1. Introduction
In recent years, coronary heart disease has become the leading cause of death worldwide. Cardiovascular diseases affect not only stressed, overweight middle-aged men in developed countries, but also women and children in low-middle-income countries. According to the 2012 World Health Statistics report by the WHO, the largest proportion of non-communicable disease deaths is caused by cardiovascular diseases (48%). It is projected that the annual number of deaths due to cardiovascular disease will increase from 17 million in year 2008 to 25 million in 2030 [
1]. Therefore, it is essential to continuously monitor cardiovascular function to allow for the early detection and treatment of cardiovascular anomalies, especially in high-risk patients.
An electrocardiogram (ECG) measures the electric current generated by the heart muscle during a heartbeat. Thus, it provides useful diagnostic information about the cardiovascular system and is a strong indicator of several specific physiological and pathological conditions in humans. Conventionally, Ag/AgCl electrodes employ a conductive adhesive to ensure good contact between the electrode and subject’s bare skin, which causes skin irritation and discomfort. Therefore, a noncontact capacitive coupled ECG monitoring technique has been proposed for long-term daily health monitoring [
2]. The principal goal of non-invasive measuring methods is to monitor health-related information reliably without interrupting the subject’s daily life.
Noncontact ECG monitoring has the distinct advantage of not requiring direct contact; it can measure biomedical signals through a layer of insulator such as clothes. This property allows for the integration of the measurement system into everyday objects and makes continuous measurement possible without placing constraints on the patients. Since its introduction by Lopez and Richardson [
2], the applications of capacitive coupled ECG monitoring methods have been extended to various environments. For instance, Lim
et al., Steffen
et al. and Aleksandrowicz
et al. developed an unconstrained ECG measurement system built into an office chair [
3,
4,
5] and a bed [
6]; Park
et al. applied ECG sensors on clothing [
7]; Fuhrhop
et al. integrated flexible electrodes into a belt [
8]; Kato
et al. demonstrated electrocardiography of neonates and infants using an incubator mattress system [
9]; Leonhardt
et al. proposed noncontact ECG monitoring in automobiles [
10];
Despite these numerous advances, ECG signals remain very susceptible to noise from a variety of sources such as motion artifacts, electromagnetic noise, power line noise, and electrostatic charge noise. In non-contact capacitive coupled ECG measurement methods, there is no skin contact; thus, no direct connection can be made between the subject’s body and the sensor electrode. The subject’s clothing acts as an insulator between the body and sensor electrode. Occasionally, static charge builds up on the subject’s clothing. Because there is no direct physical contact between the subject and any grounding point, there is no discharge path for any static build-up; therefore, the ECG signal quality deteriorates and the signal-to-noise ratio (SNR) decreases. Furthermore, a long settling time is required to obtain a stable ECG signal.
One of the possible solutions of this problem is with advances in the design of the electrodes. Wartzek
et al. [
11] tried to design grid electrodes to overcome the noise coming from the electrostatic (triboelectric effect), however the P and T waves are not distinguishable from their acquired ECG signals. Researchers in [
12,
13,
14] also tried to build soft dry electrodes to improve hairy skin contact, yet this method still needs direct contact with the skin. Only a few researchers in [
15,
16,
17] have very recently called attention to the moisturization of the electrodes. In this study, we developed a fabric electrode with embedded polymer (FEEP) that takes into account the relative humidity of the environment and sensor electrode characteristics to remove the static charge rapidly and obtain a clear ECG signal. The designed FEEP with sandwiched layers ensures a high relative humidity because less static charge builds up and electrostatic charge discharges quickly. Our results show that a good quality ECG signal can be recorded with good SNR, making this system a very promising non-contact biomedical signal acquisition technology for use in ubiquitous home healthcare applications.
