Synthesis and Gas Sensing Properties of Single La-Doped SnO2 Nanobelts
Abstract
:1. Introduction
2. Experimental Section
2.1. Synthesis of SnO2 NBs and La-SnO2 NBs
2.2. The Characterization and Preparation of a Single Nanobelt Device
2.3. The Measure of Gas Sensitivity
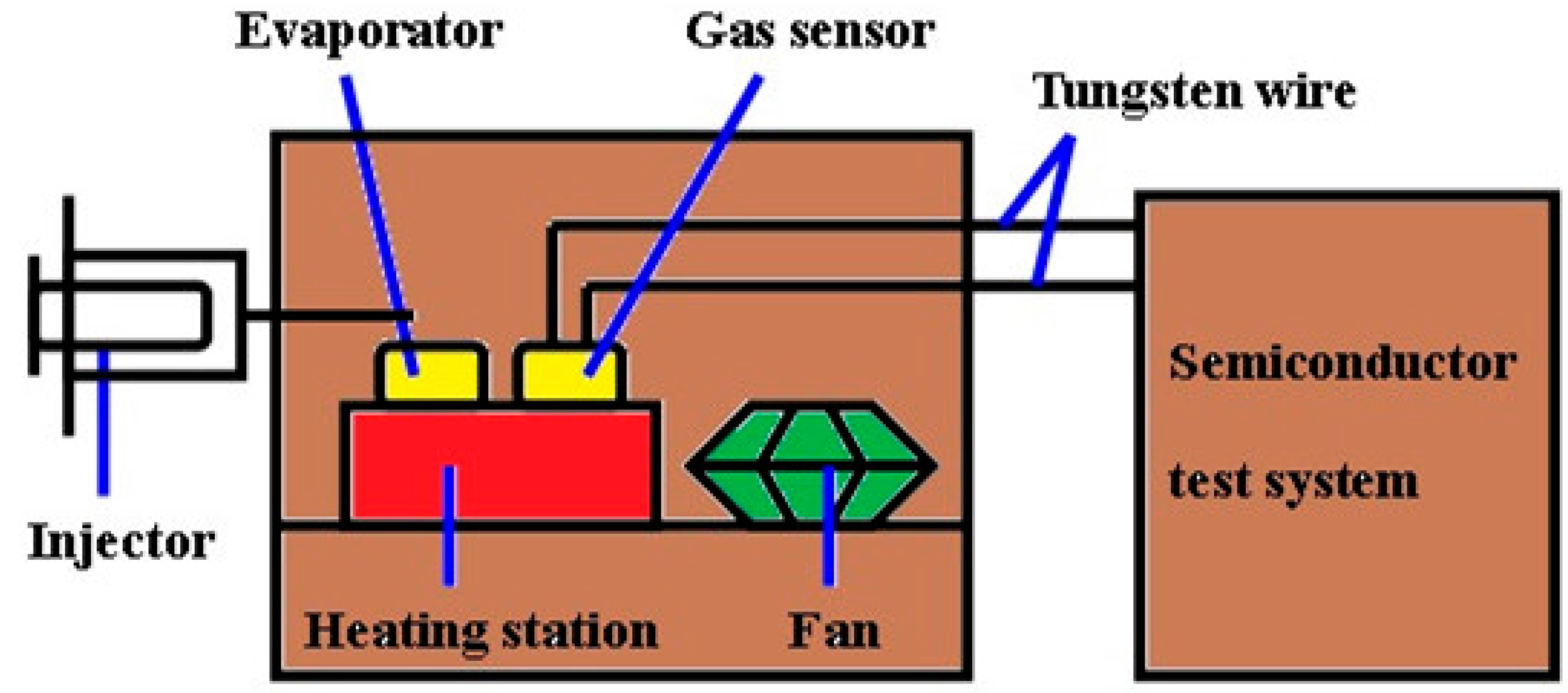
3. Results and Discussion
3.1. Structural Characterization and Microstructure Analysis
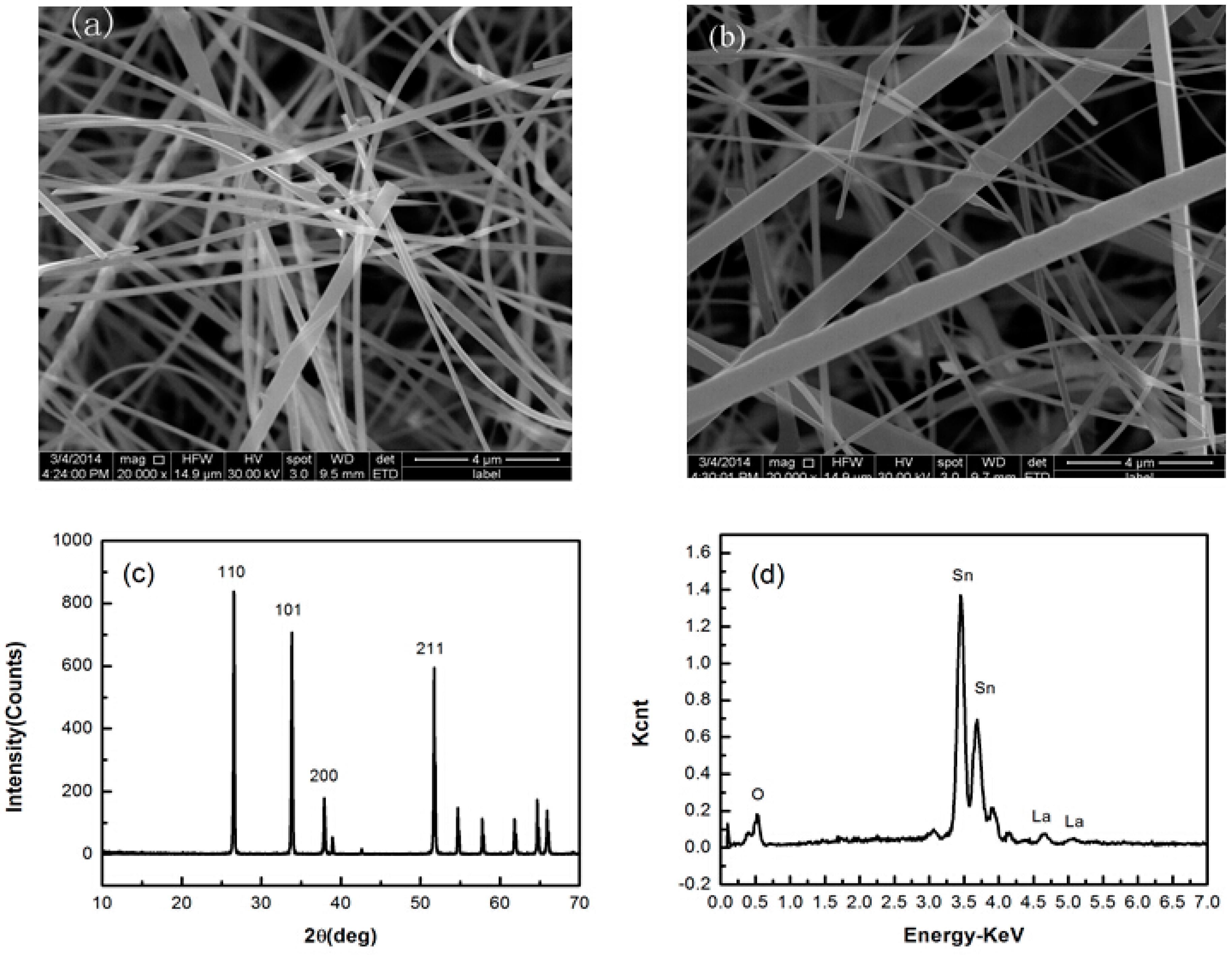
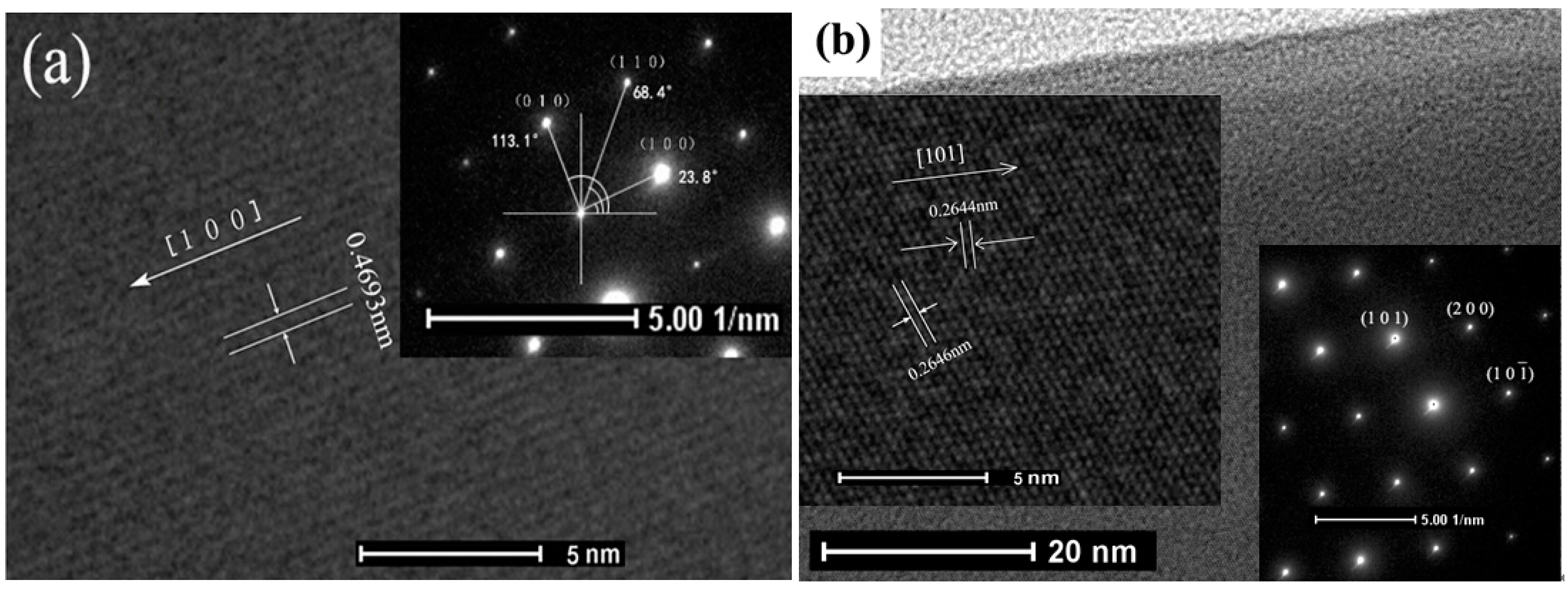
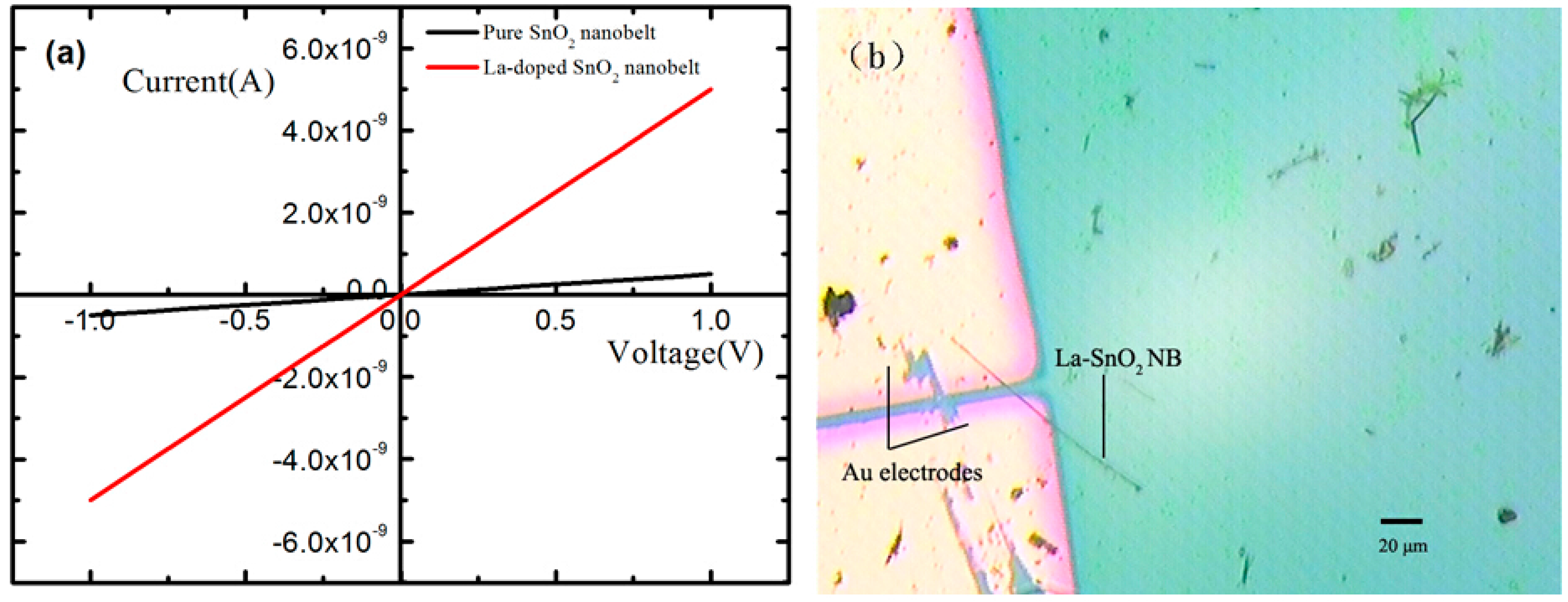
3.2. Sensing Properties of Single La-SnO2 NB Device
3.2.1. Working Temperature
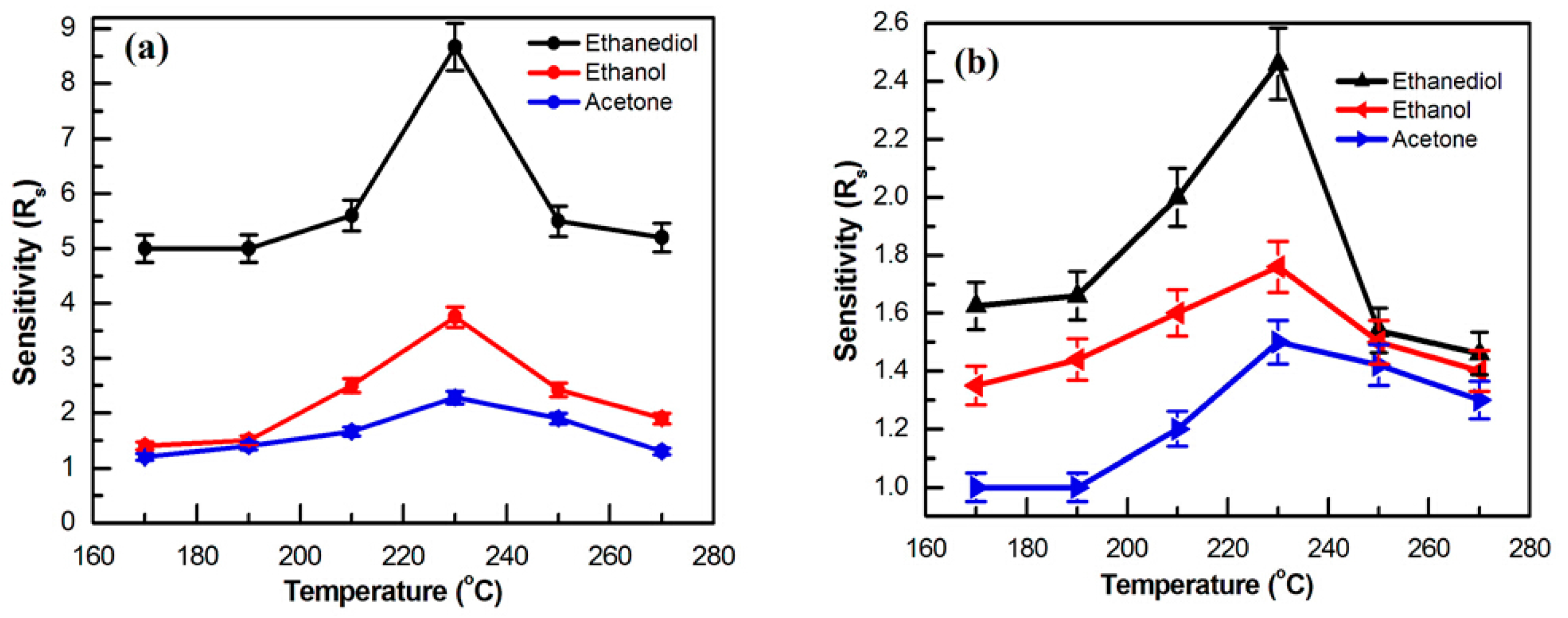
3.2.2. High Response


3.2.3. Response Time and Recovery Time
| Concentrations | 70 ppm | 100 ppm | 200 ppm | 300 ppm | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Time | Tres (s) | Trec (s) | Tres (s) | Trec (s) | Tres (s) | Trec (s) | Tres (s) | Trec (s) | Average Tres(s) | Average Trec(s) | |
| Ethanediol | doped | 9 | 7 | 12 | 4 | 15 | 5 | 16 | 6 | 13 | 5.5 |
| pure | 4 | 9 | 5 | 3 | 3 | 17 | 10 | 9 | 5.5 | 9.5 | |
| Ethanol | doped | 6 | 6 | 5 | 10 | 3 | 6 | 8 | 10 | 5.5 | 8.0 |
