Low-Coherence Reflectometry for Refractive Index Measurements of Cells in Micro-Capillaries
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Models
2.2. Normal Cells
2.3. Transformed Cells
2.4. Red Blood Cells
2.5. Cell Staining
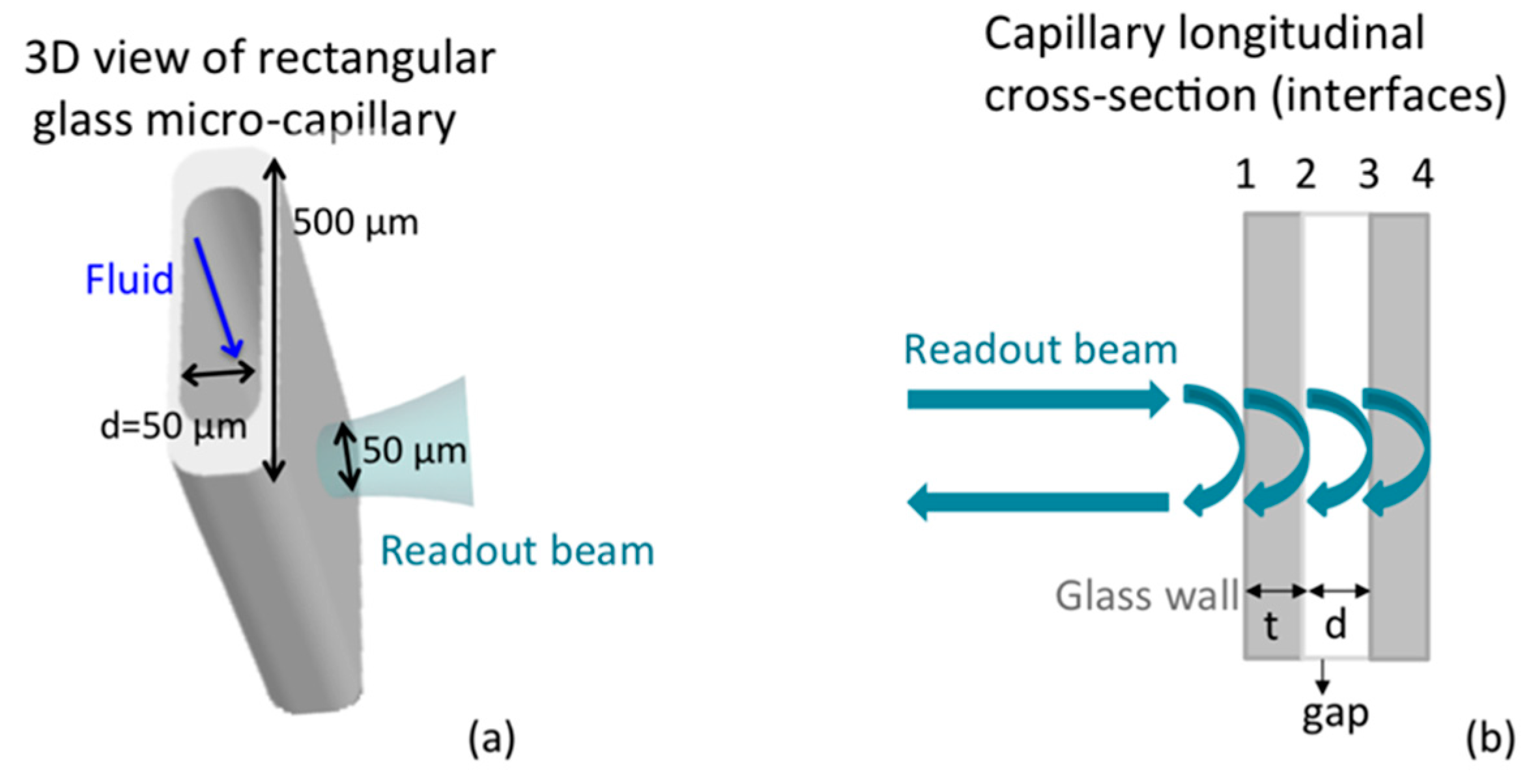
2.6. Capillary Preparation
2.7. Microscopy Analyses of Cell Distribution Inside the Capillary
2.8. Instrumental Configuration
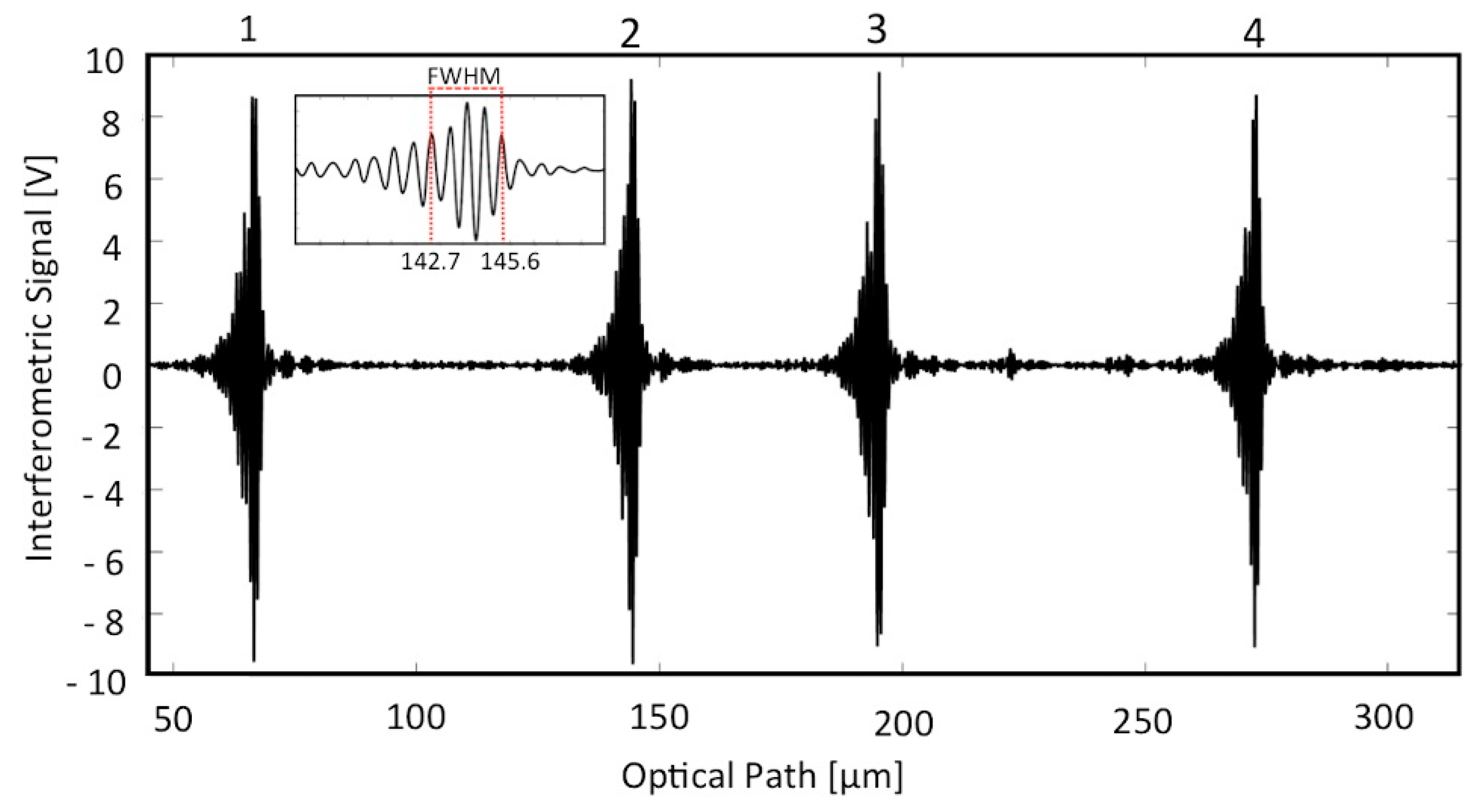
3. Results
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, P.Y.; Chin, L.K.; Ser, W.; Chen, H.F.; Hsieh, C.-M.; Lee, C.-H.; Sung, K.-B.; Ayi, T.C.; Yap, P.H.; Liedberg, B.; et al. Cell refractive index for cell biology and disease diagnosis: Past, present and future. Lab Chip 2016, 16, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Jacques, S.L. Optical Properties of Biological Tissues: A Review. Phys. Med. Biol. 2013, 58, R37–R61. [Google Scholar] [CrossRef] [PubMed]
- Barer, R. Determination of dry mass, thickness, solid and water concentration in living cells. Nature 1953, 172, 1097–1098. [Google Scholar] [CrossRef] [PubMed]
- Song, W.Z.; Zhang, X.M.; Liu, A.Q.; Lim, C.S.; Yap, P.H.; Hosseini, H.M.M. Refractive index measurement of single living cells using on-chip Fabry-Pérot cavity. Appl. Phys. Lett. 2006, 89, 203901-1–203901-3. [Google Scholar] [CrossRef]
- Lue, N.; Choi, W.; Popescu, G.; Yaqoob, Z.; Badizadegan, K.; Dasari, R.R.; Feld, M.S. Live cell refractometry using hilbert phase microscopy and confocal reflectance microscopy. J. Phys. Chem. A 2009, 113, 13327–13330. [Google Scholar] [CrossRef] [PubMed]
- Yim, J.; Kim, H.; Ryu, S.; Song, S.; Kim, H.O.; Hyun, K.A.; Jung, H.I.; Joo, C. Photothermal spectral-domain optical coherence reflectometry for direct measurement of hemoglobin concentration of erythrocytes. Biosens. Bioelectron. 2014, 57, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.J.; Jeon, D.I.; Ahn, S.-G.; Yoon, J.-H.; Kim, S.; Lee, B.H. Full-field optical coherence microscopy for identifying live cancer cells by quantitative measurement of refractive index distribution. Opt. Express 2010, 18, 23285–23295. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Tangella, K.; Balla, A.; Popescu, G. Tissue refractive index as marker of disease. J. Biomed. Opt. 2011, 16, 116017-1–116017-7. [Google Scholar] [CrossRef] [PubMed]
