QD-Based FRET Probes at a Glance
Abstract
:1. Introduction
2. FRET Principle
3. The Response Mechanisms of QD-Based FRET Sensors: Displacement
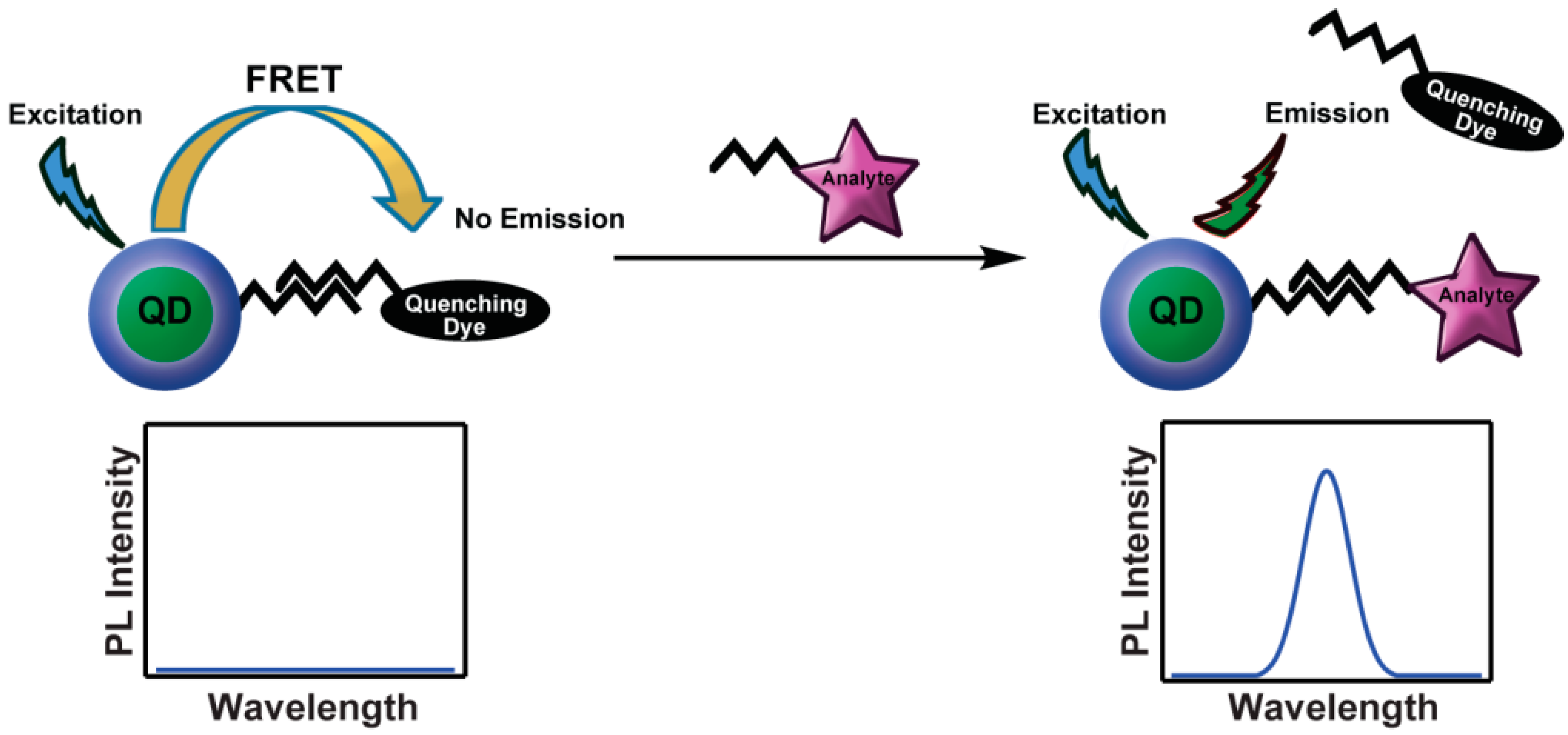
3.1. Turn-On Sensors
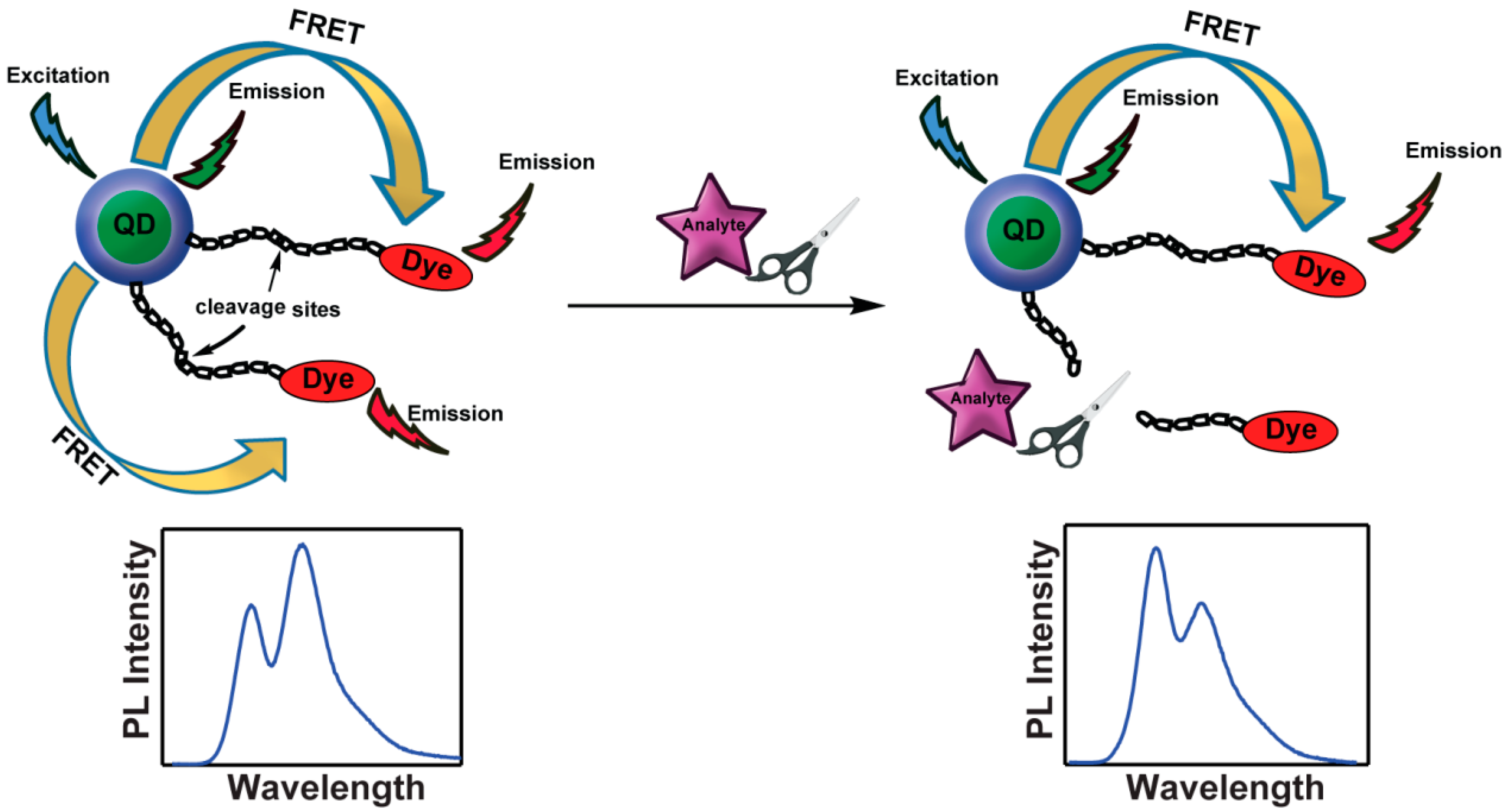
3.2. Ratiometric Sensors, Cleaving the Link
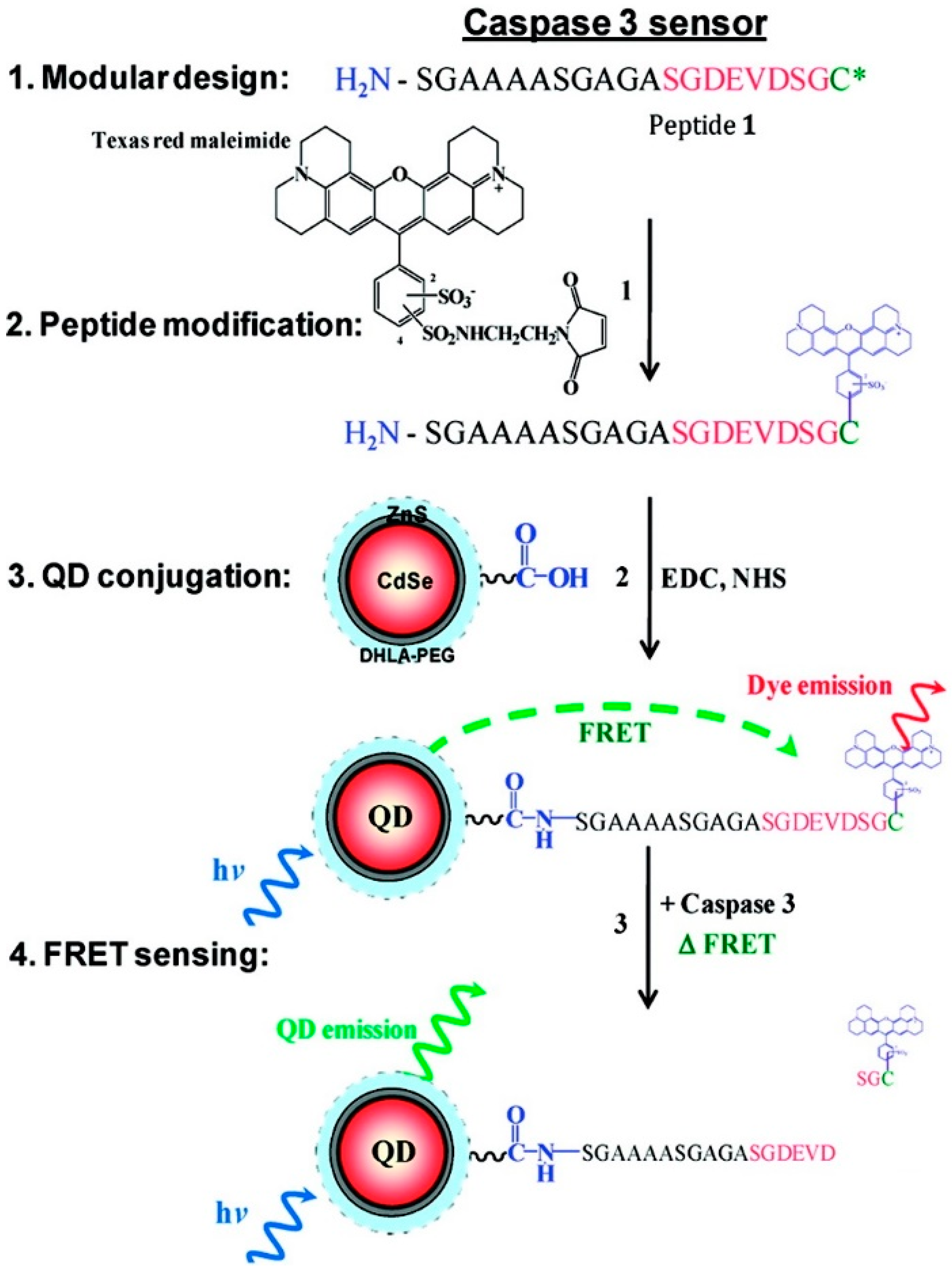

3.3. Ratiometric Sensors, Structural Rearrangement

4. The Response Mechanisms of QD-Based FRET Sensors: Spectral Overlap
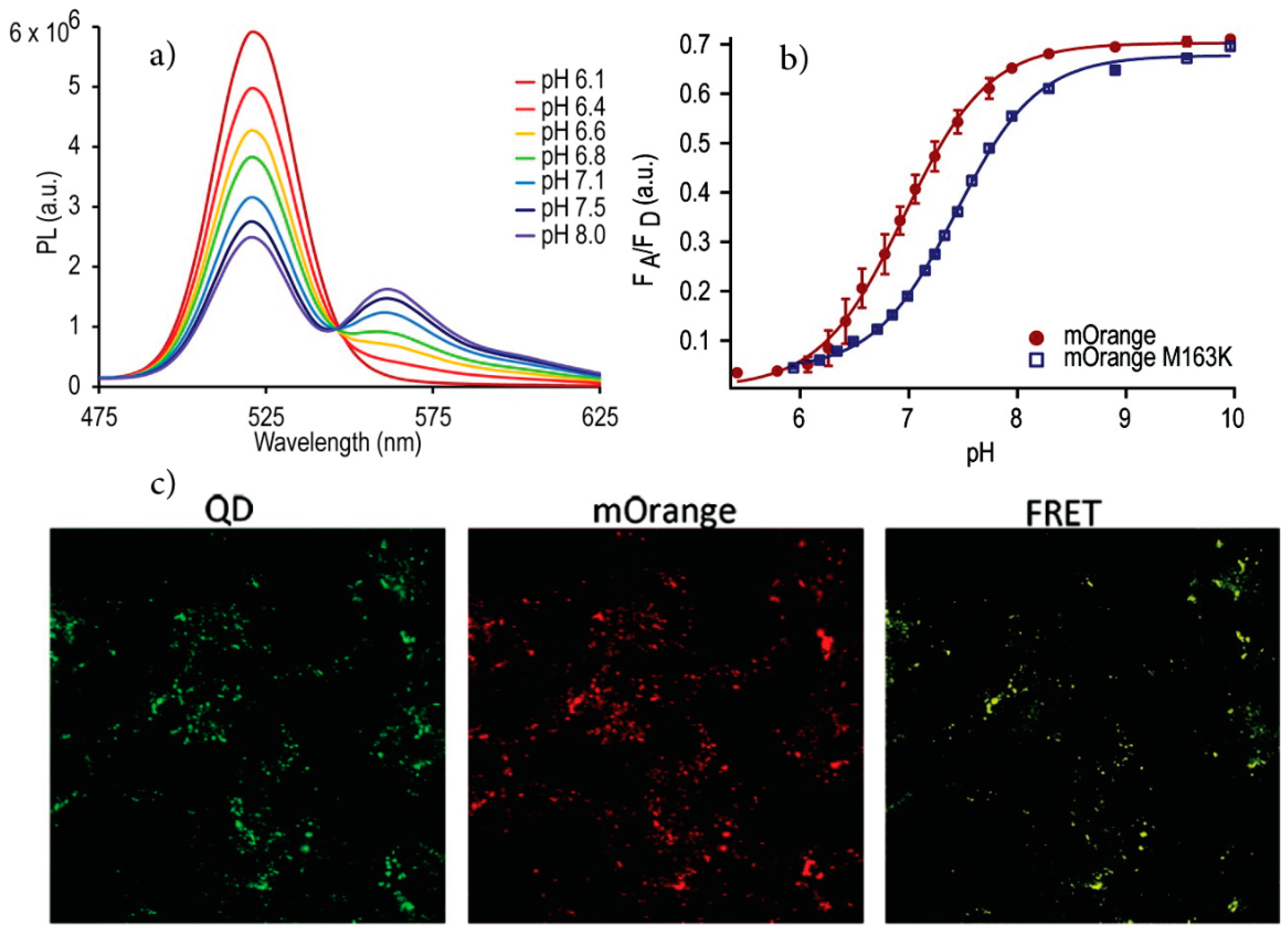
4.1. pH

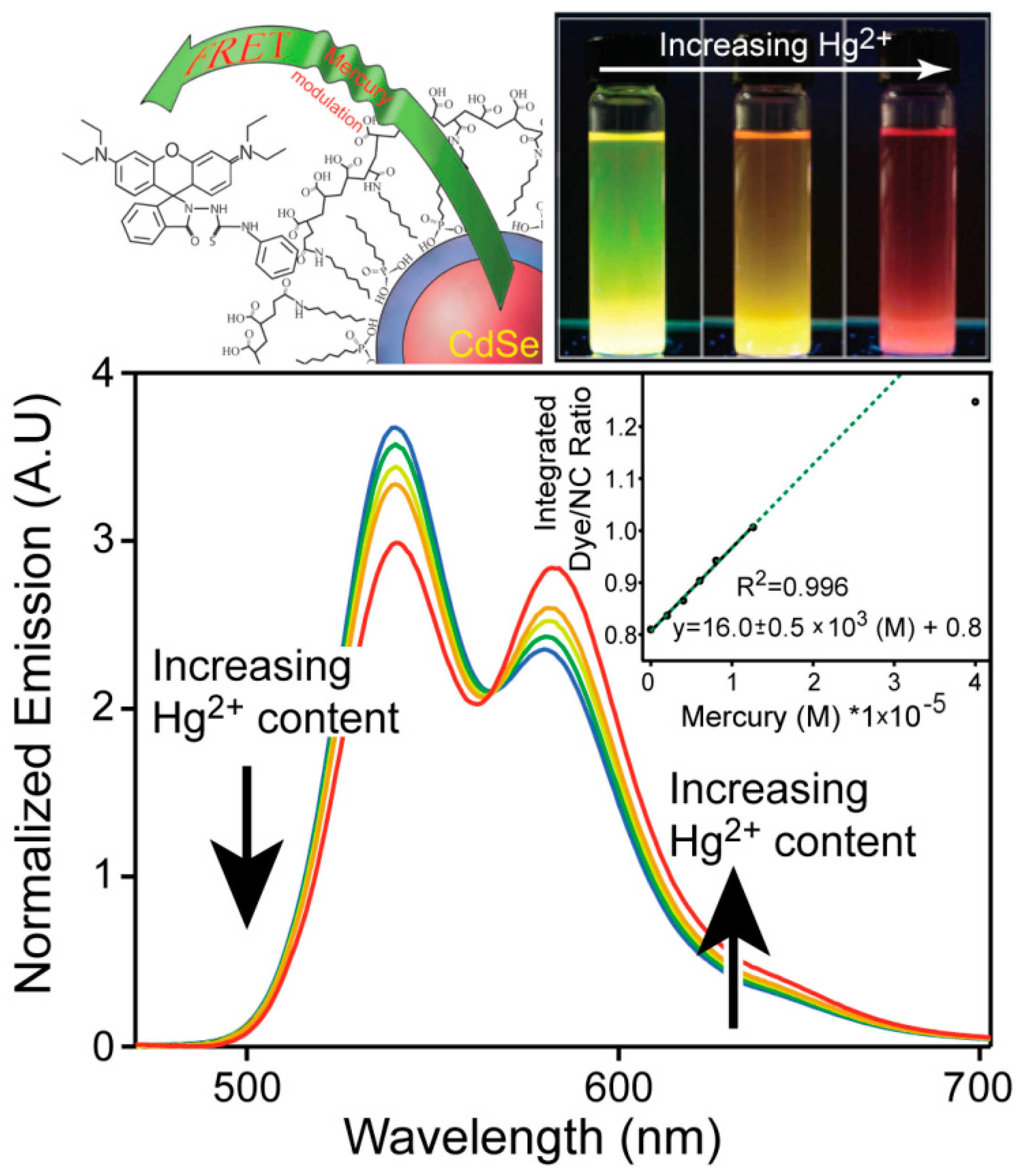
4.2. Metals
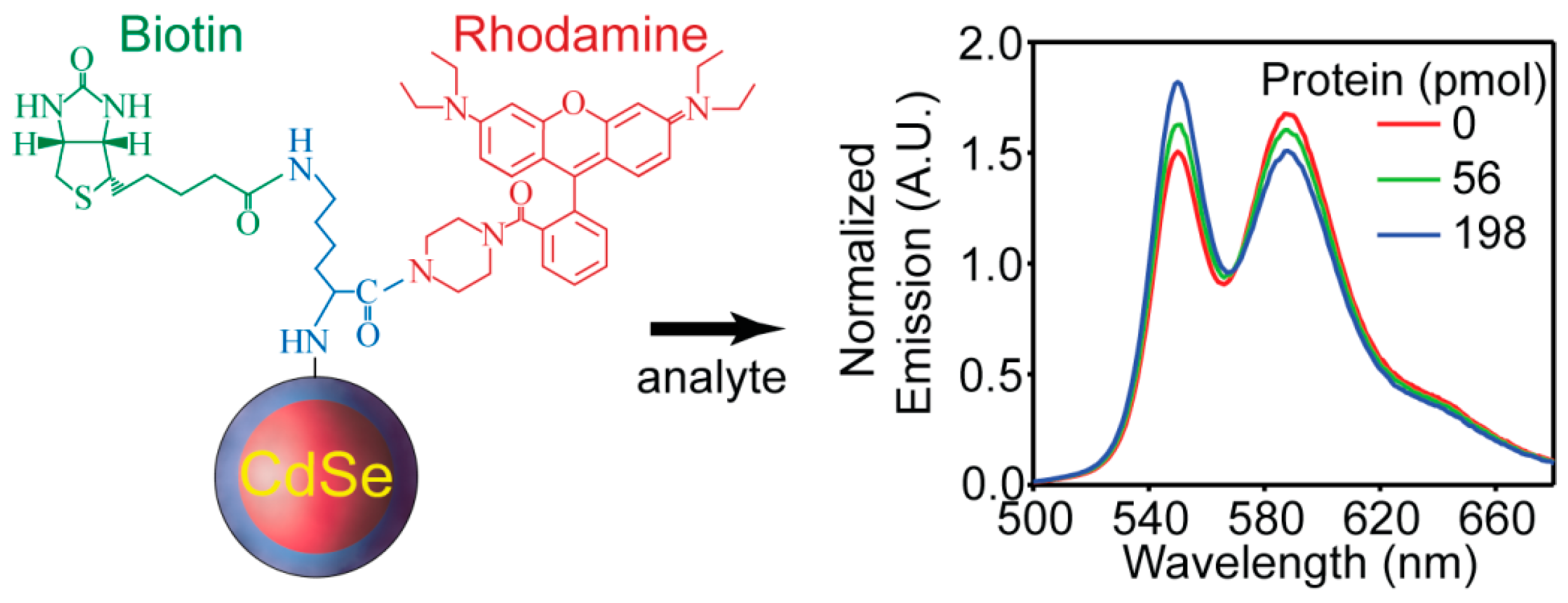
4.3. Proteins and Enzymes
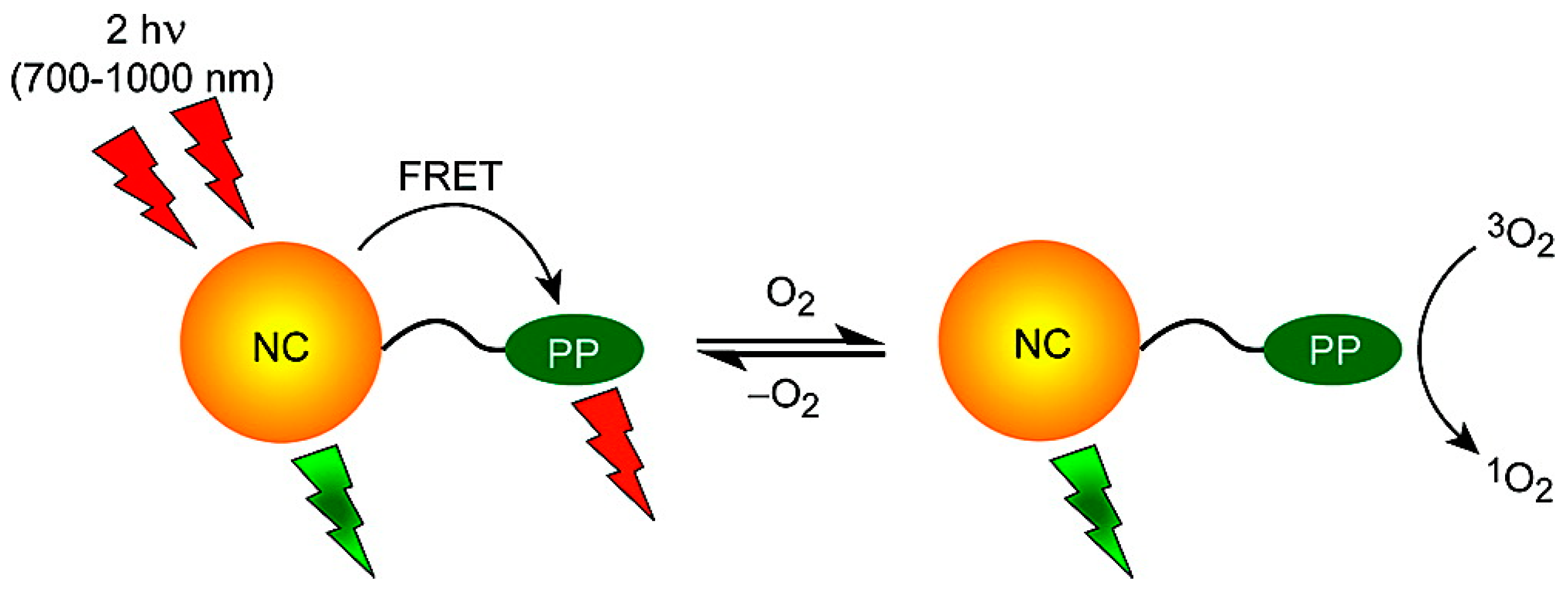
4.4. Oxygen
5. The Response Mechanisms of QD-Based FRET Sensors: High Dye Loading Levels

6. Conclusions and Outlook
Acknowledgments
Conflicts of Interest
References
- Lakowicz, J. Fluorescence sensing. In Principles of Fluorescence Spectroscopy; Lakowicz, J., Ed.; Springer: New York, NY, USA, 2006; pp. 623–673. [Google Scholar]
- Mason, W.T. Fluorescent and Luminescent Probes for Biological Activity, 2nd ed.; Academic Press: London, UK, 1999. [Google Scholar]
- Campbell, J.C. Recent advances in telecommunications avalanche photodiodes. J. Lightwave Technol. 2007, 25, 109–121. [Google Scholar] [CrossRef]
- Chan, W.C.; Maxwell, D.J.; Gao, X.; Bailey, R.E.; Han, M.; Nie, S. Luminescent quantum dots for multiplexed biological detection and imaging. Curr. Opin. Biotechnol. 2002, 13, 40–46. [Google Scholar] [CrossRef]
- Brus, L.E. A simple model for the ionization potential, electron affinity, and aqueous redox potentials of small semiconductor crystallites. J. Chem. Phys. 1983, 79, 5566–5571. [Google Scholar] [CrossRef]
- Jaiswal, J.K.; Simon, S.M. Potentials and pitfalls of fluorescent quantum dots for biological imaging. Trends Cell. Biol. 2004, 14, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Bruchez, M., Jr.; Moronne, M.; Gin, P.; Weiss, S.; Alivisatos, A.P. Semiconductor nanocrystals as fluorescent biological labels. Science 1998, 281, 2013–2016. [Google Scholar] [CrossRef] [PubMed]
- Chan, W.C.; Nie, S. Quantum dot bioconjugates for ultrasensitive nonisotopic detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef] [PubMed]
- Frasco, M.; Chaniotakis, N. Semiconductor quantum dots in chemical sensors and biosensors. Sensors 2009, 9, 7266–7286. [Google Scholar] [CrossRef] [PubMed]
- Costa-Fernández, J.M.; Pereiro, R.; Sanz-Medel, A. The use of luminescent quantum dots for optical sensing. Trends Anal. Chem. 2006, 25, 207–218. [Google Scholar] [CrossRef]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum dots versus organic dyes as fluorescent labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.B.; Norris, D.J.; Bawendi, M.G. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc. 1993, 115, 8706–8715. [Google Scholar] [CrossRef]
