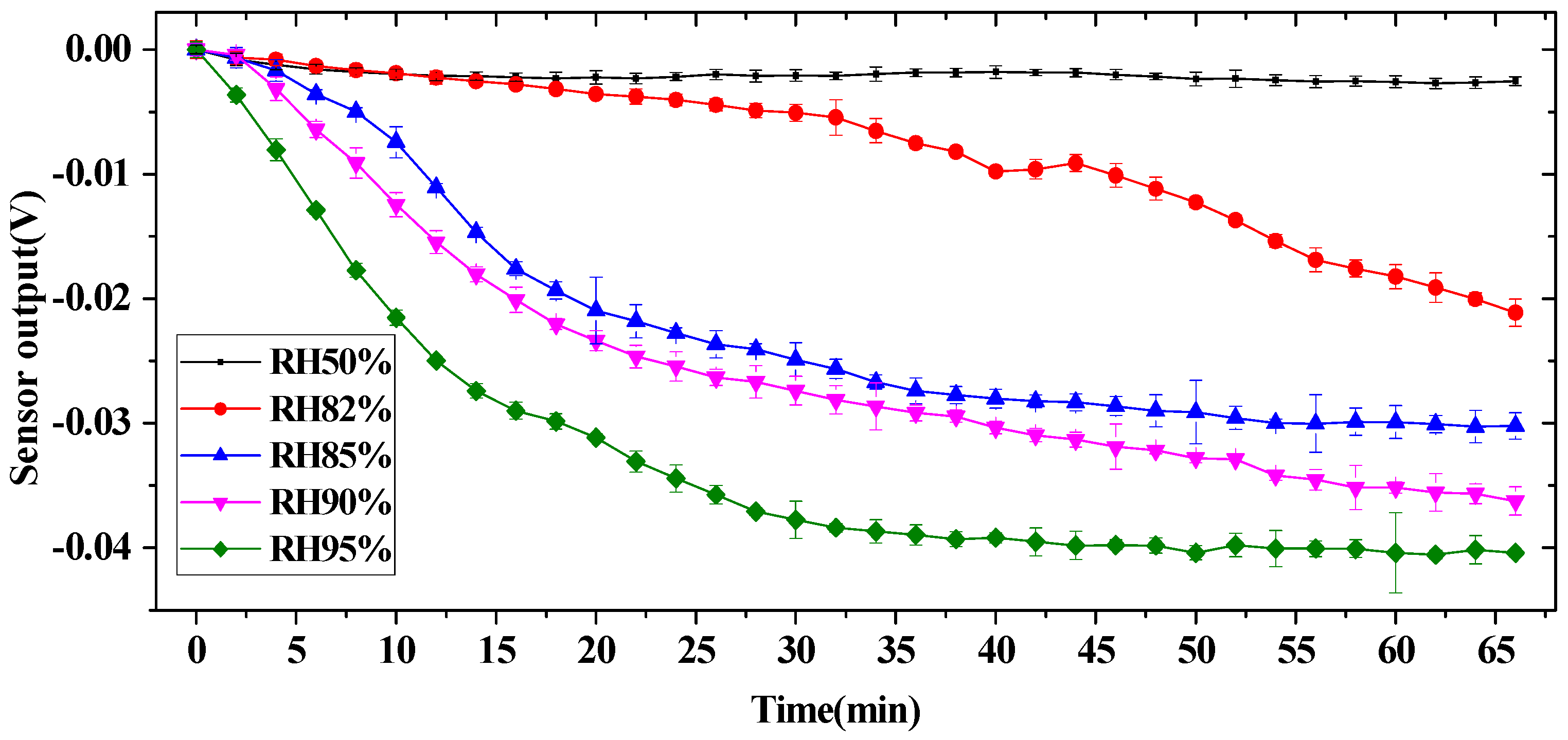
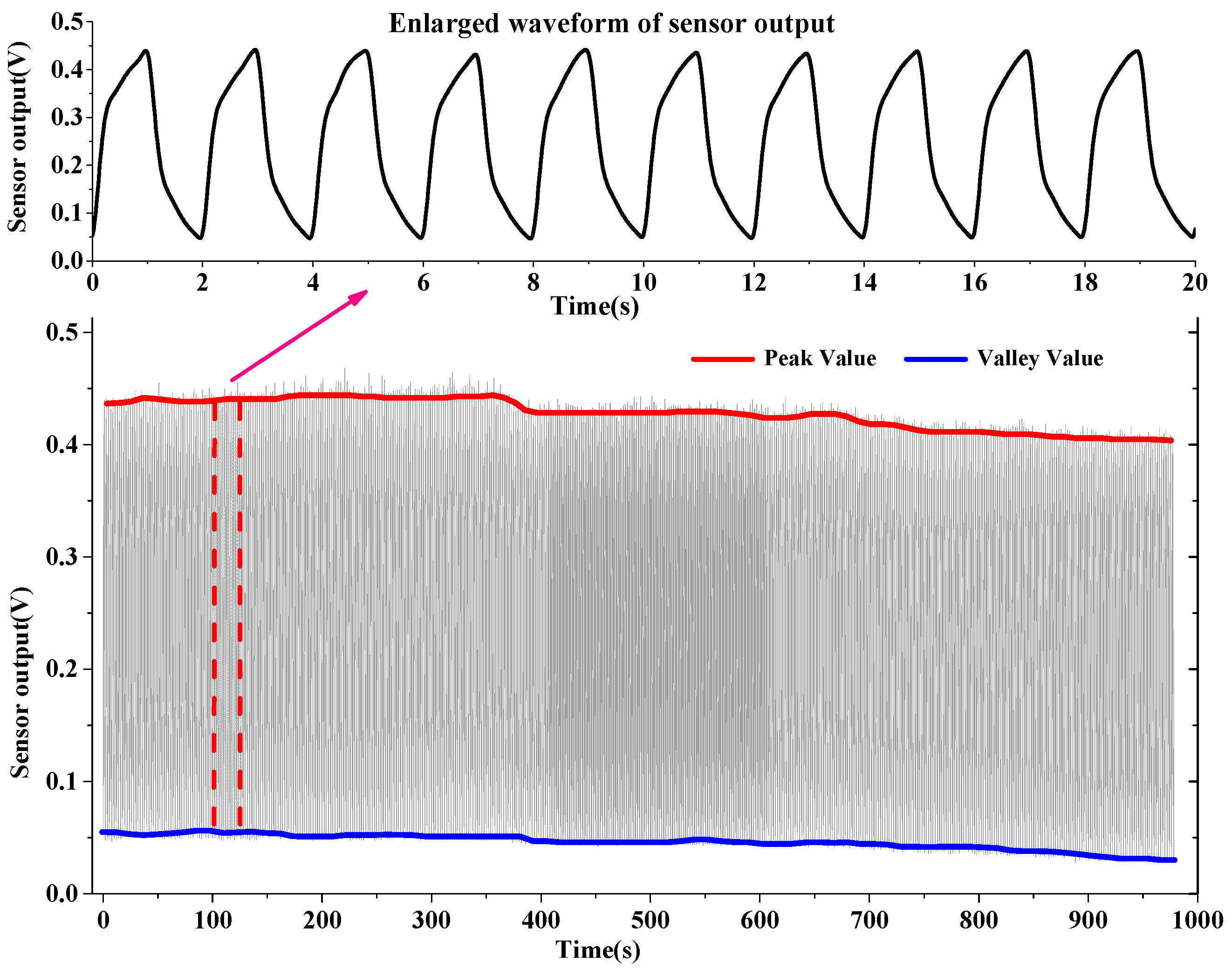
2.3. Experiment and Results
3. Remove Drifts for Respiratory Sensors
3.1. Wavelet Signal Processing
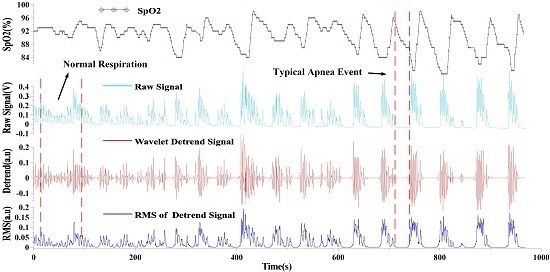
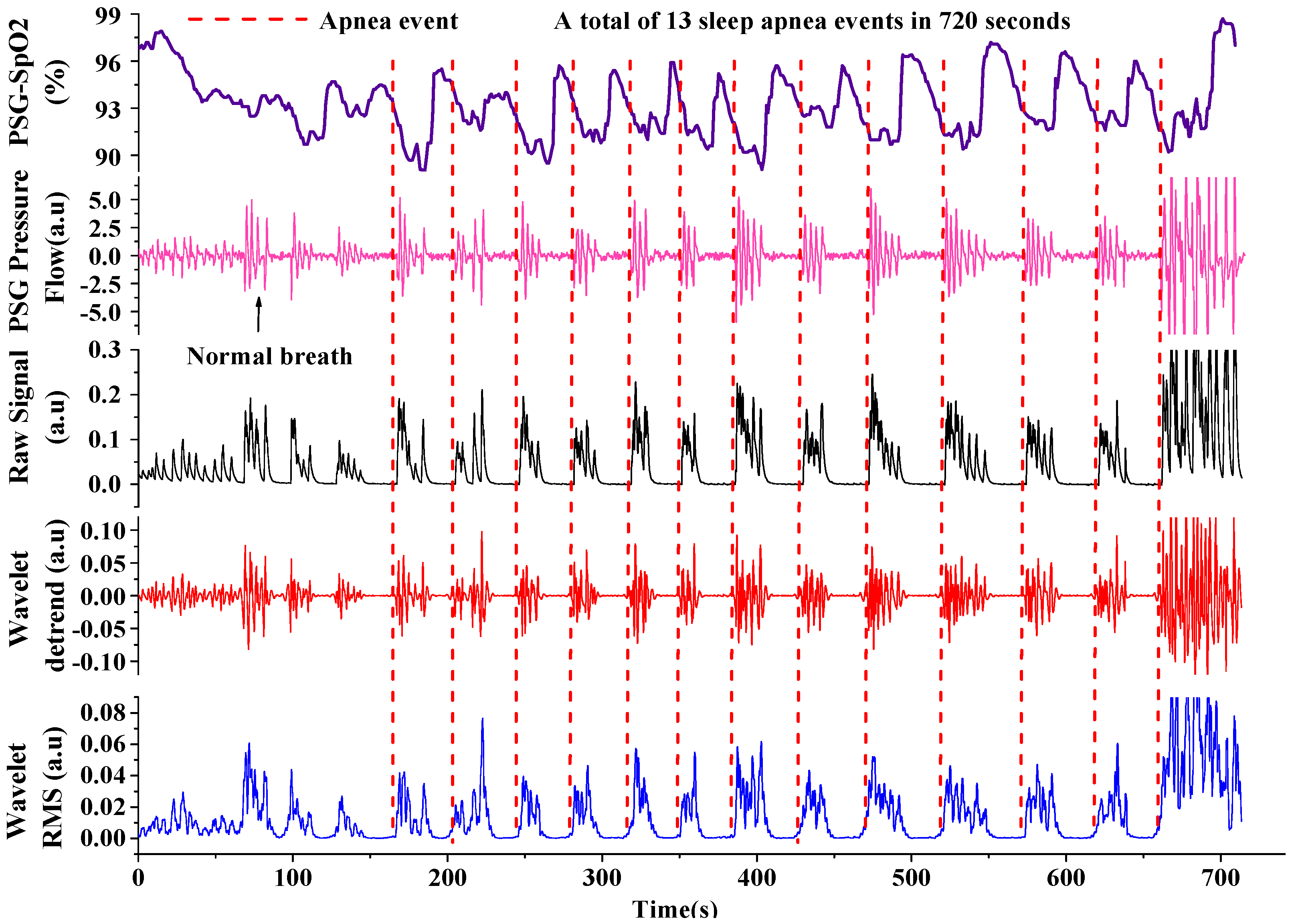
3.2. Extracting Apnea-Hypopnea Events and Results
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Martens, S.M.M.; Rabotti, C.; Mischi, M.; Sluijter, R.J. A robust fetal ECG detection method for abdominal recordings. Physiol. Meas. 2007, 28, 373. [Google Scholar] [CrossRef] [PubMed]
- Libourel, P.-A.; Corneyllie, A.; Luppi, P.-H.; Chouvet, G.; Gervasoni, D. Unsupervised online classifier in sleep scoring for sleep deprivation studies. Sleep 2015, 38, 815–828. [Google Scholar] [CrossRef] [PubMed]
- Addison, P.S.; Watson, J.N.; Mestek, M.L.; Ochs, J.P.; Uribe, A.A.; Bergese, S.D. Pulse oximetry-derived respiratory rate in general care floor patients. J. Clin. Monit. Comput. 2015, 29, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Hu, X.; Ye, M.; Cheng, X.; Li, F. Gas Recognition under Sensor Drift by Using Deep Learning. Int. J. Intell. Syst. 2015, 30, 907–922. [Google Scholar] [CrossRef]
