Visual and Plasmon Resonance Absorption Sensor for Adenosine Triphosphate Based on the High Affinity between Phosphate and Zr(IV)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Apparatus
2.3. The Preparation of AuNPs
2.4. General Procedures of ATP Detection
2.5. ATP Detection in Synthetic Mixture
3. Results and Discussions
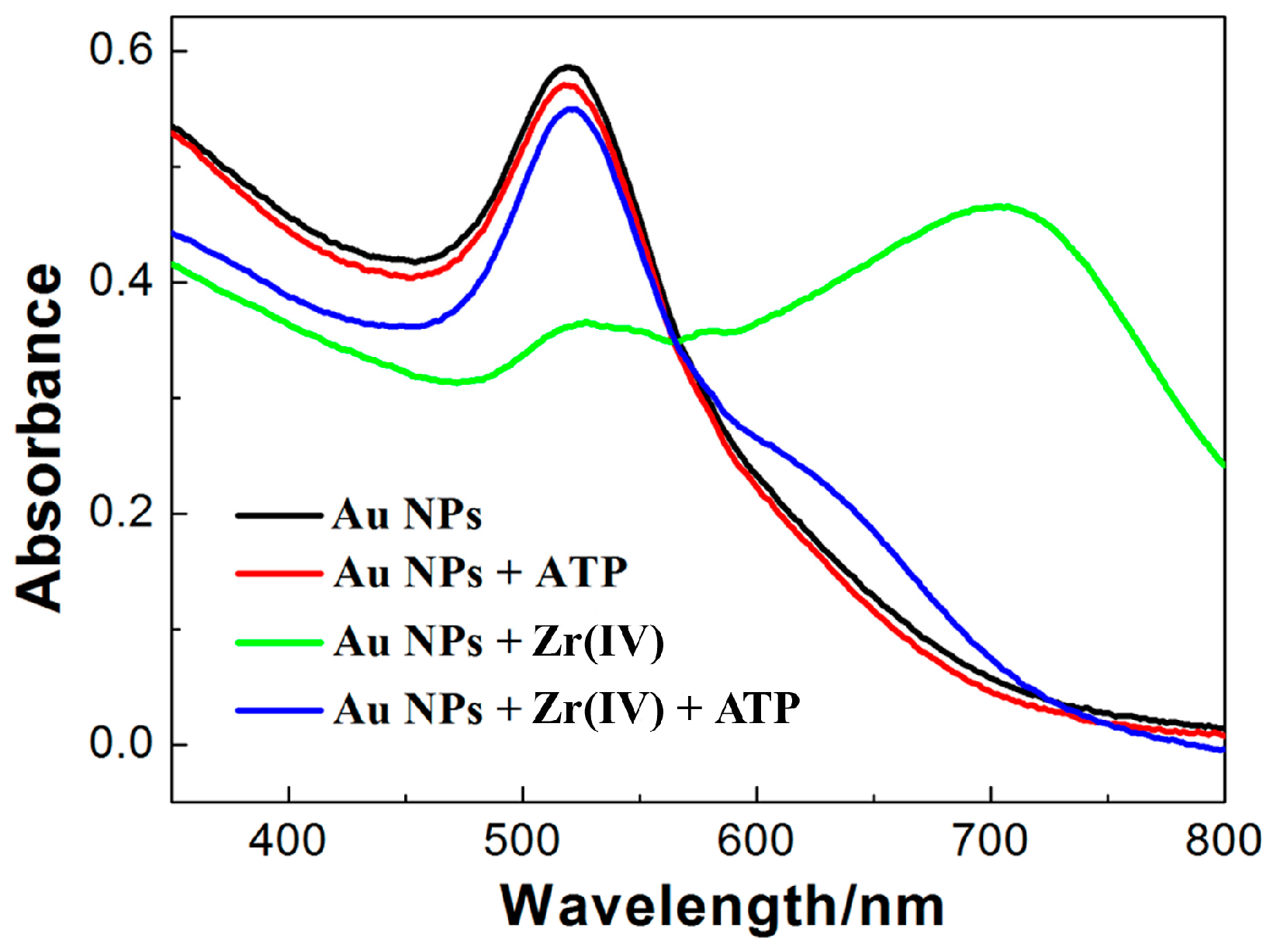
3.1. State of AuNPswith ATP in the Presence of Zr(IV)
3.2. Kinetic Behavior of PRA Sensor for ATP
3.3. Visual Sensor for ATP
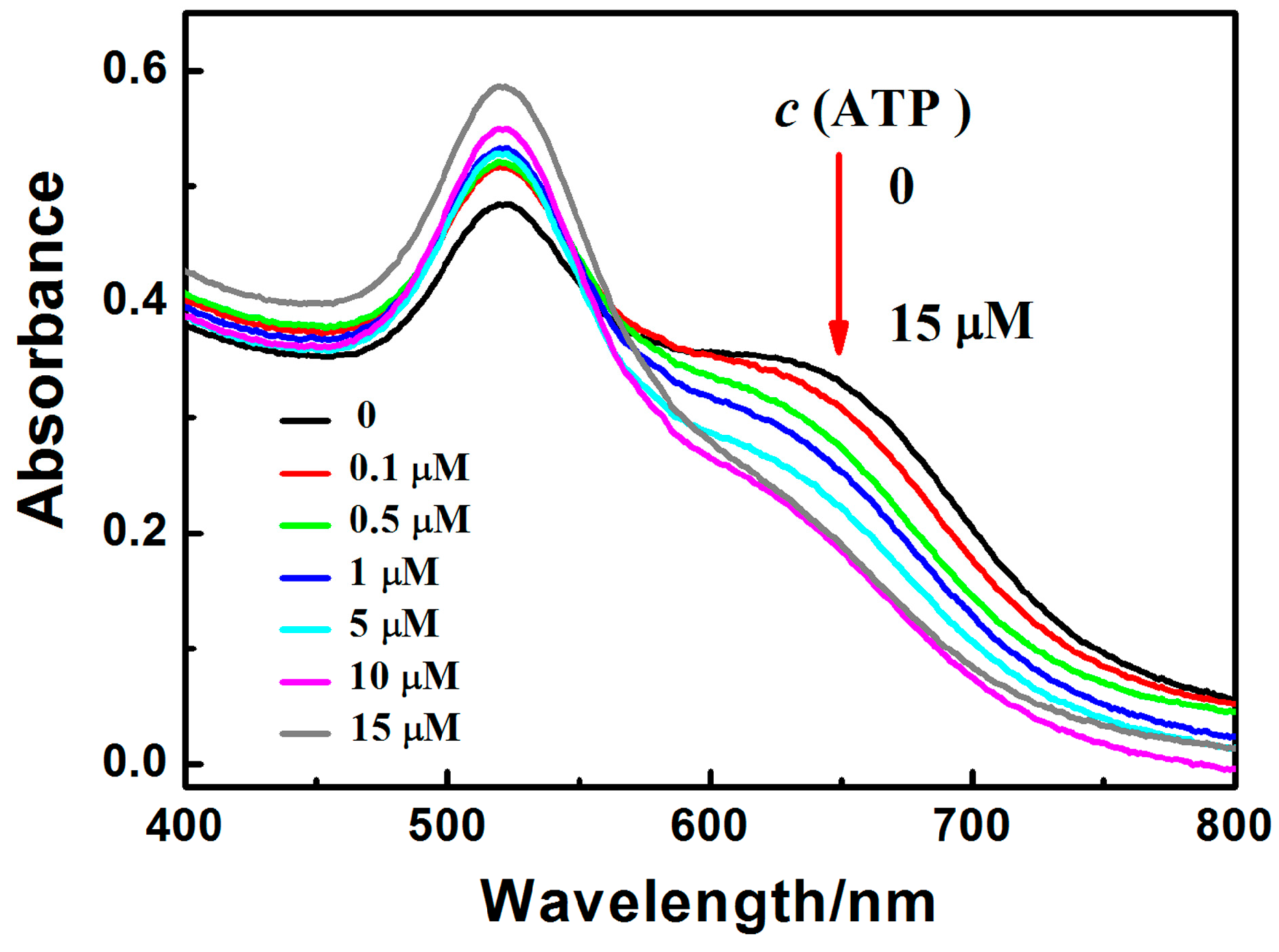
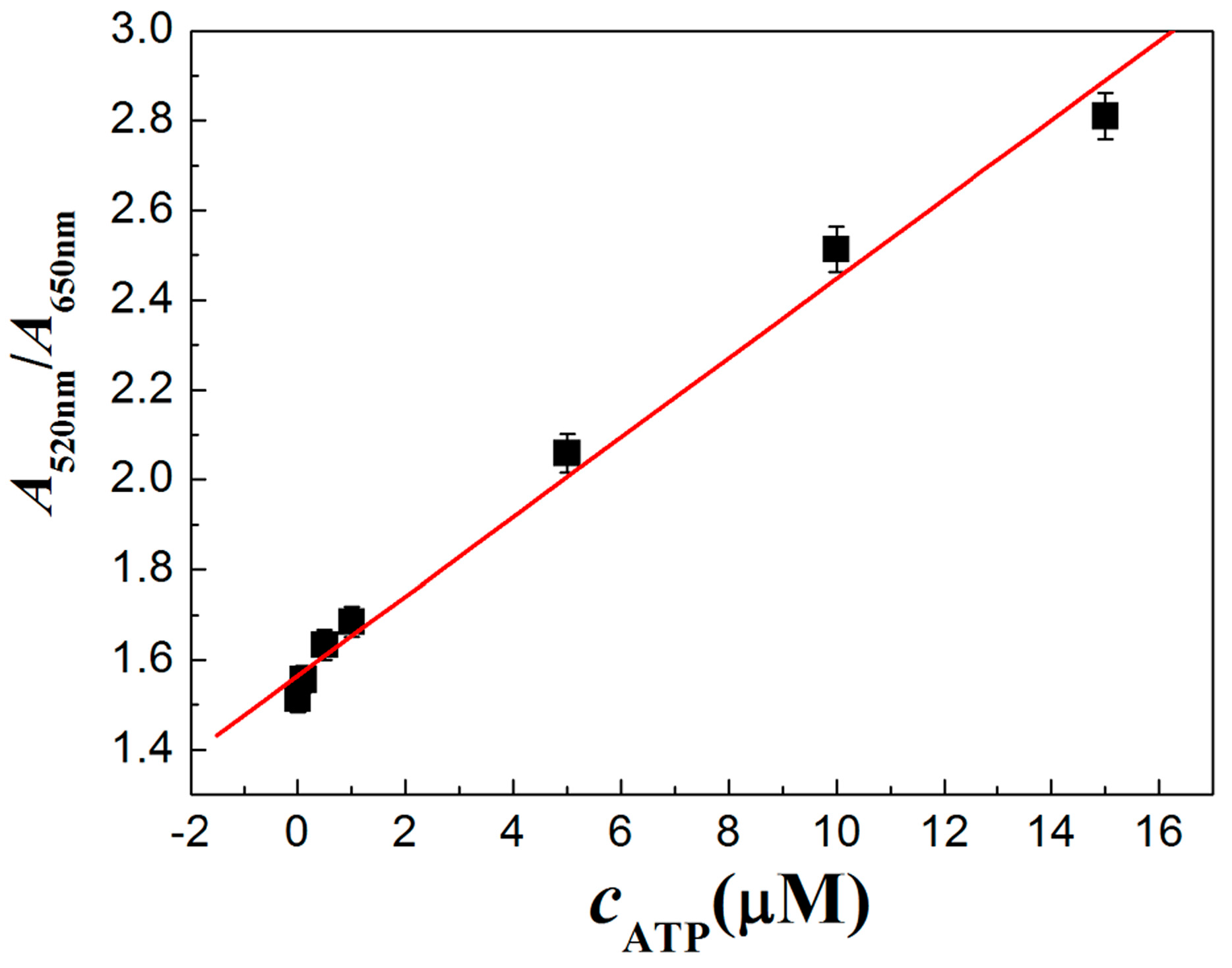
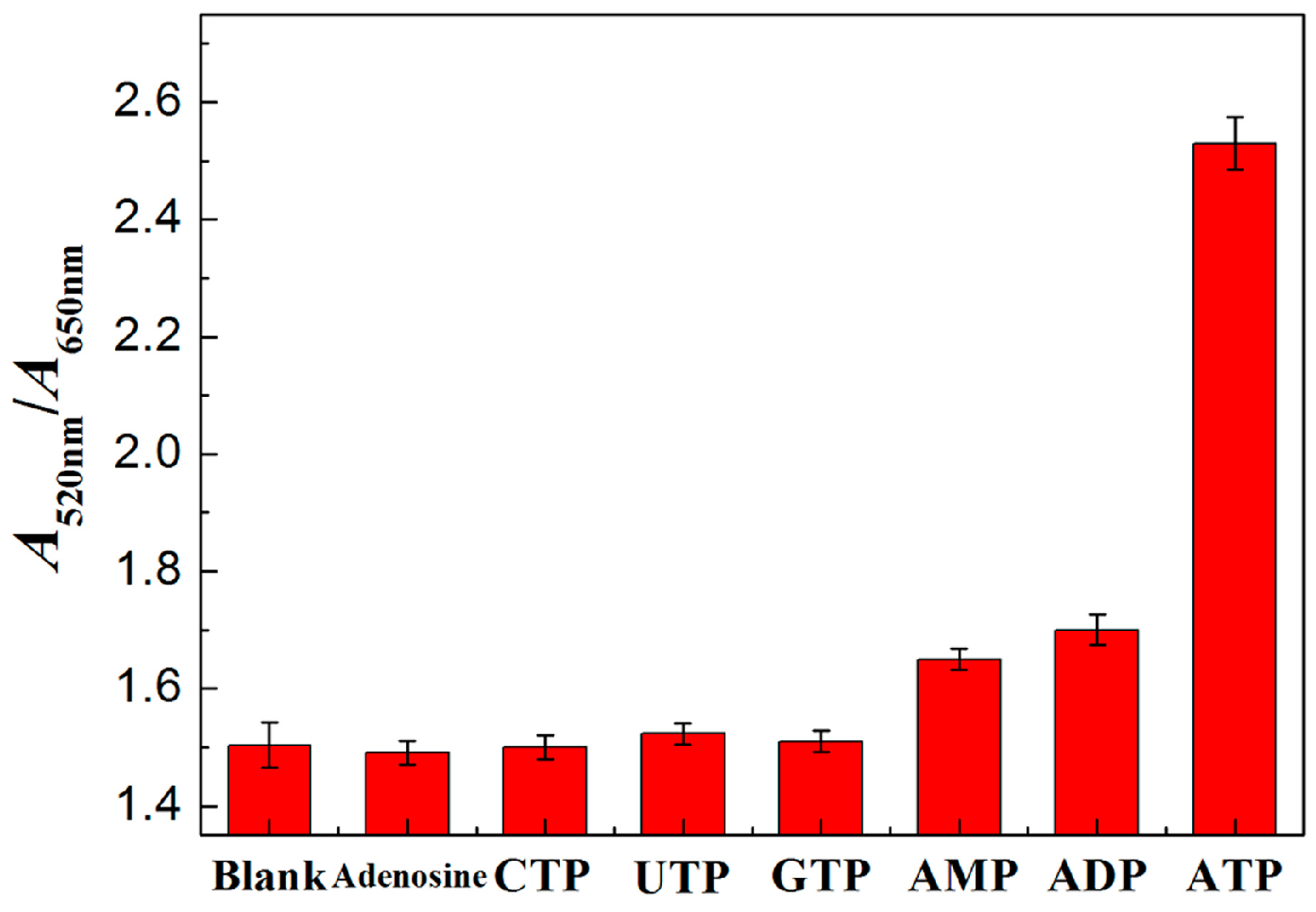
3.4. Characterization of PRA Sensor for ATP
3.5. Detection of ATP in Synthetic Mixture
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Park, J.-H.; Byun, J.-Y.; Shim, W.-B.; Kim, S.U.; Kim, M.-G. High-sensitivity detection of ATP using a localized surface plasmon resonance (LSPR) sensor and split aptamers. Biosens. Bioelectron. 2015, 73, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liao, J.; Yang, X.; Zhao, M.; Chen, M.; Yao, W.; Tan, W.; Lan, X. A label-free aptasensor for highly sensitive detection of ATP and thrombin based on metal-enhanced PicoGreen fluorescence. Biosens. Bioelectron. 2015, 63, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Chen, Y.; Li, H.; Shuang, S.; Dong, C.; Wang, G. An exonuclease I-based label-free fluorometric aptasensor for adenosine triphosphate (ATP) detection with a wide concentration range. Biosens. Bioelectron. 2015, 63, 311–316. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-Q.; Guo, S.-M.; Zuo, P.; Ye, B.-C. A cost-effective Z-folding controlled liquid handling microfluidic paper analysis device for pathogen detection via ATP quantification. Biosens. Bioelectron. 