| pure | 5 | 8 | 8 | 4 | 10 | 15 | 3 | 12 | 6.5 | 9.75 | |
| Acetone | doped | 5 | 6 | 5 | 5 | 3 | 7 | 3 | 10 | 4.0 | 7.0 |
| pure | 5 | 6 | 6 | 6 | 7 | 5 | 4 | 6 | 5.5 | 5.75 | |
3.3. Gas Sensing Mechanism
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ahn, J.H.; Yun, J.; Choi, Y.K.; Park, I. Palladium nanoparticle decorated silicon nanowire field-effect transistor with side-gates for hydrogen gas detection. Appl. Phys. Lett. 2014, 104, 013508. [Google Scholar] [CrossRef]
- Comini, E.; Baratto, C.; Concina, I.; Faglia, G.; Falasconi, M.; Ferroni, M.; Galstyan, V.; Gobbi, E.; Ponzoni, A.; Vomiero, A.; et al. Metal oxide nanoscience and nanotechnology for chemical sensors. Sens. Actuators B Chem. 2013, 179, 3–20. [Google Scholar] [CrossRef]
- Hieu, N.V.; Duc, N.A.P.; Trung, T.; Tuan, M.A.; Chien, N.D. Gas-sensing properties of tin oxide doped with metal oxides and carbon nanotubes: A competitive sensor for ethanol and liquid petroleum gas. Sens. Actuators B Chem. 2010, 144, 450–456. [Google Scholar] [CrossRef]