- Deshmukh, S.; Bandyopadhyay, R.; Bhattacharyya, N.; Pandey, R.; Jana, A. Application of electronic nose for industrial odors and gaseous emissions measurement and monitoring, an overview. Talanta 2015, 144, 329–340. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. The 10 Leading Causes of Death in the World, 2000 and 2012; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Jain, C.D.; Bhaskar, D.J.; Kalra, M.; Gandhi, R.; Bohra, P. Recent Improvements in the Management of Obstructive Sleep Apnea: The Dental Perception. Int. J. Adv. Health Sci. 2015, 1, 7–10. [Google Scholar]
- Vaessen, T.J.; Overeem, S.; Sitskoorn, M.M. Cognitive complaints in obstructive sleep apnea. Sleep Med. Rev. 2015, 19, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.-L.; Gozal, D.; Ramirez, H.M.; Bandla, H.P.; Kheirandish-Gozal, L. Overnight polysomnography versus respiratory polygraphy in the diagnosis of pediatric obstructive sleep apnea. Sleep 2014, 37, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Tardi, G.; Massaroni, C.; Saccomandi, P.; Schena, E. Experimental Assessment of a Variable Orifice Flowmeter for Respiratory Monitoring. J. Sens. 2015, 2015, 752540. [Google Scholar] [CrossRef]
- Schena, E.; Massaroni, C.; Saccomandi, P.; Cecchini, S. Flow measurement in mechanical ventilation: A review. Med. Eng. Phys. 2015, 37, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Snepvangers, Y.; de Winter, P.; Burger, H.; Brouwers, H.A.; Bogaard, J.M.; van der Ent, K. Correction factors for oxygen and flow-rate effects on neonatal Fleisch and Lilly pneumotachometers. Pediatr. Crit. Care Med. 2003, 4, 227–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schena, E.; Cecchini, S.; Silvestri, S. An orifice meter for bidirectional air flow measurements: Influence of gas thermo-hygrometric content on static response and bidirectionality. Flow Meas. Instrum. 2013, 34, 105–112. [Google Scholar] [CrossRef]
- Konvalina, G.; Haick, H. Sensors for breath testing: from nanomaterials to comprehensive disease detection. Acc. Chem. Res. 2013, 47, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zhu, R.; Que, R. A Wireless Portable System with Micro Sensors for Monitoring Respiratory Diseases. IEEE Trans. Biomed. Eng. 2012, 59, 3110–3116. [Google Scholar] [PubMed]
- Jiang, P.; Zhao, S.; Zhu, R. Smart Sensing Strip Using Monolithically Integrated Flexible Flow Sensor for Noninvasively Monitoring Respiratory Flow. Sensors 2015, 15, 31738–31750. [Google Scholar] [CrossRef] [PubMed]
- Hramov, A.E.; Koronovskii, A.A.; Makarov, V.A.; Pavlov, A.N.; Sitnikova, E. Wavelets in Neuroscience; Springer: Berlin, Germany, 2015. [Google Scholar]
- Zhu, R.; Que, R.; Cao, Z. Micro thermal flow sensors/systems on flexible PCB and extensive applications. In Proceedings of the 8th IEEE International Conference on Nano/Micro Engineered and Molecular Systems (NEMS), Suzhou, China, 7–10 April 2013; pp. 179–182.