2. System Design
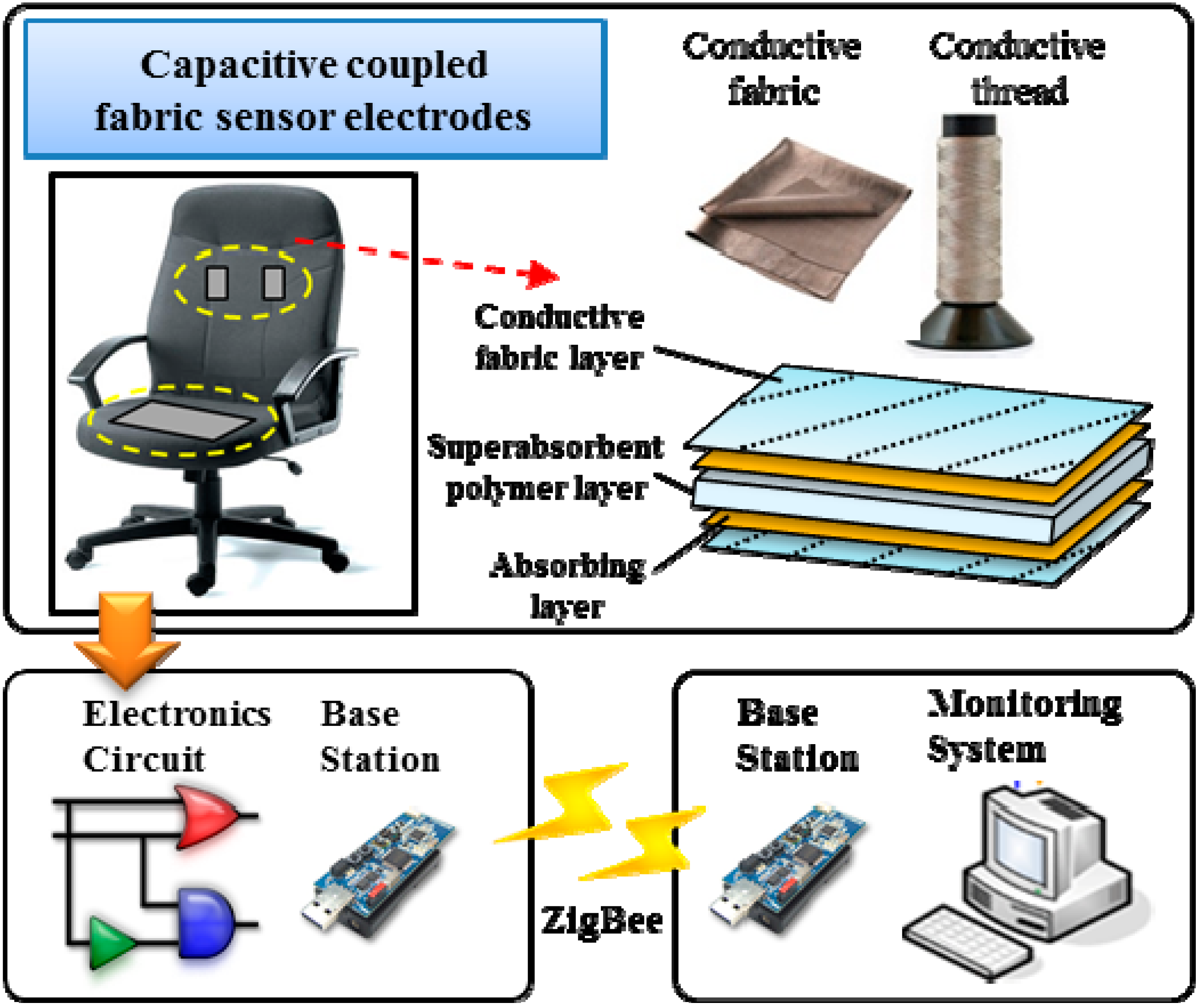
The overall system architecture for our non-contact healthcare monitoring system, shown in
Figure 1, comprises a pair of capacitive electrodes, a piece of conductive textile, an electronic circuit, and wireless modules to transmit and receive biomedical data wirelessly via IEEE 802.15.4. The biomedical signals transmitted from the wireless sensor node are saved and analyzed for healthcare monitoring purposes [
18].
Figure 1.
Non-contact ECG monitoring system.
Figure 1.
Non-contact ECG monitoring system.
