1. Introduction
The blood oxygen saturation level indicates the percentage of hemoglobin molecules in the arterial blood which are saturated with oxygen, and has been identified as an indicator of risk in chronic diseases of the circulatory and respiratory system. Pulse oximetry was introduced in 1983 as a noninvasive method for monitoring the arterial oxygen saturation of a patient’s blood [
1]. Oxygen saturation (SpO2) is defined as the measurement of the amount of oxygen dissolved in blood, based on the detection of oxygenated hemoglobin (HbO
2) and deoxygenated hemoglobin (Hb).
Two different light wavelengths are used to obtain the SpO2 value. The absorption of HbO
2 and Hb differs greatly at the first wavelength, and is approximately equal at the second wavelength. Typically 660 nm (red) and 940 nm (infrared) wavelengths are used [
1].
The signal obtained from a single wavelength is called the Photoplethysmogram (PPG), which is used for heart rate monitoring [
2]. It consists of a small pulsatile component due to the light absorption by arterial blood, and a large static component due to light absorption by bone, tissue, skin, hair and venous blood. The dual wavelength pulse oximeter calculates the absorbance ratio (R) which indicates the magnitude of SpO2 and is given by Equation (2):
where the symbols
Iac and
IDC denote the ac component and dc component of the red and infrared (IR) PPG signals respectively. Typically
R ranges from 0.5 to 2 [
1] and is a useful measurement as it is independent of the absolute light intensity of the LED and the optical properties of the tissue without arterial blood. It is then compared to look up tables that are based on a calibration curve developed empirically from various known SpO2 levels to relate
R to oxygen saturation.
There are two main configurations of pulse oximetry namely “transmission” or “reflectance” [
3,
4]. In transmission mode, the device is placed peripherally on a finger, toe, or earlobe where light is transmitted through the tissue having pulsatile blood flow and is detected on the other side of the tissue. In reflectance mode, the photodetector and light source are both on the same side. The reflectance pulse oximeter allows measurements to be taken from areas of the body in which transmittance based pulse oximetry cannot be applied, making it more suitable for ambulatory monitoring such as the abdomen and forehead [
5].
Recently miniature designs of the reflectance pulse oximeter have been developed for wearable health monitoring. Haahr
et al. [
6] designed an electronic patch with a size of 88 mm by 60 mm and Li and Warren’s design [
7] is 41 mm by 36 mm. Huang
et al. [
8] developed a ring-type optical probe with an external circuit board of approximately 40 mm by 20 mm. Recent development of pulse oximeters also includes interfacing a conventional clinical oximeter finger sensor to smart-phone devices [
9]. For developing a small, robust and low cost device a single chip design is an attractive solution. However, the reported work [
10,
11,
12,
13,
14] on the chip level designs of a pulse oximeter and the PPG focus on low power circuits with the systems operating in transmission mode. For example the designs reported in [
10,
11,
12] are purely analogue (except for the LED timing circuit) and the chip outputs are analogue PPG or SpO2 signal. In [
13] an external analogue to digital converter (ADC) and a PC are used for digitization and signal processing whilst [
14] also reports off-chip digital signal processing. Moreover, all of these chip designs require an external photodiode for light detection.
This paper describes a single-chip pulse oximeter consisting of photodiode, transimpedance amplifier, analogue band-pass filters, analogue-to-digital converters, digital signal processor (including lock-in) and LED timing control. The novelty of the design lies in the fully integrated circuit implementation which enables a compact, robust and low cost design solution providing the potential for it to be used as a wearable device for health monitoring. The device can be operated in reflection or transmission geometry and incorporates on chip modulated LED illumination drive circuitry and integrated lock-in detection so that the sensor effectively suppresses the ambient light and 1/f noise of the transimpedance amplifier.
The paper is broken down into
Section 2 that describes the design and system configuration of the sensor.