2015, 63, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Si, J.C.; Gao, Z.F.; Zhang, Y.; Lei, J.L.; Luo, H.Q.; Li, N.B. Highly selective and sensitive electrochemical biosensor for ATP based on the dual strategy integrating the cofactor-dependent enzymatic ligation reaction with self-cleaving DNAzyme-amplified electrochemical detection. Biosens. Bioelectron. 2015, 63, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Kahlin, J.; Mkrtchian, S.; Ebberyd, A.; Hammarstedt-Nordenvall, L.; Nordlander, B.; Yoshitake, T.; Kehr, J.; Prabhakar, N.; Poellinger, L.; Fagerlund, M.J.; et al. The human carotid body releases acetylcholine, ATP and cytokines during hypoxia. Exp. Physiol. 2014, 99, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.B.; Yang, X.H.; Wang, K.M.; Tang, Z.W.; Li, W.; Tan, W.H.; Lv, X.Y. A novel kinase-based ATP assay using molecular beacon. Anal. Biochem. 2008, 372, 131–133. [Google Scholar] [CrossRef] [PubMed]
- He, H.-Z.; Ma, P.-Y.; Leung, K.-H.; Chan, S.-H.; Yang, H.; Cheng, Z.; Leung, C.-H.; Ma, D.-L. A label-free G-quadruplex-based switch-on fluorescence assay for the selective detection of ATP. Analyst 2012, 137, 1538–1540. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, W.; Qi, W.; Gao, W.; Hanif, S.; Saqib, M.; Xu, G. Label-free signal-on ATP aptasensor based on the remarkable quenching of tris(2,2′-bipyridine)ruthenium(II) electrochemiluminescence by single-walled carbon nanohorn. Chem. Commun. 2015, 51, 4256–4258. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Bian, Z.; Li, H.; Han, S.; Yuan, Y.; Gao, L.; Xu, G. [Ru(bpy)2dppz]2+ electrochemiluminescence switch and Its applications for DNA interaction study and label-free ATP aptasensor. Anal. Chem. 2009, 81, 9807–9811. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhang, W.; Hu, L.; Li, H.; Zhu, S.; Xu, G. Label-free and signal-on electrochemiluminescence aptasensor for ATP based on target-induced linkage of split aptamer fragments by using [Ru(phen)3]2+ intercalated into double-strand DNA as a probe. Chem. Eur. J. 2010, 16, 13356–13359. [Google Scholar] [CrossRef] [PubMed]