- Dabbousi, B.O.; Rodriguez-Viejo, J.; Mikulec, F.V.; Heine, J.R.; Mattoussi, H.; Ober, R.; Jensen, K.F.; Bawendi, M.G. (CdSe)ZnS core–shell quantum dots: Synthesis and characterization of a size series of highly luminescent nanocrystallites. J. Phys. Chem. B 1997, 101, 9463–9475. [Google Scholar] [CrossRef]
- Hines, M.A.; Guyot-Sionnest, P. Synthesis and characterization of strongly luminescing ZnS-capped CdSe nanocrystals. J. Phys. Chem. 1996, 100, 468–471. [Google Scholar] [CrossRef]
- Snee, P.T.; Tyrakowski, C.M.; Page, L.E.; Isovic, A.; Jawaid, A.M. Quantifying quantum dots through Förster resonant energy transfer. J. Phys. Chem. C 2011, 115, 19578–19582. [Google Scholar] [CrossRef]
- Bilan, R.; Fleury, F.; Nabiev, I.; Sukhanova, A. Quantum dot surface chemistry and functionalization for cell targeting and imaging. Bioconjugate Chem. 2015, 26. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Liu, H.; Liu, J.; Haley, K.N.; Treadway, J.A.; Larson, J.P.; Ge, N.; Peale, F.; Bruchez, M.P. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat. Biotechnol. 2003, 21, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Thakar, R.; Snee, P.T. Imparting nanoparticle function with size-controlled amphiphilic polymers. J. Am. Chem. Soc. 2008, 130, 3744–3745. [Google Scholar] [CrossRef] [PubMed]
- Dubertret, B.; Skourides, P.; Norris, D.J.; Noireaux, V.; Brivanlou, A.H.; Libchaber, A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science 2002, 298, 1759–1762. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.W.; Chang, E.; Falkner, J.C.; Zhang, J.; Al-Somali, A.M.; Sayes, C.M.; Johns, J.; Drezek, R.; Colvin, V.L. Forming biocompatible and nonaggregated nanocrystals in water using amphiphilic polymers. J. Am. Chem. Soc. 2007, 129, 2871–2879. [Google Scholar] [CrossRef] [PubMed]
- Algar, W.R.; Krull, U.J. Luminescence and stability of aqueous thioalkyl acid capped CdSe/ZnS quantum dots correlated to ligand ionization. ChemPhysChem 2007, 8, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.J.; Hollmann, C.A.; Zhang, Z.; Suffern, D.; Bradforth, S.E.; Dimitrijevic, N.M.; Minarik, W.G.; Nadeau, J.L. Photophysics of dopamine-modified quantum dots and effects on biological systems. Nat. Mater. 2006, 5, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Mattoussi, H.; Mauro, J.M.; Goldman, E.R.; Anderson, G.P.; Sundar, V.C.; Mikulec, F.V.; Bawendi, M.G. Self-assembly of CdSe–ZnS quantum dot bioconjugates using an engineered recombinant protein. J. Am. Chem. Soc. 2000, 122, 12142–12150. [Google Scholar] [CrossRef]
- Aldana, J.; Wang, Y.A.; Peng, X. Photochemical instability of CdSe nanocrystals coated by hydrophilic thiols. J. Am. Chem. Soc. 2001, 123, 8844–8850. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Snee, P.T. Water-soluble semiconductor nanocrystals cap exchanged with metalated ligands. ACS Nano 2011, 5, 546–550. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zrazhevskiy, P.; Gao, X. Encapsulation of single quantum dots with mesoporous silica. Ann. Biomed. Eng. 2009, 37, 1960–1966. [Google Scholar] [CrossRef] [PubMed]