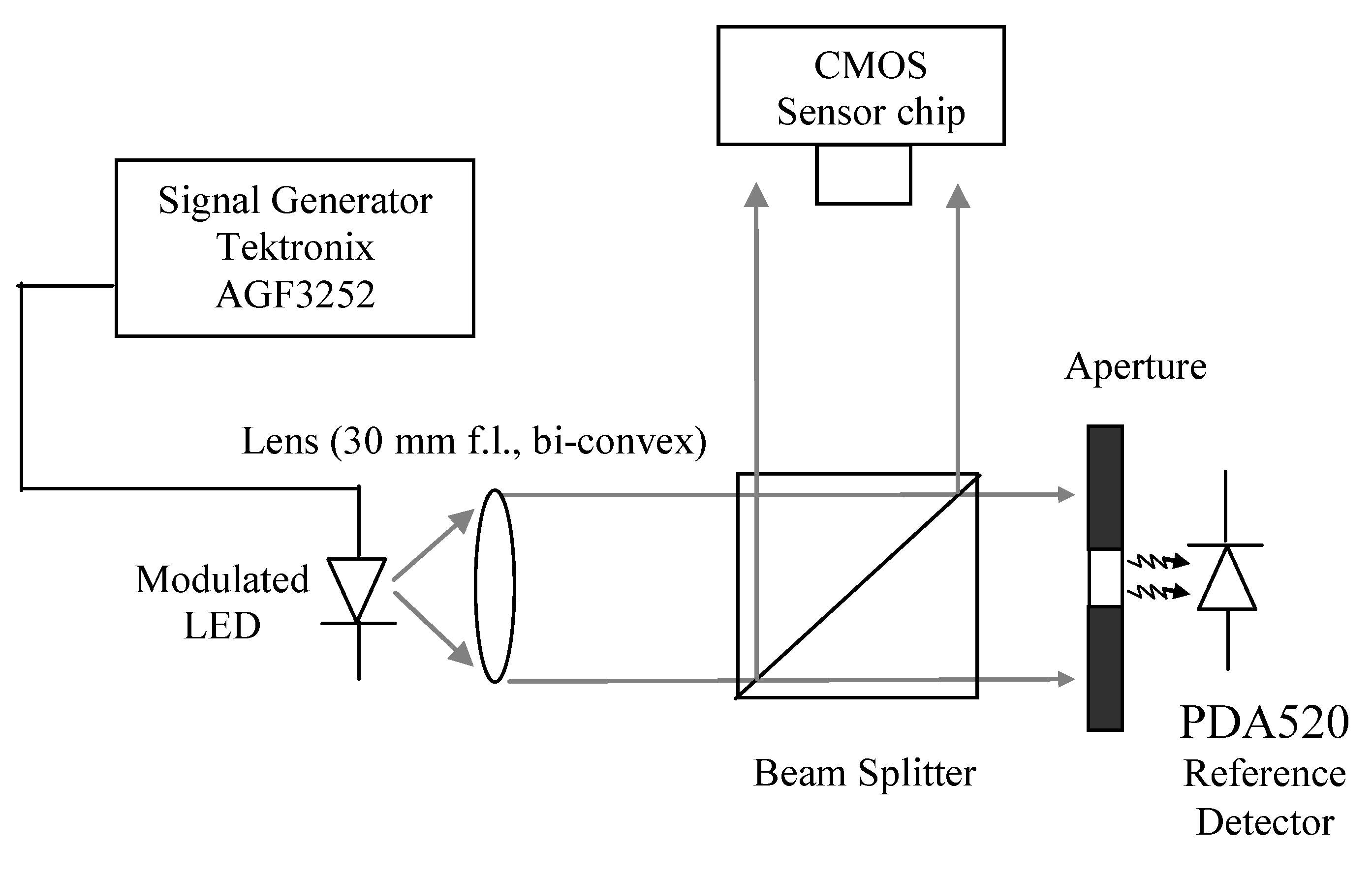
Section 3 describes the experimental set up and methods deployed to characterize the CMOS sensor.
Section 4 describes the experimental results of the analogue circuit characterization,
in vivo measurements for the complete analogue and digital CMOS design where the results are compared with commercial CE marked and FDA approved devices. Discussion and conclusions follow in
Section 5.
2. Sensor Design and PCB Design
Figure 1a illustrates a block diagram of the pulse oximeter sensor. It consists of a 1 mm × 2.5 mm n-well-p-substrate photodiode, a linear transimpedance amplifier having an 8 M Ohm feedback resistor and 30 KHz bandwidth, two Sallen-Key band-pass filters having 2 KHz (band-pass1) and 4 KHz (band-pass2) filter bandwidths respectively, two 10-bit successive approximation register (SAR) ADCs running at a sampling rate of 640 KHz. The digital signal processor includes a quadrature demodulator and LED timing control unit generating 10 KHz and 20 KHz square waves. The LEDs shown in the figure are off-chip components whilst all other components illustrated inside the dotted line are all integrated into a single semi-custom CMOS chip.