- Multiphysics, C.; Module, C.M.H.T. COMSOL Multiphysics User’s Guide. In Version: COMSOL Multiphysics; COMSOL Inc.: Burlington, MA, USA, 2014. [Google Scholar]
- Addison, P.S. The Illustrated Wavelet Transform Handbook: Introductory Theory and Applications in Science, Engineering, Medicine And Finance; IOP Publishing: Bristol, UK, 2002. [Google Scholar]
- Daubechies, I. Ten Lectures on Wavelets; Society for Industrial and Applied Mathematics: Philadelphia, PA, USA, 1992; Volume 61. [Google Scholar]
- Abo-Zahhad, M.; Al-Ajlouni, A.F.; Ahmed, S.M.; Schilling, R.J. A new algorithm for the compression of ECG signals based on mother wavelet parameterization and best-threshold levels selection. Digit. Signal Process. 2013, 23, 1002–1011. [Google Scholar] [CrossRef]
- Berry, R.B.; Brooks, R.; Gamaldo, C.E.; Harding, S.M.; Marcus, C.L.; Vaughn, B.V. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications; American Academy of Sleep Medicine: Darien, IL, USA, 2012. [Google Scholar]
- De Backer, W. Obstructive sleep apnea/hypopnea syndrome. Panminerva Med. 2013, 55, 191–195. [Google Scholar] [PubMed]







| Patients | Infomation | PSG Total Times | Our Device Total Times | AHI (times/h) | Effective Ratio | ||
|---|---|---|---|---|---|---|---|
| Age | Sex | PSG | Device | ||||
| Patient 1 | 31 | Female | 259 | 266 | 37 | 38 | 97.3% Good |
| Patient 2 | 34 | Male | 517 | 507 | 70 | 68 | 98.1% Good |
| Patient 3 | 70 | Male | 115 | 117 | 27 | 27 | 98.3% Good |
| Patient 4 | 55 | Female | 327 | 336 | 54 | 55 | 97.2% Good |
| Patient 5 | 45 | Male | 125 | 129 | 20 | 21 | 96.8% Good |
| Patient 6 | 73 | Male | 356 | 345 | 56 | 54 | 96.9 % Good |
| Patient 7 | 60 | Male | 393 | 410 | 59 | 61 | 95.7% Good |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, P.; Zhu, R. Elimination of Drifts in Long-Duration Monitoring for Apnea-Hypopnea of Human Respiration. Sensors 2016, 16, 1779. https://doi.org/10.3390/s16111779
Jiang P, Zhu R. Elimination of Drifts in Long-Duration Monitoring for Apnea-Hypopnea of Human Respiration. Sensors. 2016; 16(11):1779. https://doi.org/10.3390/s16111779
Chicago/Turabian StyleJiang, Peng, and Rong Zhu. 2016. "Elimination of Drifts in Long-Duration Monitoring for Apnea-Hypopnea of Human Respiration" Sensors 16, no. 11: 1779. https://doi.org/10.3390/s16111779