- Selvan, S.T.; Tan, T.T.; Ying, J.Y. Robust, non-cytotoxic, silica-coated CdSe quantum dots with efficient photoluminescence. Adv. Mater. 2005, 17, 1620–1621. [Google Scholar] [CrossRef]
- Gerion, D.; Pinaud, F.; Williams, S.C.; Parak, W.J.; Zanchet, D.; Weiss, S.; Alivisatos, A.P. Synthesis and properties of biocompatible water-soluble silica-coated CdSe/ZnS semiconductor quantum dots. J. Phys. Chem. B 2001, 105, 8861–8871. [Google Scholar] [CrossRef]
- East, D.A.; Todd, M.; Bruce, I.J. Quantum dot-antibody conjugates via carbodiimide-mediated coupling for cellular imaging. Methods Mol. Biol. 2014, 1199, 67–83. [Google Scholar] [PubMed]
- Shen, H.; Jawaid, A.M.; Snee, P.T. Poly(ethylene glycol) carbodiimide coupling reagents for the biological and chemical functionalization of water-soluble nanoparticles. ACS Nano 2009, 3, 915–923. [Google Scholar] [CrossRef] [PubMed]
- Susumu, K.; Mei, B.C.; Mattoussi, H. Multifunctional ligands based on dihydrolipoic acid and polyethylene glycol to promote biocompatibility of quantum dots. Nat. Protoc. 2009, 4, 424–436. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.; Lai, E.P. Capillary electrophoresis for the characterization of quantum dots after non-selective or selective bioconjugation with antibodies for immunoassay. J. Nanobiotechnol. 2008, 6. [Google Scholar] [CrossRef] [PubMed]
- Goldman, E.R.; Balighian, E.D.; Mattoussi, H.; Kuno, M.K.; Mauro, J.M.; Tran, P.T.; Anderson, G.P. Avidin: A natural bridge for quantum dot-antibody conjugates. J. Am. Chem. Soc. 2002, 124, 6378–6382. [Google Scholar] [CrossRef] [PubMed]
- Berti, L.; D’Agostino, P.; Boeneman, K.; Medintz, I. Improved peptidyl linkers for self-assembly of semiconductor quantum dot bioconjugates. Nano Res. 2009, 2, 121–129. [Google Scholar] [CrossRef]
- Prasuhn, D.E.; Blanco-Canosa, J.B.; Vora, G.J.; Delehanty, J.B.; Susumu, K.; Mei, B.C.; Dawson, P.E.; Medintz, I.L. Combining chemoselective ligation with polyhistidine-driven self-assembly for the modular display of biomolecules on quantum dots. ACS Nano 2010, 4, 267–278. [Google Scholar] [CrossRef] [PubMed]
- Aldeek, F.; Safi, M.; Zhan, N.; Palui, G.; Mattoussi, H. Understanding the self-assembly of proteins onto gold nanoparticles and quantum dots driven by metal-histidine coordination. ACS Nano 2013, 7, 10197–10210. [Google Scholar] [CrossRef] [PubMed]
- Rogach, A.L.; Klar, T.A.; Lupton, J.M.; Meijerink, A.; Feldmann, J. Energy transfer with semiconductor nanocrystals. J. Mater. Chem. 2009, 19, 1208–1221. [Google Scholar] [CrossRef]
- Somers, R.C.; Bawendi, M.G.; Nocera, D.G. CdSe nanocrystal based chem-/bio- sensors. Chem. Soc. Rev. 2007, 36, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Willard, D.; Mutschler, T.; Yu, M.; Jung, J.; Van Orden, A. Directing energy flow through quantum dots: Towards nanoscale sensing. Anal. Bioanal. Chem. 2006, 384, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Crooker, S.A.; Hollingsworth, J.A.; Tretiak, S.; Klimov, V.I. Spectrally resolved dynamics of energy transfer in quantum-dot assemblies: Towards engineered energy flows in artificial materials. Phys. Rev. Lett. 2002, 89. [Google Scholar] [CrossRef]