The capacitive coupled electrodes carry an alternating bioelectric current through the capacitive coupling formed by the conductive electrode, an insulator, and the skin of the subject [
2]. This combination forms a capacitive coupling between the subject’s body and the sensor electrode. Our method is operational through a pair of capacitive coupled electrodes installed on the chair back and a conductive textile installed on the seat for capacitive driven-right-leg (DRL) grounding. A DRL circuit [
19] has been designed as an additional reference to suppress the interference caused by the finite common-mode rejection ratio (CMRR) of the instrumental amplifiers. In the system, a hygroscopic capacitive coupled fabric sensor electrode is designed as a multiple layer sandwich structure using conductive fabric.
The capacitive electrodes are connected to an electronic circuit including high input impedance amplifiers and band-pass filters for analog signal processing. These include a low-pass filter (100 Hz), high-pass filter (0.04 Hz), and notch filter (60 Hz). In addition, the system included a sensor node with an integrated ultra-low-power microcontroller. The MSP430 digitizes the ECG signals with its built-in 12-bit analog-to-digital converter and the CC2420 wireless transceiver in the wireless sensor node transmits the biomedical signal data through via 802.15.4 using a ZigBee-based radio protocol (frequency band: 2.4 GHz to 2.485 GHz) at a transceiver rate of 250 Kbps to a PC-based monitoring system.
4. Experimental Results
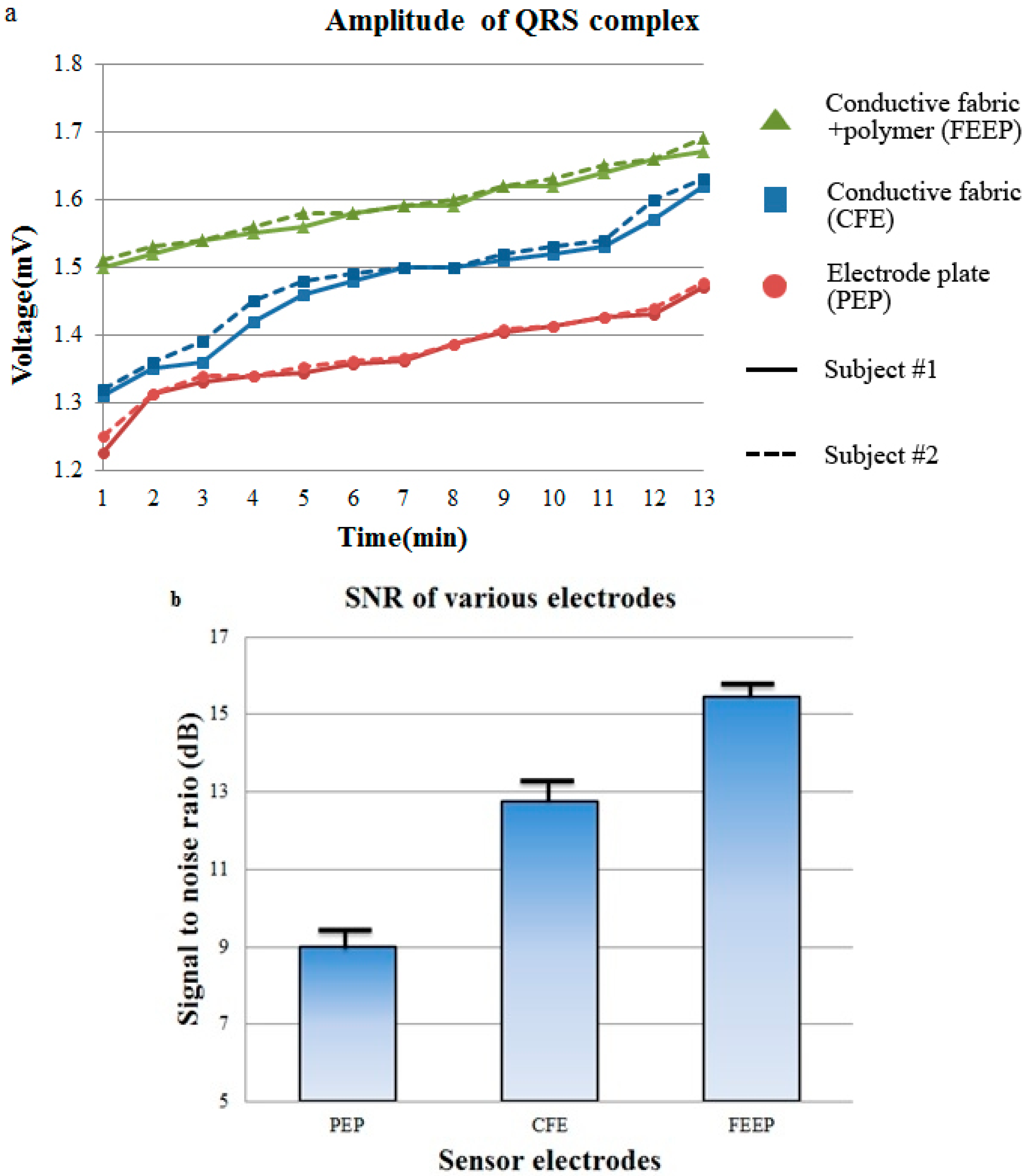
All experiments were carried out in a laboratory. The temperature was kept constant at approximately 25 °C with a relative humidity of approximately 50%. Subjects wore a cotton t-shirt and jeans. A PEP, a CFE, and a conductive FEEP were used as sensor materials. The electrode size was designed to be 4 cm × 4 cm and the total thickness of the sensor electrode was approximately 20 mm. They were fixed with a vertical center-to-center distance of 10 cm. The sensor electrodes were attached at a fixed position on the chair. For the driven ground plane, a conductive textile with a size of 30 cm × 30 cm was employed.
4.3. Comparison with Other Sensor Electrodes
The non-contact capacitive biopotential measurement technique has been widely used. At the same time, non-contact electrodes with the objective to improve electrode-body interface have been presented by many researchers. This is important to eliminate triboelectricity effects due to friction and static charge, and to suppress motion artifacts to minimal values.