- Jie, G.; Yuan, J.; Zhang, J. Quantum dots-based multifunctional dendritic superstructure for amplified electrochemiluminescence detection of ATP. Biosens. Bioelectron. 2012, 31, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Tan, Y.; Shi, J.; Liang, G.; Zhu, J.-J. DNA aptasensor for the detection of ATP based on quantum dots electrochemiluminescence. Nanoscale 2010, 2, 606–612. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Sun, X.; Yang, G.; Liu, J. Ultrasensitive detection of ATP based on ATP regeneration amplification and its application in cell homogenate and human serum. Chem. Commun. 2014, 50, 7659–7662. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Han, Y.; Guo, Y.; Dong, C. Porphyrin functionalized graphene nanosheets-based electrochemical aptasensor for label-free ATP detection. J. Mater. Chem. 2012, 22, 23900–23905. [Google Scholar] [CrossRef]
- Soldatkin, O.O.; Schuvailo, O.M.; Marinesco, S.; Cespuglio, R.; Soldatkin, A.P. Microbiosensor based on glucose oxidase and hexokinase co-immobilised on platinum microelectrode for selective ATP detection. Talanta 2009, 78, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, X.; Wang, K.; Ni, X. A sensitive ligase-based ATP electrochemical assay using molecular beacon-like DNA. Biosens. Bioelectron. 2010, 25, 2101–2106. [Google Scholar] [CrossRef] [PubMed]
- Bao, T.; Shu, H.; Wen, W.; Zhang, X.; Wang, S. A sensitive electrochemical aptasensor for ATP detection based on exonuclease III-assisted signal amplification strategy. Anal. Chim. Acta 2015, 862, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.-L.; Li, C.-Y.; Li, Y.-F.; Zou, C.-X. A ratiometric fluorescent probe with unexpected high selectivity for ATP and its application in cell imaging. Chem. Commun. 2014, 50, 15411–15414. [Google Scholar] [CrossRef] [PubMed]