- Clapp, A.R.; Medintz, I.L.; Mauro, J.M.; Fisher, B.R.; Bawendi, M.G.; Mattoussi, H. Fluorescence resonance energy transfer between quantum dot donors and dye-labeled protein acceptors. J. Am. Chem. Soc. 2004, 126, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Pons, T.; Medintz, I.L.; Wang, X.; English, D.S.; Mattoussi, H. Solution-phase single quantum dot fluorescence resonance energy transfer. J. Am. Chem. Soc. 2006, 128, 15324–15331. [Google Scholar] [CrossRef] [PubMed]
- Clapp, A.R.; Medintz, I.L.; Fisher, B.R.; Anderson, G.P.; Mattoussi, H. Can luminescent quantum dots be efficient energy acceptors with organic dye donors? J. Am. Chem. Soc. 2005, 127, 1242–1250. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, N.; Charbonnière, L.J.; Löhmannsröben, H.-G. Time-resolved analysis of a highly sensitive Förster resonance energy transfer immunoassay using terbium complexes as donors and quantum dots as acceptors. J. Biomed. Biotechnol. 2007, 2007. [Google Scholar] [CrossRef] [PubMed]
- Charbonnière, L.J.; Hildebrandt, N.; Ziessel, R.F.; Löhmannsröben, H.-G. Lanthanides to quantum dots resonance energy transfer in time-resolved fluoro-immunoassays and luminescence microscopy. J. Am. Chem. Soc. 2006, 128, 12800–12809. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.L.; Uyeda, H.T.; Goldman, E.R.; Mattoussi, H. Quantum dot bioconjugates for imaging, labelling and sensing. Nat. Mater. 2005, 4, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.L.; Konnert, J.H.; Clapp, A.R.; Stanish, I.; Twigg, M.E.; Mattoussi, H.; Mauro, J.M.; Deschamps, J.R. A fluorescence resonance energy transfer-derived structure of a quantum dot-protein bioconjugate nanoassembly. Proc. Natl. Acad. Sci. USA 2004, 101, 9612–9617. [Google Scholar] [CrossRef] [PubMed]
- Clapp, A.R.; Medintz, I.L.; Mattoussi, H. Förster resonance energy transfer investigations using quantum-dot fluorophores. ChemPhysChem 2006, 7, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Basko, D.M.; Agranovich, V.M.; Bassani, F.; Rocca, G.C.L. Energy transfer from a semiconductor quantum dot to an organic matrix. Eur. Phys. J. B 2000, 13, 653–659. [Google Scholar] [CrossRef]
- Willard, D.M.; Carillo, L.L.; Jung, J.; Van Orden, A. CdSe–ZnS quantum dots as resonance energy transfer donors in a model protein–protein binding assay. Nano Lett. 2001, 1, 469–474. [Google Scholar] [CrossRef]
- Medintz, I.L.; Clapp, A.R.; Mattoussi, H.; Goldman, E.R.; Fisher, B.; Mauro, J.M. Self-assembled nanoscale biosensors based on quantum dot fret donors. Nat. Mater. 2003, 2, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Goldman, E.R.; Medintz, I.L.; Whitley, J.L.; Hayhurst, A.; Clapp, A.R.; Uyeda, H.T.; Deschamps, J.R.; Lassman, M.E.; Mattoussi, H. A hybrid quantum dot–antibody fragment fluorescence resonance energy transfer-based TNT sensor. J. Am. Chem. Soc. 2005, 127, 6744–6751. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Zhang, Y.; Xiao, F.; Xia, Z.; Rao, J. Quantum dot/bioluminescence resonance energy transfer based highly sensitive detection of proteases. Angew. Chem. Int. Ed. Engl. 2007, 46, 4346–4349. [Google Scholar] [CrossRef] [PubMed]