Table 2 provides an overview of existing sensor electrodes developed by researchers. Del Re
et al. [
29] enhanced a bio-electrode with a a cushioning layer placed between the body and electrode whereby this enhancement layer can carry and release water or moisture to reduce triboelectric noise. Chi
et al. [
30] mentioned the poor settling times but the solution provided distorted the signal waveform. Grützmann
et al. [
12] presented a soft dry electrode where a soft foam cushioning layer is constructed on top of a SiO
2 electrode to improve the contact on hairy skin to diminish motion artifacts. A passive filter network is also integrated into the new electrodes to suppress the slow offset fluctuation of the ECG signal. Wartzek
et al. [
11] also described the triboelectricity effects. Four different types of sensor electrodes were constructed to analyze the electrostatic voltage generated and the discharging behavior. Wartzek
et al. proposed an insulated electrode, metal-coated electrode, no-isolation electrode and grid electrode to reduce triboelectricity charges. Experimental analysis proved that a metal layer provides a conductive back-path for separated charges and the grid on top of the electrode produces much less electrostatic voltages as charges are separated on the material’s surface, so the grid can drain them off. The discharge behaviors of different electrodes are different. Leicht
et al. [
15] and Weder
et al. [
16] used superabsorber or absorbent layers to moisturize the electrodes to reduce the motion artifact noise and to get long time signal stabilization in recent work.
However in [
11,
25], the researchers showed a fluctuating ECG signal as shown in
Figure 6.
Figure 6a shows the QRS complexes are rarely visible when the grid is turned off. Meanwhile, even the grid electrode produces much less electrostatic voltage and the discharge slope is much larger (can quickly drain off the charge and discharge the cloth), and the ECG signals measured with a grid electrode do not show a clear ECG signal with clear P peaks, and T peaks. QRS complexes with small ampliyude are visible, as shown in
Figure 6b. From a medical field point of view, the P waves and T waves are important for the diagnosis of diseases such as arrhythmia, Wolff-Parkinson-White syndrome, myocardial ischemia and infarction. As compared to ECG signals measured with the proposed FEEP electrodes, P waves, QRS complexes, T waves are clearly distinguishable which are very useful for cardiological diagnostics during the interpretation of abnormal ECGs.