- Shao, F.; Hoffmann, M.W.G.; Prades, J.D.; Zamani, R.; Arbiol, J.; Morante, J.R.; Varechkina, E.; Rumyantseva, M.; Gaskov, A.; Giebelhaus, I.; et al. Heterostructured p-CuO (nanoparticle)/n-SnO2 (nanowire) devices for selective H2S detection. Sens. Actuators B Chem. 2013, 181, 130–135. [Google Scholar] [CrossRef]
- Quang, V.V.; Dung, N.V.; Trong, N.S.; Hoa, N.D.; Duy, N.V.; Hieu, N.V. Outstanding gas-sensing performance of graphene/SnO2 nanowire Schottky junctions. Appl. Phys. Lett. 2014, 105, 013107. [Google Scholar] [CrossRef]
- Manjula, P.; Arunkumar, S.; Manorama, S.V. Au/SnO2 an excellent material for room temperature carbon monoxide sensing. Sens. Actuators B Chem. 2011, 152, 168–175. [Google Scholar] [CrossRef]
- Wang, K.W.; Kang, W.D.; Wei, Y.C.; Liu, C.W.; Su, P.C.; Chen, H.S.; Chung, S.R. Promotion of Pd Cu/C Catalysts for Ethanol Oxidation in Alkaline Solution by SnO2 Modifiter. ChemCatChem 2011, 4, 1154–1161. [Google Scholar] [CrossRef]
- Fang, L.M.; Zu, X.T.; Li, Z.J.; Zhu, S.; Liu, C.M.; Zhou, W.L.; Wang, L.M. Synthesis and Characterization of Fe3+-doped SnO2 nanoparticles via sol-gel-calcination or sol-gel-hydrothermal route. J. Alloy Compd. 2008, 454, 261–267. [Google Scholar] [CrossRef]