- Chin, L.K.; Liu, A.Q.; Lim, C.S.; Zhang, X.M.; Ng, J.H.; Hao, J.Z.; Takahashi, S. Differential single living cell refractometry using grating resonant cavity with optical trap. Appl. Phys. Lett. 2007, 91, 243901-1–243901-3. [Google Scholar] [CrossRef]
- Curl, C.L.; Bellair, C.J.; Harris, T.; Allman, B.E.; Harris, P.J.; Stewart, A.G.; Roberts, A.; Nugent, K.A.; Delbridge, L.M.D. Refractive index measurement in viable cells using quantitative phase-amplitude microscopy and confocal microscopy. Cytom. Part A 2005, 65, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Backman, V.; Wallace, M.B.; Perelman, L.T.; Arendt, J.T.; Gurjar, R.; Müller, M.G.; Zhang, Q.; Zonios, G.; Kline, E.; McGilligan, J.A.; et al. Detection of preinvasive cancer cells. Nature 2000, 406, 35–36. [Google Scholar] [PubMed]
- Liu, Y.; Uttam, S.; Alexandrov, S.; Bista, R.K. Investigation of nanoscale structural alterations of cell nucleus as an early sign of cancer. BMC Biophys. 2014, 7, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.J.; Liu, A.Q.; Lim, C.S.; Ayi, T.C.; Yap, P.H. Determining refractive index of single living cell using an integrated microchip. Sens. Actuators A Phys. 2007, 133, 349–354. [Google Scholar] [CrossRef]
- Wang, P.; Bista, R.; Bhargava, R.; Brand, R.E.; Liu, Y. Spatial-domain low-coherence quantitative phase microscopy for cancer diagnosis. Opt. Lett. 2010, 35, 2840–2842. [Google Scholar] [CrossRef] [PubMed]
- Bista, R.K.; Uttam, S.; Wang, P.; Staton, K.; Choi, S.; Bakkenist, C.J.; Hartman, D.J.; Brand, R.E.; Liu, Y. Quantification of nanoscale nuclear refractive index changes during the cell cycle. J. Biomed. Opt. 2011, 16, 70503-1–70503-3. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Song, M.; Zhang, Y.; Yan, M.; Zhang, M.; Bi, H. Nickel nanowires induce cell cycle arrest and apoptosis by generation of reactive oxygen species in HeLa cells. Toxicol. Rep. 2014, 1, 114–121. [Google Scholar] [CrossRef]
- Wu, X. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med. Sci. Monit. Basic Res. 2015, 21, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Bista, R.K.; Qiu, W.; Khalbuss, W.E.; Zhang, L.; Brand, R.E.; Liu, Y. An insight into statistical refractive index properties of cell internal structure via low-coherence statistical amplitude microscopy. Opt. Express 2010, 18, 21950–21958. [Google Scholar] [CrossRef] [PubMed]