Figure 6c shows the ECG signals measured with the proposed sensor electrode FEEP where the P waves, QRS complexes, T waves are clearly visible. Secondly, the authors in [
23] mentioned that if charges are separated between fibers, the grid does not improve the results as it does not reduce electrostatic charge.
Meanwhile, some researchers [
14] developed a sensor electrode with on-board electronics components. From a long term point of view, this may result in a high cost sensor electrode replacement when the electronics component or op-amp is not functioning. Users have to replace the sensor electrodes with on-board components. The proposed FEEP electrode however can be replaced easily at very low cost. Besides, FEEP sensor electrodes are easy to build using conductive fabric and superabsorbent polymers. They are also very flexible and it can bend according to the body curvature and therefore, very minimal movement artifacts occur once the coupling capacitance is formed.
Table 2.
Overview of existing sensor electrodes.
Table 2.
Overview of existing sensor electrodes.
| Author [Ref.] | Problem Statement | Solutions | Features |
|---|
| Brun del Re [29] | Triboelectric noise with orders of magnitude larger than the desired body signal arises when obtain signal. | A cushioning layer is placed intermediate the body and electrode where this enhancement layer to carry and release water or moisture
To reduce triboelectric noise. | On-board electronic components on electrode will make electrode replacement expensive. |
| Chi [30] | Poor settling times due to the high-pass characteristic at the electrode. Recovery times of upwards of 10 s. | Shifting the corner frequency of the high-pass filter to improve settling time, but at a cost of distorting the signal waveform. | - |
| Wartzek [11] | Global triboelectricity – Common-mode interference on the whole body
Local triboelectricity – Electrode-body interface. | Electrode design
Insulated electrode
Metal-coated electrode
No-isolation electrode
Grid electrode. | P peaks and T peaks of ECG signal are not distinguishable. |
| Gruetzmann [12] | Motion artifacts such as walking, breathing introduce fluctuations in the zero line. | Soft dry electrode to improve the contact on hairy skin to reduce the electrode impedance, to diminish motion artifacts.
Passive filter network to suppress slow offset fluctuation of the ECG signal. | Soft dry electrodes are developed to improve hairy skin contact, which is not applicable for non-contact measurement technique. |
| Chi [14] | Dry electrodes are prone to poor signal quality due to unstable offsets, high drifts, long settling times, movement artifacts. | Conductive media layer
To aid in conduction due to its high ionic content.
Ionic exchange media
To protect conductive media from damage
To help retain its moisture content. | Dry electrodes are built for direct contact ECG measurements. |
| Leicht [15] | Strong artifact noise is generated at capacitive electrodes for a car seat due to driver movement | Superabsorber to moisturize the electrodes to reduce strong artifacts and triboelectricity | Superabsorber layer integrated in the electrode generates moisture to reduce motion artifacts and triboelectricity. |
| Weder [16] | Water vapor has a positive effect on ECG quality in a breast belt for the long time monitoring of ECG | Absorbent layer to keep condensed sweat water at flexible water tank in a form of absorbent layer to moisturize electrodes with a very low amount of water vapor | Electrodes are moisturized with a very low amount of water vapor from the integrated reservoir |
| The proposed electrode FEEP | Long stabilization time is needed to allow for static discharge. | Sandwiched layer hygroscopic FEEPConductive fabric electrode with embedded superabsorbent polymers (acts as sponge humidifiers)
To shorten stabilization time.
To rapidly discharge any accumulated charge. | Low cost
Flexibility
Easy to build
Sensor electrode can be replaced easily |
Figure 6.
Comparison of ECG signals measured from grid-electrode (with permission from [
11]) and the proposed FEEP. (
a) The active driven grid is switched off (it acts as metal-coated electrodes); (
b) The active driven grid is switched on; (
c) The proposed sensor electrodes FEEP.
Figure 6.
Comparison of ECG signals measured from grid-electrode (with permission from [
11]) and the proposed FEEP. (
a) The active driven grid is switched off (it acts as metal-coated electrodes); (
b) The active driven grid is switched on; (
c) The proposed sensor electrodes FEEP.