- Sapsford, K.E.; Granek, J.; Deschamps, J.R.; Boeneman, K.; Blanco-Canosa, J.B.; Dawson, P.E.; Susumu, K.; Stewart, M.H.; Medintz, I.L. Monitoring botulinum neurotoxin a activity with peptide-functionalized quantum dot resonance energy transfer sensors. ACS Nano 2011, 5, 2687–2699. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.B.; Kim, Y.P. Analysis of protease activity using quantum dots and resonance energy transfer. Theranostics 2012, 2, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Miller, J.S.; Sun, J.; Yu, W.W.; Colvin, V.L.; Drezek, R.; West, J.L. Protease-activated quantum dot probes. Biochem. Biophys. Res. Commun. 2005, 334, 1317–1321. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Rosenzweig, N.; Rosenzweig, Z. Luminescent quantum dots fluorescence resonance energy transfer-based probes for enzymatic activity and enzyme inhibitors. Anal. Chem. 2007, 79, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Algar, W.R.; Malonoski, A.; Deschamps, J.R.; Blanco-Canosa, J.B.; Susumu, K.; Stewart, M.H.; Johnson, B.J.; Dawson, P.E.; Medintz, I.L. Proteolytic activity at quantum dot-conjugates: Kinetic analysis reveals enhanced enzyme activity and localized interfacial “hopping”. Nano Lett. 2012, 12, 3793–3802. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xia, J. Capillary electrophoretic studies on displacement and proteolytic cleavage of surface bound oligohistidine peptide on quantum dots. Anal. Chim. Acta 2012, 709, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Seker, U.O.; Ozel, T.; Demir, H.V. Peptide-mediated constructs of quantum dot nanocomposites for enzymatic control of nonradiative energy transfer. Nano Lett. 2011, 11, 1530–1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, R.; Freeman, R.; Xu, J.P.; Willner, I.; Winograd, S.; Shweky, I.; Banin, U. Probing biocatalytic transformations with CdSe-ZnS QDs. J. Am. Chem. Soc. 2006, 128, 15376–15377. [Google Scholar] [CrossRef] [PubMed]
- Biswas, P.; Cella, L.N.; Kang, S.H.; Mulchandani, A.; Yates, M.V.; Chen, W. A quantum-dot based protein module for in vivo monitoring of protease activity through fluorescence resonance energy transfer. Chem. Commun. Camb. 2011, 47, 5259–5261. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, J.; Kim, K.; Kim, H.; Sommer, P.; Song, R. Fluorogenic assay and live cell imaging of HIV-1 protease activity using acid-stable quantum dot-peptide complex. Chem. Commun. Camb. 2010, 46, 9146–9148. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; De Paoli, V.; Rosenzweig, N.; Rosenzweig, Z. Synthesis and application of quantum dots FRET-based protease sensors. J. Am. Chem. Soc. 2006, 128, 10378–10379. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Xing, Y.; So, M.K.; Koh, A.L.; Sinclair, R.; Rao, J. Multiplex detection of protease activity with quantum dot nanosensors prepared by intein-mediated specific bioconjugation. Anal. Chem. 2008, 80, 8649–8655. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.P.; Oh, Y.H.; Oh, E.; Ko, S.; Han, M.K.; Kim, H.S. Energy transfer-based multiplexed assay of proteases by using gold nanoparticle and quantum dot conjugates on a surface. Anal. Chem. 2008, 80, 4634–4641. [Google Scholar] [CrossRef] [PubMed]
- Boeneman, K.; Mei, B.C.; Dennis, A.M.; Bao, G.; Deschamps, J.R.; Mattoussi, H.; Medintz, I.L. Sensing caspase 3 activity with quantum dot-fluorescent protein assemblies. J. Am. Chem. Soc. 2009, 131, 3828–3829. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.L.; Clapp, A.R.; Brunel, F.M.; Tiefenbrunn, T.; Tetsuo Uyeda, H.; Chang, E.L.; Deschamps, J.R.; Dawson, P.E.; Mattoussi, H. Proteolytic activity monitored by fluorescence resonance energy transfer through quantum-dot-peptide conjugates. Nat. Mater. 2006, 5, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Xing, B.; Rao, J. A self-assembled quantum dot probe for detecting β-lactamase activity. Biochem. Biophys. Res. Commun. 2006, 344, 931–935. [Google Scholar] [CrossRef] [PubMed]
- Ghadiali, J.E.; Lowe, S.B.; Stevens, M.M. Quantum-dot-based FRET detection of histone acetyltransferase activity. Angew. Chem. Int. Ed. 2011, 50, 3417–3420. [Google Scholar] [CrossRef] [PubMed]
- Lowe, S.B.; Dick, J.A.; Cohen, B.E.; Stevens, M.M. Multiplex sensing of protease and kinase enzyme activity via orthogonal coupling of quantum dot-peptide conjugates. ACS Nano 2012, 6, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.M. Caspases: The executioners of apoptosis. Biochem. J. 1997, 326, 1–16. [Google Scholar] [PubMed]
- Hunter, A.M.; LaCasse, E.C.; Korneluk, R.G. The inhibitors of apoptosis (IAPs) as cancer targets. Apoptosis 2007, 12, 1543–1568. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.; Schulze-Osthoff, K. Apoptosis-based therapies and drug targets. Cell Death Differ. 2005, 12, 942–961. [Google Scholar] [CrossRef] [PubMed]
- Louneva, N.; Cohen, J.W.; Han, L.-Y.; Talbot, K.; Wilson, R.S.; Bennett, D.A.; Trojanowski, J.Q.; Arnold, S.E. Caspase-3 is enriched in postsynaptic densities and increased in Alzheimer’s Disease. Am. J. Pathol. 2008, 173, 1488–1495. [Google Scholar] [CrossRef] [PubMed]
- Prasuhn, D.E.; Feltz, A.; Blanco-Canosa, J.B.; Susumu, K.; Stewart, M.H.; Mei, B.C.; Yakovlev, A.V.; Loukov, C.; Mallet, J.M.; Oheim, M.; et al. Quantum dot peptide biosensors for monitoring caspase 3 proteolysis and calcium ions. ACS Nano 2010, 4, 5487–5497. [Google Scholar] [CrossRef] [PubMed]
- Breger, J.C.; Sapsford, K.E.; Ganek, J.; Susumu, K.; Stewart, M.H.; Medintz, I.L. Detecting kallikrein proteolytic activity with peptide-quantum dot nanosensors. ACS Appl. Mater. Interfaces 2014, 6, 11529–11535. [Google Scholar] [CrossRef] [PubMed]