- Rappaz, B.; Marquet, P.; Cuche, E.; Emery, Y.; Depeursinge, C.; Magistretti, P. Measurement of the integral refractive index and dynamic cell morphometry of living cells with digital holographic microscopy. Opt. Express 2005, 13, 9361–9373. [Google Scholar] [CrossRef] [PubMed]
- Rappaz, B.; Barbul, A.; Emery, Y.; Korenstein, R.; Depeursinge, C.; Magistretti, P.J.; Marquet, P. Comparative study of human erythrocytes by digital holographic microscopy, confocal microscopy, and impedance volume analyzer. Cytom. Part A 2008, 73, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Uttam, S.; Bista, R.K.; Staton, K.; Alexandrov, S.; Choi, S.; Bakkenist, C.J.; Hartman, D.J.; Brand, R.E.; Liu, Y. Investigation of depth-resolved nanoscale structural changes in regulated cell proliferation and chromatin decondensation. Biomed. Opt. Express 2013, 4, 596–613. [Google Scholar] [CrossRef] [PubMed]
- Choma, M.A.; Ellerbee, A.K.; Yang, C.; Creazzo, T.L.; Izatt, J.A. Spectral-domain phase microscopy. Opt. Lett. 2005, 30, 1162–1164. [Google Scholar] [CrossRef] [PubMed]
- Joo, C.; Akkin, T.; Cense, B.; Park, B.H.; de Boer, J.F. Spectral-domain optical coherence phase microscopy for quantitative phase-contrast imaging. Opt. Lett. 2005, 30, 2131–2133. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Hyun, K.-A.; Heo, J.; Jung, H.-I.; Joo, C. Label-free cell-based assay with spectral-domain optical coherence phase microscopy. J. Biomed. Opt. 2014, 19, 46003-1–46003-6. [Google Scholar] [CrossRef] [PubMed]
- Song, W.Z.; Liu, A.Q.; Swaminathan, S.; Lim, C.S.; Yap, P.H.; Ayi, T.C. Determination of single living cell’s dry/water mass using optofluidic chip. Appl. Phys. Lett. 2007, 91, 89–92. [Google Scholar] [CrossRef]
- Yasokawa, T.; Ishimaru, I.; Kondo, M.; Kuriyama, S.; Masaki, T.; Takegawa, K.; Tanaka, N. A method for measuring the three-dimensional refractive-index distribution of single cells using proximal two-beam optical tweezers and a phase-shifting Mach-Zehnder interferometer. Opt. Rev. 2007, 14, 161–164. [Google Scholar] [CrossRef]
- Tung, Y.C.; Huang, N.T.; Oh, B.R.; Patra, B.; Pan, C.C.; Qiu, T.; Chu, P.K.; Zhang, W.; Kurabayashi, K. Optofluidic detection for cellular phenotyping. Lab Chip 2012, 12, 3552–3565. [Google Scholar] [CrossRef] [PubMed]