5. Conclusions
Using the capacitively coupled ECG measurement system presented here, ECG signals can be obtained without direct skin contact, and consequently without causing skin irritation. By integrating these technologies into a chair system in a home healthcare environment, biomedical data can be acquired through clothing in a nonintrusive fashion. By adapting a hygroscopic FEEP, high humidity conditions can be ensured. The stabilization time is shortened and a clear, stable ECG signal quality with high QRS amplitude and SNR can be obtained, even in environments with relative humidity lower than 55%–60%. Thus, problems regarding static charge and long stabilization times in ubiquitous healthcare systems can be resolved
Acknowledgments
This research was supported by Research Grant of BB (Brain Busan) 21 project of 2015.
Author Contributions
All authors contributed extensively to the work presented in this paper. Wan-Young Chung and Ee-May Fong designed the sensors, conducted the experiments and assembled input data. Ee-May Fong analyzed output data as well as wrote the manuscript draft. Wan-Young Chung administered the experiments and carefully proofread this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. World Health Statistics 2012. Available online: http://www.who.int/gho/publications/world_health_statistics/EN_WHS2012_Full.pdf/ (accessed on 16 May 2012).
- Lopez, A.J.; Richardson, P.C. Capacitive electrocardiographic and bioelectric electrodes. IEEE Trans. Biomed. Eng. 1969, 16, 99. [Google Scholar]
- Lim, Y.G.; Kim, K.K.; Park, K.S. ECG measurement on a chair without conductive contact. IEEE Trans. Biomed. Eng. 2006, 53, 956–959. [Google Scholar]
- Steffen, M.; Aleksandrowicz, A.; Leonhardt, S. Mobile non-contact monitoring of heart and lung activity. IEEE Trans. Biomed. Circuits Syst. 2007, 1, 250–257. [Google Scholar] [PubMed]
- Aleksandrowicz, A.; Leonhardt, S. Wireless and Non-Contact ECG Measurement System—The “Aachen SmartChair”. Acta Polytechnica 2007, 47, 5–8. [Google Scholar]
- Lim, Y.G.; Kim, K.K.; Park, K.S. ECG recording on a bed during sleep without direct skin-contact. IEEE Trans. Biomed. Eng. 2007, 54, 718–725. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Chou, P.H.; Bai, Y.; Matthews, R.; Hibbs, A. An ultra-wearable, wireless, low power ECG monitoring system. In Proceedings of IEEE Biomedical Circuits and Systems Conference (BioCAS 2006), London, UK, 29 Novermber–1 December 2006; pp. 241–244.
- Fuhrhop, S.; Lamparth, S.; Heuer, S. A textile integrated long-term ECG monitor with capacitively coupled electrodes. In Proceedings of IEEE Biomedical Circuits and Systems Conference (BioCAS 2009), Beijing, China, 26–28 November 2009; pp. 21–24.
- Kato, T.; Ueno, A.; Kataoka, S.; Hoshino, H.; Ishiyama, Y. An application of capacitive electrode for detecting electrocardiogram of neonates and infants. In Proceedings of 28th Annual International Conference on IEEE Engineering in Medicine and Biology Society (EMBS), New York, NY, USA, 30 August–3 September 2006; pp. 916–919.
- Leonhardt, S.; Aleksandrowicz, A. Non-contact ECG monitoring for automotive application. In Proceedings of 5th International Summer School and Symposium on Medical Devices and Biosensors (ISSS-MDBS 2008), Hong Kong, China, 1–3 June 2008; pp. 183–185.