Light sources with two different wavelengths (λ1 = 660 nm, λ2 = 940 nm) are modulated with square waves at 10 KHz and 20 KHz respectively to enable frequency division multiplexing and to eliminate the effects of ambient light on the detected signal. These frequencies are used because they are unlikely to be present within the operating environment and are above the 1/f noise corner frequency (~1 KHz) of the I/V converter. The light backscattered by the skin is detected by the photodiode, which is of size 1 mm × 2.5 mm and is an n-well-p-substrate type common in CMOS structures for junction isolation [
15]. The current to voltage converter is based on an operational amplifier and has a linear response to the generated photocurrent. It has an 8 M Ohm feedback resistor and a bandwidth of 30 KHz, which is above the modulation frequencies and therefore it is sufficient for detecting the 10 KHz and 20 KHz channels corresponding to the two wavelengths. The output dynamic range of the current to voltage converter is between 1.65 V to 3.17 V, and can be observed off chip through a buffer for more detailed sub-circuit characterization. The subsequent band-pass filters are of type Sallen-Key having 2 KHz (band-pass1) and 4 KHz (band-pass2) filter bandwidths centred at the modulation frequencies (10 KHz and 20 KHz respectively). These filters allow additional isolation of the two carriers (λ1 = 660 nm, λ2 = 940 nm), provide anti-alias filtering, and attenuate interfering constant and low frequency light sources at the photodiode. The output of the band-pass filters is then sampled by the 10-bit successive approximation register (SAR) ADCs running at a sampling rate of 640 KHz [
16]. The SAR ADC utilises synchronous clock generation and has differential input. For chip characterization, output signals can be taken at intermediate points in the circuit (after the transimpedance amplifier and the band pass filters). The digitized data are demodulated by the on chip digital signal processor using a quadrature demodulator (lock-in) to recover the PPG signal [
17]. The demodulator multiplies digitized data by local oscillator (LO) signals that have a 90 degree phase shift between them to generate in phase and quadrature (I and Q) channels. The root-mean-square (RMS) combination of I and Q channels provides the demodulated PPG signal. The demodulator processes 64 raw data values in real-time for the 10 KHz channel (and 32 raw data values for the 20 KHz channel), and outputs a single value for subsequent accumulation. Therefore in total it stores only 96 10-bit raw data values. As multiplications and accumulations are involved, the PPG output expands to 24-bit. The quadrature demodulation algorithm was firstly modelled in Matlab, then written in VHDL and simulated in ModelSim. The layout of analogue and digital circuits was generated in Virtuoso and Encounter (Cadence) respectively. The digital processing unit consists of 4139 standard logic gates and consumes 4.6 mW power over a 1 mm × 1 mm silicon area.
Mixed-signal simulation has been carried out to validate the system design. The VerilogA models of analogue circuits and the VerilogAMS model of the ADC were simulated in Advanced Design System (Keysight Technologies, Belgium). SystemVue (Keysight Technologies) captured the output, together with the VHDL simulation of ModelSim, to run a mixed-signal simulation for the whole system. For a simulated time of 1 s, an execution time of 30 min was needed on a PC (Windows 7, i7 2.7 GHz CPU, 16 GB RAM). In order to accelerate the simulation speed, the VerilogA and VerilogAMS models were translated into native SystemVue models, and the VHDL code was translated into C++ models by HIF Suite (EDALab) and imported into SystemVue. The execution time was reduced to 8 min.
The chip layout shown in
Figure 1b is of size 3.2 mm × 3.3 mm and was fabricated in a 0.35 µm four metal layer CMOS process (Austriamicrosystems). Regions of the chip other than the photodiodes were covered by a top (4th) metal layer to prevent illuminating parts of the chip that are not intended to be light sensitive.