- Pu, W.D.; Zhang, L.; Huang, C.Z. Graphene oxide as a nano-platform for ATP detection based on aptamer chemistry. Anal. Methods 2012, 4, 1662–1666. [Google Scholar] [CrossRef]
- Cheng, S.; Zheng, B.; Wang, M.; Lam, M.H.-W.; Ge, X. A target-triggered strand displacement reaction cycle: The design and application in adenosine triphosphate sensing. Anal. Biochem. 2014, 446, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Xu, J.; Xiang, Y.; Yuan, R.; Chai, Y. Target-induced structure switching of hairpin aptamers for label-free and sensitive fluorescent detection of ATP via exonuclease-catalyzed target recycling amplification. Biosens. Bioelectron. 2014, 51, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.P.; Wang, Z.X.; Zhang, L.; Qiu, J.D. Label-free colorimetric detection of arsenite utilizing G-/T-rich oligonucleotides and unmodified Au nanoparticles. Chem. Eur. J. 2013, 19, 5029–5033. [Google Scholar] [CrossRef] [PubMed]
- Zhan, S.; Wu, Y.; He, L.; Wang, F.; Zhan, X.; Zhou, P.; Qiu, S. A silver-specific DNA-based bio-assay for Ag(Ι) detection via the aggregation of unmodified gold nanoparticles in aqueous solution coupled with resonance Rayleigh scattering. Anal. Methods 2012, 4, 3997–4002. [Google Scholar] [CrossRef]
- Du, G.; Zhang, D.; Xia, B.; Xu, L.; Wu, S.; Zhan, S.; Ni, X.; Zhou, X.; Wang, L. A label-free colorimetric progesterone aptasensor based on the aggregation of gold nanoparticles. Microchim. Acta 2016, 183, 2251–2258. [Google Scholar] [CrossRef]
- Xing, H.; Zhan, S.; Wu, Y.; He, L.; Zhou, P. Sensitive colorimetric detection of melamine in milk with an aptamer-modified nanogold probe. RSC Adv. 2013, 3, 17424–17430. [Google Scholar] [CrossRef]