- Mu, H.C.; Zhang, Z.Q.; Zhao, X.J.; Feng, L.; Wang, K.K.; Xie, H.F. High sensitive formaldehyde graphene gas sensor modified by atomic layer deposition zinc oxide films. Appl. Phys. Lett. 2014, 105, 033107. [Google Scholar] [CrossRef]
- Sun, P.; Yu, Y.S.; Xu, J.; Sun, Y.F.; Ma, J.; Lu, G.Y. One-Step synthesis and gas sensing characteristics of hierarchical SnO2 nanorods modified by Pd loading. Sens. Actuators B Chem. 2011, 160, 244–250. [Google Scholar] [CrossRef]
- Kim, K.M.; Choi, K.I.; Jeong, H.M.; Kim, H.J.; Kim, H.R.; Lee, J.H. Highly sensitive and selective trimethylamine sensors using Ru-doped SnO2 hollow spheres. Sens. Actuators B Chem. 2012, 166–167, 733–738. [Google Scholar] [CrossRef]
- Ghaddab, B.; Sanchez, J.B.; Mavon, C.; Paillet, M.; Parret, R.; Zahab, A.A.; Bantignies, J.L.; Flaud, V.; Beche, E.; Berger, F. Detection of O3 and NH3 using hybrid tin dioxide/carbon nanotubes sensors: Influence of materials and processing on sensor’s sensitivity. Sens. Actuators B Chem. 2012, 170, 67–74. [Google Scholar] [CrossRef]
- Li, X.P.; Cho, J.H.; Kurup, P.; Gu, Z.Y. Novel sensor array based on doped tin oxide nanowires for organic vapor detection. Sens. Actuators B Chem. 2012, 162, 251–258. [Google Scholar] [CrossRef]