- Wartzek, T.; Lammersen, T.; Eilebrecht, B.; Walter, M.; Leonhardt, S. Triboelectricity in capacitive biopotential measurements. IEEE Trans. Biomed. Eng. 2010, 58, 1268–1277. [Google Scholar] [CrossRef] [PubMed]
- Gruetzmann, A.; Hansen, S.; Muller, J. Novel dry electrodes for ECG monitoring. Physiol. Meas. 2007, 28, 1375. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.T.; Liao, L.D.; Liu, Y.H.; Wang, I.J.; Lin, B.S.; Chang, J.Y. Novel dry polymer form electrodes for long-term EEG measurement. IEEE Trans. Biomed. Eng. 2011, 58, 1200–1207. [Google Scholar] [PubMed]
- Chi, Y.M.; Jolla, L. Apparatuses, Systems and Methods for Biopotential Sensing with Dry Electrodes. U.S. Patent 8,798,710, 5 August 2014. [Google Scholar]
- Leicht, L.; Eilebrecht, B.; Weyer, S.; Wartzek, T.; Leonhardt, S. Active humidification for capacitive-resistive ECG-Systems. Biomed. Technol. 2014, 59, S818–S821. [Google Scholar]
- Weder, M.; Hegemann, D.; Amberg, M.; Hess, M.; Boesel, L.F.; Abacherli, R.; Meyer, V.R.; Rossi, R.M. Embroidered Electrode with Silver/Titanium Coating for Long-Term ECG Monitoring. Sensors 2015, 15, 1750–1759. [Google Scholar] [CrossRef] [PubMed]
- Eilebrecht, B.; Leicht, L.; Mathissen, M.; Lem, J.; Lindner, A.; Vogt, R.; Leonhardt, S.; Walter, M. Contactless Electrocardiographic Sensor with Moisture Generator. U.S. Patent 14/493,901, 2014. [Google Scholar]
- Fong, E.M.; Kwon, T.H.; Chung, W.Y. Non-contact biomedical signal acquisition for home healthcare service. In Proceedings of the 6th Asia-Pacific Conference of Transducers and Micro/Nano Technology (APCOT), Nanjing, China, 8–11 July 2012; pp. 8–11.
- Lim, Y.G.; Chung, G.S.; Park, K.S. Capacitive driven-right-leg grounding in indirect-contact ECG measurement. In Proceedings of the IEEE 32nd Annual International Conference Engineering in Medicine and Biology Society (EMBC), Buenos Aires, Argentina, 2010; pp. 1250–1253.
- The Triboelectric Series. Alphalab Inc. Available online: http://www.trifield.com/content/tribo-electric-series/ (accessed on 29 July 2015).
- Relationship between Humidity and Static Electricity. H. Ikeuchi & Co. Ltd., Osaka, Japan. Available online: http://www.kirinoikeuchi.co.jp/eng/technical_information/relationship_between_humidity_and_static_electricity.html (accessed on 29 July 2015).
- Lion Precision. (2006). Capacitive Sensor Operation and Optimization. Available online: http://www.lionprecision.com/tech-library/technotes/cap-0020-sensor-theory.html (accessed on 29 July 2015).
- Eren, H.; Kong, W.L. Capacitive Sensors—Displacement. In The Measurement, Instrumentation, and Sensors Handbook; CRC Press: Boca Raton, FL, USA, 1999. [Google Scholar]
- Baek, H.J.; Chung, G.S.; Kim, K.K.; Park, K.S. A smart health monitoring chair for noninstrusive measurement of biological signals. IEEE Trans. Inf. Technol. Biomed. 2012, 16, 150–158. [Google Scholar] [CrossRef] [PubMed]
- Schumm, J.; Setz, C.; Bächlin, M.; Bächler, M.; Arnrich, B.; Tröster, G. Unobtrusive physiological monitoring in an airplane seat. Pers. Ubiquit. Comput. 2010, 14, 541–550. [Google Scholar] [CrossRef]
- Lowne, A. Non-Contact ECG Measurement Using EPIC Sensors. Saelig Co. Inc., Plessey Semiconductors, UK. Available online: http://eecatalog.com/automotive/2012/10/02/non-contact-ecg-measurement-using-epic-sensors/ (accessed on 2 October 2012).
- Superabsorbent Aqueous Based Polymers for Industry. Available online: http://www.soilmoist.com/products/industrial.php (accessed on 29 July 2015).
- Diaz, D. “Doc” ed. Humidity Beads vs. Superabsorbent Polymers. Available online: http://www.stogiefresh.info/edu-humidors/articles/beads-vs-polymers.html/ (accessed on 30 April 2015).
- Del Re, R.B.; Batkin, I. Enhanced Pickup-Electrode. U.S. Patent 10/468,776, 2002. [Google Scholar]
- Chi, Y.; Jung, T.-P.; Cauwenberghs, G. Dry-contact and noncontact biopotential electrodes: Methodological review. IEEE Rev. Biomed. Eng. 2010, 3, 106–119. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).