- Chi, C.W.; Lao, Y.H.; Li, Y.S.; Chen, L.C. A quantum dot-aptamer beacon using a DNA intercalating dye as the FRET reporter: Application to label-free thrombin detection. Biosens. Bioelectron. 2011, 26, 3346–3352. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Morikis, D.; Ozkan, M. Adaptation of inorganic quantum dots for stable molecular beacons. Sens. Actuators B Chem. 2004, 102, 315–319. [Google Scholar] [CrossRef]
- Levy, M.; Cater, S.F.; Ellington, A.D. Quantum-dot aptamer beacons for the detection of proteins. ChemBioChem 2005, 6, 2163–2166. [Google Scholar] [CrossRef] [PubMed]
- Freeman, R.; Girsh, J.; Willner, I. Nucleic acid/quantum dots (QDs) hybrid systems for optical and photoelectrochemical sensing. ACS Appl. Mater. Interfaces 2013, 5, 2815–2834. [Google Scholar] [CrossRef] [PubMed]
- Sharon, E.; Freeman, R.; Willner, I. CdSe/ZnS quantum dots-G-quadruplex/hemin hybrids as optical DNA sensors and aptasensors. Anal. Chem. 2010, 82, 7073–7077. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Nam, H.Y.; Lee, E.J.; Jung, W.; Hah, S.S. Molecular beacon-based quantitiation of epithelial tumor marker mucin 1. Bioorg. Med. Chem. Lett. 2012, 22, 6081–6084. [Google Scholar] [CrossRef] [PubMed]
- Medintz, I.L.; Berti, L.; Pons, T.; Grimes, A.F.; English, D.S.; Alessandrini, A.; Facci, P.; Mattoussi, H. A reactive peptidic linker for self-assembling hybrid quantum dot-DNA bioconjugates. Nano Lett. 2007, 7, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Kay, E.R.; Lee, J.; Nocera, D.G.; Bawendi, M.G. Conformational control of energy transfer: A mechanism for biocompatible nanocrystal-based sensors. Angew. Chem. Int. Ed. 2013, 52, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Dennis, A.M.; Rhee, W.J.; Sotto, D.; Dublin, S.N.; Bao, G. Quantum dot-fluorescent protein FRET probes for sensing intracellular pH. ACS Nano 2012, 6, 2917–2924. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zeng, F.; Wu, G.; Wu, S. Nanoparticles as scaffolds for fret-based ratiometric detection of mercury ions in water with QDs as donors. Analyst 2012, 137, 3717–3724. [Google Scholar] [CrossRef] [PubMed]
- Snee, P.T.; Somers, R.C.; Nair, G.; Zimmer, J.P.; Bawendi, M.G.; Nocera, D.G. A ratiometric CdSe/ZnS nanocrystal pH sensor. J. Am. Chem. Soc. 2006, 128, 13320–13321. [Google Scholar] [CrossRef] [PubMed]
- Krooswyk, J.D.; Tyrakowski, C.M.; Snee, P.T. Multivariable response of semiconductor nanocrystal-dye sensors: The case of pH. J. Phys. Chem. C 2010, 114, 21348–21352. [Google Scholar] [CrossRef]
- Elliott, J.E.; Macdonald, M.; Nie, J.; Bowman, C.N. Structure and swelling of poly(acrylic acid) hydrogels: Effect of pH, ionic strength, and dilution on the crosslinked polymer structure. Polymer 2004, 45, 1503–1510. [Google Scholar] [CrossRef]
- Xia, Y.-S.; Cao, C.; Zhu, C.-Q. Two distinct photoluminescence responses of CdTe quantum dots to Ag (I). J. Lumin. 2008, 128, 166–172. [Google Scholar] [CrossRef]