- Kanayama, N.; Takarada, T.; Maeda, M. Rapid naked-eye detection of mercury ions based on non-crosslinking aggregation of double-stranded DNA-carrying gold nanoparticles. Chem. Commun. 2011, 47, 2077–2079. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.D.; Li, Y.F.; Ling, J.; Huang, C.Z. A localized surface plasmon rsonance light-scattering assay of mercury (II) on the basis of Hg2+-DNA complex induced aggregation of gold nanoparticles. Environ. Sci. Technol. 2009, 43, 5022–5027. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhan, S.; Wang, F.; He, L.; Zhi, W.; Zhou, P. Cationic polymers and aptamers mediated aggregation of gold nanoparticles for the colorimetric detection of arsenic(III) in aqueous solution. Chem. Commun. 2012, 48, 4459–4461. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhi, W.; Wu, Y.; Zhan, S.; Wang, F.; Xing, H.; Zhou, P. A highly sensitive resonance scattering based sensor using unmodified gold nanoparticles for daunomycin detection in aqueous solution. Anal. Methods 2012, 4, 2266–2271. [Google Scholar] [CrossRef]
- Liu, Y.; Yang, Y.; Zhao, X.; Liu, Z.; Li, Y. Responsive disassembly of the gold nanoparticle aggregates triggered by the competitive adsorption for lighting up the colorimetric sensing. Anal. Methods 2013, 5, 3242–3247. [Google Scholar] [CrossRef]
- Deng, D.; Xia, N.; Li, S.; Xu, C.; Sun, T.; Pang, H.; Liu, L. Simple, fast and selective detection of adenosine triphosphate at physiological pH using unmodified gold nanoparticles as colorimetric probes and metal ions as cross-linkers. Sensors 2012, 12, 15078–15087. [Google Scholar] [CrossRef] [PubMed]
- Huo, Y.; Qi, L.; Lv, X.-J.; Lai, T.; Zhang, J.; Zhang, Z.-Q. A sensitive aptasensor for colorimetric detection of adenosine triphosphate based on the protective effect of ATP-aptamer complexes on unmodified gold nanoparticles. Biosens. Bioelectron. 2016, 78, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.N.; Lee, K.H.; Su, X. Study of Single-Stranded DNA Binding Protein-Nucleic Acids Interactions using Unmodified Gold Nanoparticles and Its Application for Detection of Single Nucleotide Polymorphisms. Anal. Chem. 2011, 83, 4251–4257. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, Y.; Yang, X. Aptamer-based colorimetric biosensing of dopamine using unmodified gold nanoparticles. Sens. Actuators B Chem. 2011, 156, 95–99. [Google Scholar] [CrossRef]