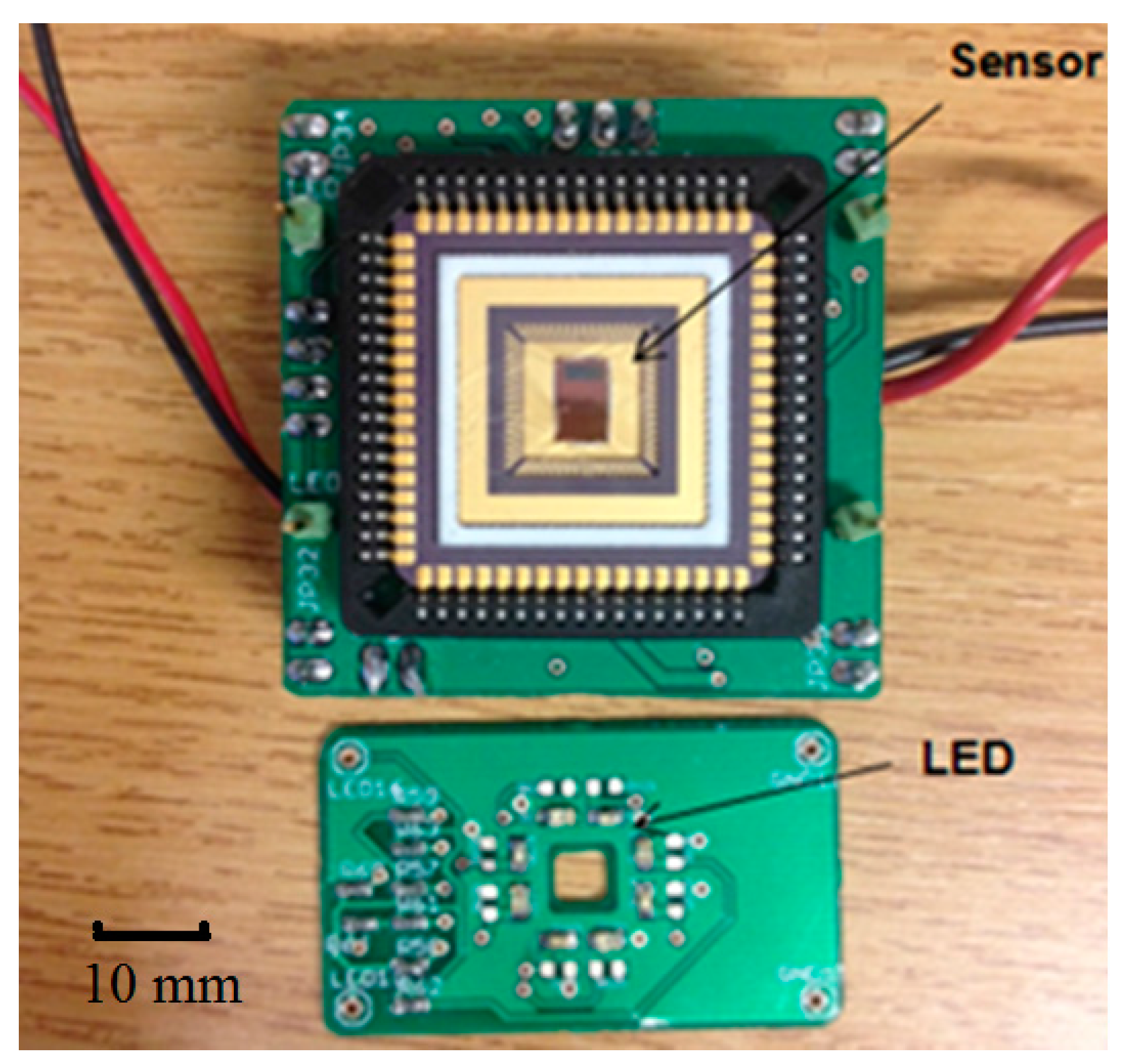
Figure 2 shows the prototype including a test board with the CMOS sensor chip and an LED board. The light source consists of four λ = 660 nm (ROHM—SML-310LTT86N) and four λ = 940 nm (KINGBRIGHT—KP-2012F3C) LEDs, providing even illumination to the tissue. There are four holes at the corners of the LED board and four corresponding pins on the CMOS sensor board for fixation. In reflection configuration, the LED board is placed at the top of the CMOS sensor board with LEDs facing upward, the light backscattered by the tissue passes through the central hole (4 mm × 4 mm) and illuminates the photodiode on the CMOS sensor. The separation of the LED and the CMOS sensor is 5 mm. The system can also work in transmission mode by flipping the LED board so that it is situated on top of the finger.
Figure 1.
(a) Block diagram of the sensor. (Photodiode, I/V—Current to Voltage Converter, ADC—analogue to digital converter, Digital signal processor, DAC—digital to analogue converter, LED—light emitting diode)—Items inside dotted region are all “CMOS on-chip”; (b) Layout of the CMOS sensor.