- Lue, N.; Popescu, G.; Ikeda, T.; Dasari, R.R.; Badizadegan, K.; Feld, M.S. Live cell refractometry using microfluidic devices. Opt. Lett. 2006, 31, 2759–2761. [Google Scholar] [CrossRef] [PubMed]
- Yashunsky, V.; Lirtsman, V.; Golosovsky, M.; Davidov, D.; Aroeti, B. Real-time monitoring of epithelial cell-cell and cell-substrate interactions by infrared surface plasmon spectroscopy. Biophys. J. 2010, 99, 4028–4036. [Google Scholar] [CrossRef] [PubMed]
- Zilbershtein, A.; Golosovsky, M.; Lirtsman, V.; Aroeti, B.; Davidov, D. Quantitative surface plasmon spectroscopy: Determination of the infrared optical constants of living cells. Vib. Spectrosc. 2012, 61, 43–49. [Google Scholar] [CrossRef]
- Choi, W.; Fang-Yen, C.; Badizadegan, K.; Oh, S.; Lue, N.; Dasari, R.R.; Feld, M.S. Tomographic phase microscopy. Nat. Methods 2007, 4, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Carpignano, F.; Rigamonti, G.; Merlo, S. Characterization of rectangular glass microcapillaries by low-coherence reflectometry. IEEE Photonics Technol. Lett. 2015, 27, 1064–1067. [Google Scholar] [CrossRef]
- Carpignano, F.; Surdo, S.; Barillaro, G.; Merlo, S. Silicon micromachined device testing by low-coherence infrared reflectometry. J. Microelectromech. Syst. 2015, 24, 1960–1964. [Google Scholar] [CrossRef]
- Tuchin, V.V. Handbook of optical sensing of glucose in biological fluids and tissues. In Handbook of Optical Sensing of Glucose in Biological Fluids and Tissues Series in Medical Physics and Biomedical Engineering; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Tuchin, V.V.; Xu, X.; Wang, R.K. Dynamic optical coherence tomography in studies of optical clearing, sedimentation, and aggregation of immersed blood. Appl. Opt. 2002, 41, 258–271. [Google Scholar] [CrossRef] [PubMed]
- Brezinski, M.; Saunders, K.; Jesser, C.; Li, X.; Fujimoto, J. Index matching to improve optical coherence tomography imaging through blood. Circulation 2001, 103, 1999–2003. [Google Scholar] [CrossRef] [PubMed]
- Mann, H.B.; Whitney, D.R. On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Merlo, S.; Barillaro, G.; Carpignano, F.; Silva, G.; Surdo, S.; Strambini, L.M.; Giorgetti, S.; Nichino, D.; Relini, A.; Mazzini, G.; et al. Fibrillogenesis of human β2-microglobulin in three-dimensional silicon microstructures. J. Biophotonics 2012, 5, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Fiorani, L.; Fantoni, R.; Lai, A.; Palucci, A. First simultaneous determination of size, refractive index, light scattering depolarization and fluorescence of phytoplankton cells by laser scanning flow cytometry. EARSeL eProceedings 2007, 6, 47–57. [Google Scholar]