- Ma, J.; Liu, Y.K.; Zhang, H.; Ai, P.; Gong, N.L.; Zhang, Y. Synthesis and high sensing properties of a single Pd-doped SnO2 Nanoribbon. Nanoscale Res. Lett. 2014, 9, 503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.P.; Zu, R.D.; Zhong, L.W. Nanobelts of Semiconducting Oxides. Science 2001, 291, 1947–1949. [Google Scholar]
- Ying, P.Z.; Ni, Z.F.; Xiu, W.J.; Jia, L.J.; Luo, Y. Study on Synthesis and Gas Sensitivity of SnO2 Nanobelts. Chin. Phys. Lett. 2006, 23, 1026. [Google Scholar]
- Huang, H.T.; Tian, S.Q.; Xu, J. Needle-like Zn-doped SnO2 nanorods with enhanced photocatalytic and gas sensing properties. Nanotechnology 2012, 23, 105502. [Google Scholar] [CrossRef] [PubMed]
- Mohanapriya, P.; Segawa, H.; Watanabe, K. Enhanced ethanol gas sensing performance of Ce-doped SnO2 hollow nanofibers prepared by electrospinning. Sens. Actuators B Chem. 2013, 188, 872–878. [Google Scholar] [CrossRef]
- Abaker, M.; Dar, G.N.; Ahmad, U.; Zaidi, S.A.; Ibrahim, A.A.; Baskoutas, S.; Hajry, A.A. CuO Nanocubes Based Highly-Sensitive 4-Nitrophenol Chemical Sensor. Sci. Adv. Mater. 2012, 4, 893–900. [Google Scholar] [CrossRef]