- Chen, S.-J.; Huang, Y.-F.; Huang, C.-C.; Lee, K.-H.; Lin, Z.-H.; Chang, H.-T. Colorimetric determination of urinary adenosine using aptamer-modified gold nanoparticles. Biosens. Bioelectron. 2008, 23, 1749–1753. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Liang, R.P.; Bai, J.M.; Qiu, J.D. Using graphene quantum dots as photoluminescent probes for protein kinase sensing. Anal. Chem. 2013, 85, 9148–9155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, Q.; Liang, Z.; Yang, K.; Sun, L.; Zhang, L.; Zhang, Y. Synthesis of adenosine functionalized metal immobilized magnetic nanoparticles for highly selective and sensitive enrichment of phosphopeptides. Chem. Commun. 2012, 48, 6274–6276. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhou, X.; Xing, D. Highly sensitive protein kinase activity assay based on electrochemiluminescence nanoprobes. Biosens. Bioelectron. 2012, 31, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.J.; Wu, D.; Ling, J.; Huang, C.Z. Visual and light scattering spectrometric detections of melamine with polythymine-stabilized gold nanoparticles through specific triple hydrogen-bonding recognition. Chem. Commun. 2010, 46, 4893–4895. [Google Scholar] [CrossRef] [PubMed]
- Qi, W.; Zhao, J.; Zhang, W.; Liu, Z.; Xu, M.; Anjum, S.; Majeed, S.; Xu, G. Visual and surface plasmon resonance sensor for zirconium based on zirconium-induced aggregation of adenosine triphosphate-stabilized gold nanoparticles. Anal. Chim. Acta 2013, 787, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Feng, L.; Ren, J.; Wu, L.; Qu, X. Visual Detection of Glucose Using Conformational Switch of I-Motif DNA and Non-Crosslinking Gold Nanoparticles. Chem. Eur. J. 2012, 18, 12637–12642. [Google Scholar] [CrossRef] [PubMed]