Figure 1.
(a) Block diagram of the sensor. (Photodiode, I/V—Current to Voltage Converter, ADC—analogue to digital converter, Digital signal processor, DAC—digital to analogue converter, LED—light emitting diode)—Items inside dotted region are all “CMOS on-chip”; (b) Layout of the CMOS sensor.
Figure 2.
CMOS Sensor chip and LED illumination PCBs.
Figure 2.
CMOS Sensor chip and LED illumination PCBs.
5. Discussion and Conclusions
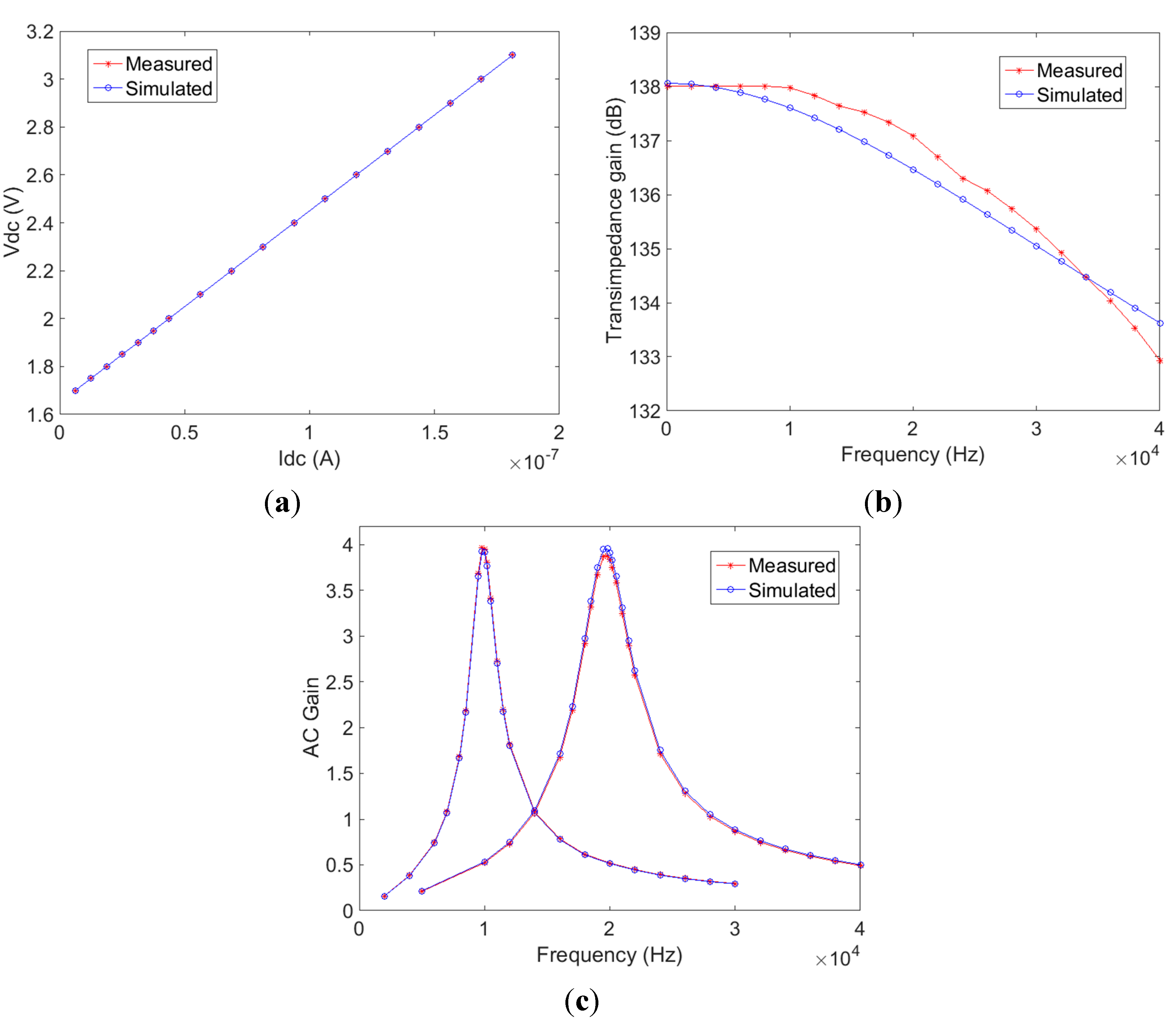
A single-chip CMOS pulse oximeter has been designed, fabricated and tested. The CMOS chip consists of photodiode, I/V converter, analogue band-pass filters, ADCs, digital signal processor (with digital lock-in detection) and LED timing control. The I/V converter and band-pass filters were optically and electrically characterised and the measured results compare well with the design specifications (<1% difference). The modulated light sources and integrated digital lock-in detection enables the sensor to effectively suppress interference from ambient light and 1/f noise. PPG signals using 660 nm and 940 nm wavelengths are presented and an empirical calibration curve for transmission geometry was derived using commercially available dye based translucent phantom SpO2 fingers.