- Ahmad, U.; Akhtar, M.S.; Dar, G.N.; Baskoutas, S. Low-Temperature synthesis of α-Fe2O3 hexagonal nanoparticles for environmental remediation and smart sensor applications. Talanta 2013, 116, 1060–1066. [Google Scholar]
- Dar, G.N.; Ahmad, U.; Zaidi, S.A.; Ibrahim, A.A.; Abaker, M.; Baskoutas, S.; Al-Assiri, M.S. Ce-doped ZnO nanorods for the detection of hazardous chemical. Sens. Actuators B Chem. 2012, 173, 72–78. [Google Scholar] [CrossRef]
- Torsi, L.; Dodabalapur, A.; Sabbatini, L.; Zambonin, P.G. Multi-Parameter gas sensors based on organic thin-film-transistors. Sens. Actuators B Chem. 2000, 67, 312–316. [Google Scholar] [CrossRef]
- Cordero, S.R.; Carson, P.J.; Estabrook, R.A.; Strouse, G.F.; Buratto, S.K. Photo-Activated luminescence of CdSe quantum dot monolayers. Phys. Chem. B 2000, 104, 12137–12142. [Google Scholar] [CrossRef]
- Jin, Z.Z.; Wang, M.S. Modern Sensor Technology, 1st ed.; Electronic Industry Press: Beijing, China, 1995; p. 3. [Google Scholar]
- Zhang, G.Z.; Zhang, S.P.; Yang, L.; Zou, Z.J.; Zeng, D.W.; Xie, C.S. La2O3-Sensitized SnO2 nanocrystalline porous film gas sensors and sensing mechanism toward formaldehyde. Sens. Actuators B Chem. 2013, 188, 137–146. [Google Scholar] [CrossRef]