- Larkey, N.E.; Brucks, C.N.; Lansing, S.S.; Le, S.D.; Smith, N.M.; Tran, V.; Zhang, L.; Burrows, S.M. Molecular structure and thermodynamic predictions to create highly sensitive microRNA biosensors. Anal. Chim. Acta 2016, 909, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Goswami, N.; Giri, A.; Bootharaju, M.S.; Xavier, P.L.; Pradeep, T.; Pal, S.K. Copper quantum clusters in protein matrix: Potential sensor of Pb2+ ion. Anal. Chem. 2011, 83, 9676–9680. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Hu, Y.; Cen, Y.; Chu, X.; Lu, Y. A dual-emission fluorescent nanocomplex of gold-cluster-decorated silica particles for live cell imaging of highly reactive oxygen species. J. Am. Chem. Soc. 2013, 135, 11595–11602. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Irudayaraj, J. Fluorescent Ag clusters via a protein-directed approach as a Hg(II) ion sensor. Anal. Chem. 2011, 83, 2883–2889. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Lee, T.M.H.; Leung, S.S.Y.; Hsing, I.M. Tunable stabilization of gold nanoparticles in aqueous solutions by mononucleotides. Langmuir 2007, 23, 7143–7147. [Google Scholar] [CrossRef] [PubMed]
- Sitaula, S.; Branch, S.D.; Ali, M.F. GOx signaling triggered by aptamer-based ATP detection. Chem. Commun. 2012, 48, 9284–9286. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.; Pyatibrat, L.; Kalendo, G. Irradiation with He-Ne laser increases ATP level in cells cultivated in vitro. J. Photochem. Photobiol. B 1995, 27, 219–223. [Google Scholar] [CrossRef]




| Methods | Materials | Linear Range (μM) | LOD (nM) | Refs. |
|---|---|---|---|---|
| ECL(using aptamer) | CdSe/ZnSquantum dots | 0.018~90.7 | 6 | [13] |
| ECL(using aptamer) | Magnetic nanoparticles-CdSe/CdS quantum dots | 0.01~0.8 | 3 | [12] |
| ECL(using aptamer) | [Ru(bpy)3]2+, single-walled carbonnanohorn | 0.005~50 | 1 | [9] |