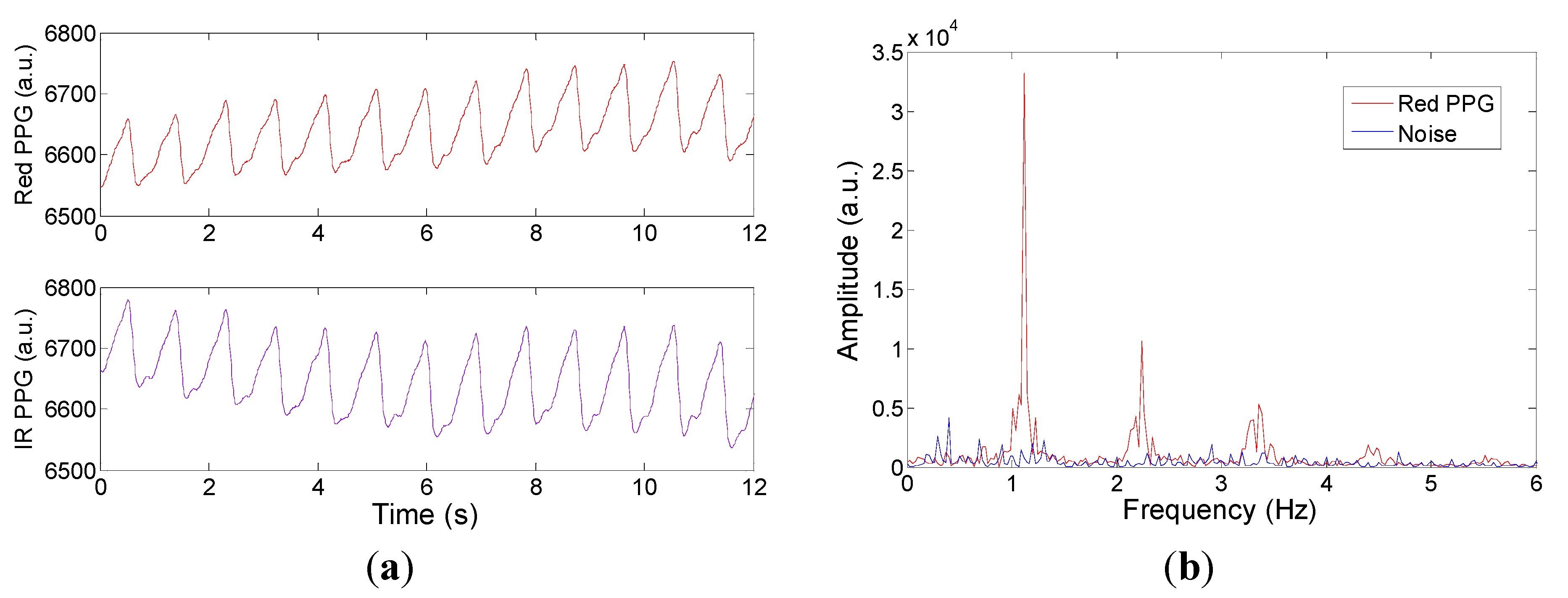
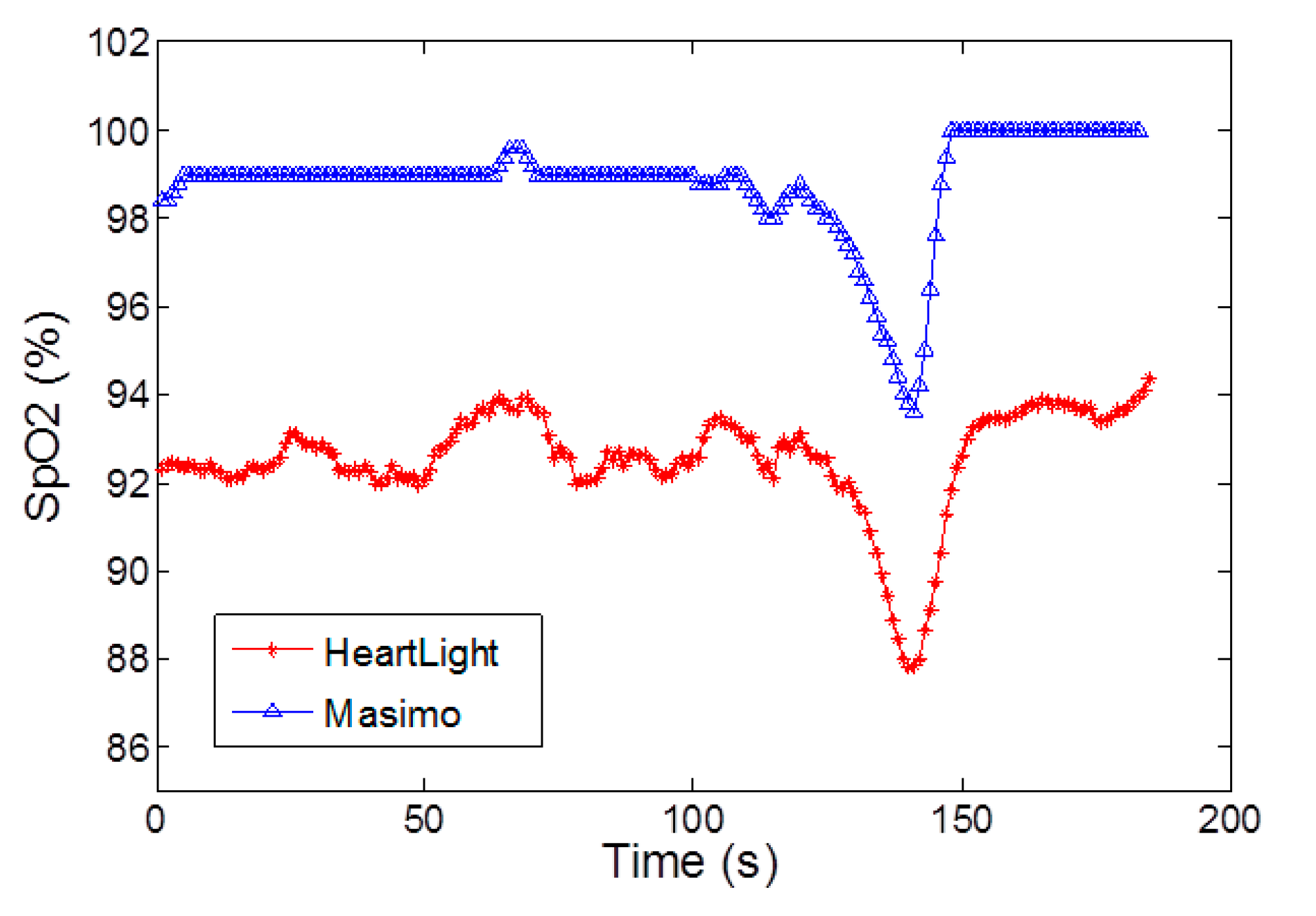
During in vivo breath-holding experiments, changes in SpO2 can be observed and the trends and absolute values show good agreement with two commercial devices namely the Masimo Radical 7 and the Nexus-10 in transmission mode. In reflection geometry however, the phantom fingers are not valid and hence the calibration curve of the CMOS sensor possesses an offset when compared with the commercially calibrated reflectance mode device (Masimo Radical 7). This latter point will be addressed in future by comparing the reflectance measurements with realistic blood samples and blood gas analysis.
The overall packaging is relatively large (25 mm × 25 mm) as it uses a JLCC68 packaging for test purposes. In subsequent prototypes, quad flat no-leads (QFN) or chip-scale packaging can be employed in order to miniaturize the device footprint. This will reduce the overall size to 5 mm × 5 mm. Future versions of the chip will also investigate a fully integrated chip in which the SpO2 calibration curve is implemented on-chip along with a heart rate extraction algorithm.