- Ackleson, S.G.; Spinrad, R.W. Size and refractive index of individual marine participates: A flow cytometric approach. Appl. Opt. 1988, 27, 1270–1277. [Google Scholar] [CrossRef] [PubMed]
- Green, R.E.; Sosik, H.M.; Olson, R.J.; DuRand, M.D. Flow cytometric determination of size and complex refractive index for marine particles: Comparison with independent and bulk estimates. Appl. Opt. 2003, 42, 526–541. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Bowman, L.; Zhang, X.; Shi, X.; Jiang, B.; Castranova, V.; Ding, M. Metallic nickel nano- and fine particles induce JB6 cell apoptosis through a caspase-8/AIF mediated cytochrome c-independent pathway. J. Nanobiotechnol. 2009, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Granchi, D.; Cenni, E.; Ciapetti, G. Cell death induced by metal ions: Necrosis or apoptosis? J. Mater. Sci. 1998, 9, 31–37. [Google Scholar]
- Oblak, A.; Pohar, J.; Jerala, R. MD-2 Determinants of Nickel and Cobalt-mediated activation of human TLR4. PLoS ONE 2015, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Foladori, P.; Quaranta, A.; Ziglio, G. Use of silica microspheres having refractive index similar to bacteria for conversion of flow cytometric forward light scatter into biovolume. Water Res. 2008, 42, 3757–3766. [Google Scholar] [CrossRef] [PubMed]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carpignano, F.; Rigamonti, G.; Mazzini, G.; Merlo, S. Low-Coherence Reflectometry for Refractive Index Measurements of Cells in Micro-Capillaries. Sensors 2016, 16, 1670. https://doi.org/10.3390/s16101670
Carpignano F, Rigamonti G, Mazzini G, Merlo S. Low-Coherence Reflectometry for Refractive Index Measurements of Cells in Micro-Capillaries. Sensors. 2016; 16(10):1670. https://doi.org/10.3390/s16101670
Chicago/Turabian StyleCarpignano, Francesca, Giulia Rigamonti, Giuliano Mazzini, and Sabina Merlo. 2016. "Low-Coherence Reflectometry for Refractive Index Measurements of Cells in Micro-Capillaries" Sensors 16, no. 10: 1670. https://doi.org/10.3390/s16101670