| ECL(using aptamer) | [Ru(bpy)2dppz]2+ | 0.2~1 | 100 | [10] |
| FRET(using aptamer) | FAM-labelled DNA, graphene oxide | 3~320 | 450 | [20] |
| FRET(using aptamer) | FAM using SDR amplification | 0.02~0.6 | 20 | [21] |
| FRET(no aptamer) | The prepared ratiometric fluorescent probe: naphthalimide-rhodaminecompound | 0.1~10 | 100 | [19] |
| Fluorescence(using aptamer) | SYBR Green I using exonuclease-catalyzed target recycling amplification | 0.01~2 | 9.5 | [22] |
| LSPR(using aptamer) | Gold nanorod, TAMRA dye | 0.00001~10 | 10 pM | [1] |
| ITC(using aptamer) | Glucose oxidase | 10~100 | 10 μM | [48] |
| DPV(using aptamer) | Porphyrin functionalized graphene nanosheets | 0.0022~1.3 | 0.7 | [15] |
| Amperometry(no aptamer) | Glucose oxidase and hexokinase co-immobilizedPt electrode | 100~16000 | 2500 | [16] |
| PRA(no aptamer) | AuNPs, Zr(IV) | 0.1~15 | 28 | This work |
| Samples | The Added ATP (μM) | The Total ATP (μM) | Mean Recoveries (%) |
|---|---|---|---|
| 1 | 1 | 0.98, 1.03, 1.05 | 102.0 |
| 2 | 5 | 5.04, 5.01, 5.11 | 101.1 |
| 3 | 10 | 9.87, 9.93, 10.05 | 95.3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, W.; Liu, Z.; Zhang, W.; Halawa, M.I.; Xu, G. Visual and Plasmon Resonance Absorption Sensor for Adenosine Triphosphate Based on the High Affinity between Phosphate and Zr(IV). Sensors 2016, 16, 1674. https://doi.org/10.3390/s16101674
Qi W, Liu Z, Zhang W, Halawa MI, Xu G. Visual and Plasmon Resonance Absorption Sensor for Adenosine Triphosphate Based on the High Affinity between Phosphate and Zr(IV). Sensors. 2016; 16(10):1674. https://doi.org/10.3390/s16101674
Chicago/Turabian StyleQi, Wenjing, Zhongyuan Liu, Wei Zhang, Mohamed Ibrahim Halawa, and Guobao Xu. 2016. "Visual and Plasmon Resonance Absorption Sensor for Adenosine Triphosphate Based on the High Affinity between Phosphate and Zr(IV)" Sensors 16, no. 10: 1674. https://doi.org/10.3390/s16101674