- Gaik, T.A.; Geik, H.T.; Mohamad, Z.A.B.; Ahmad, Z.A.; Mohd, R.O. High sensitivity and fast response SnO2 and La-SnO2 catalytic pellet sensors in detecting volatile organic compounds. Process. Saf. Environ. 2011, 89, 186–192. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Zhang, H.; Liu, Y.; Chen, W.; Ma, J.; Li, S.; Qin, Z. Synthesis and Gas Sensing Properties of Single La-Doped SnO2 Nanobelts. Sensors 2015, 15, 14230-14240. https://doi.org/10.3390/s150614230
Wu Y, Zhang H, Liu Y, Chen W, Ma J, Li S, Qin Z. Synthesis and Gas Sensing Properties of Single La-Doped SnO2 Nanobelts. Sensors. 2015; 15(6):14230-14240. https://doi.org/10.3390/s150614230
Chicago/Turabian StyleWu, Yuemei, Heng Zhang, Yingkai Liu, Weiwu Chen, Jiang Ma, Shuanghui Li, and Zhaojun Qin. 2015. "Synthesis and Gas Sensing Properties of Single La-Doped SnO2 Nanobelts" Sensors 15, no. 6: 14230-14240. https://doi.org/10.3390/s150614230
APA StyleWu, Y., Zhang, H., Liu, Y., Chen, W., Ma, J., Li, S., & Qin, Z. (2015). Synthesis and Gas Sensing Properties of Single La-Doped SnO2 Nanobelts. Sensors, 15(6), 14230-14240. https://doi.org/10.3390/s150614230