- Gonçalves, H.; Mendonça, C.; Esteves da Silva, J.G. Parafac analysis of the quenching of EEM of fluorescence of glutathione capped CdTe quantum dots by Pb(II). J. Fluoresc. 2009, 19, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Ma, Q.; Li, Y.; Lin, Z.H.; Tang, G.; Su, X.G. A novel ascorbic acid sensor based on the Fe3+/Fe2+ modulated photoluminescence of CdTe quantum dots@SiO2 nanobeads. Nanoscale 2013, 5, 9726–9731. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Argüelles, M.T.; Jin, W.J.; Costa-Fernández, J.M.; Pereiro, R.; Sanz-Medel, A. Surface-modified cdse quantum dots for the sensitive and selective determination of Cu(II) in aqueous solutions by luminescent measurements. Anal. Chim. Acta 2005, 549, 20–25. [Google Scholar] [CrossRef]
- Xie, H.-Y.; Liang, J.-G.; Zhang, Z.-L.; Liu, Y.; He, Z.-K.; Pang, D.-W. Luminescent CdSe-ZnS quantum dots as selective Cu2+ probe. Spectrochim. Acta A 2004, 60, 2527–2530. [Google Scholar] [CrossRef]
- Xia, Y.S.; Zhu, C.Q. Use of surface-modified CdTe quantum dots as fluorescent probes in sensing mercury (II). Talanta 2008, 75, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Son, D.H.; Hughes, S.M.; Yin, Y.; Paul Alivisatos, A. Cation exchange reactions in ionic nanocrystals. Science 2004, 306, 1009–1012. [Google Scholar] [CrossRef] [PubMed]
- Page, L.E.; Zhang, X.; Jawaid, A.M.; Snee, P.T. Detection of toxic mercury ions using a ratiometric CdSe/ZnS nanocrystal sensor. Chem. Commun. Camb. 2011, 47, 7773–7775. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mei, F.; Li, W.-Y.; He, X.-W.; Zhang, Y.-K. Study on the fluorescence resonance energy transfer between CdTe QDs and butyl-rhodamine B in the presence of CTMAB and its application on the detection of Hg(II). Spectrochim. Acta A 2008, 70, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yu, P.; Du, D.; He, C.; Qiu, B.; Chen, X.; Chen, G. Rhodamine-based ratiometric fluorescence sensing for the detection of mercury(II) in aqueous solution. Talanta 2010, 81, 433–437. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Li, Y.; Xu, H.; Ma, Y.; Zhu, W.; Zhong, X. Quantum dots-based ratiometric fluorescence probe for mercuric ions in biological fluids. Talanta 2014, 119, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Engvall, E.; Jonsson, K.; Perlmann, P. Enzyme-linked immunosorbent assay. II. Quantitative assay of protein antigen, immunoglobulin G, by means of enzyme-labelled antigen and antibody-coated tubes. Biochim. Biophys. Acta 1971, 251, 427–428. [Google Scholar] [CrossRef]
- Choi, J.H.; Chen, K.H.; Strano, M.S. Aptamer-capped nanocrystal quantum dots: A new method for label-free protein detection. J. Am. Chem. Soc. 2006, 128, 15584–15585. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.K.; Su, H.; Wang, Y.A.; Yu, H.Z. Aptamer-based detection of epithelial tumor marker mucin 1 with quantum dot-based fluorescence readout. Anal. Chem. 2009, 81, 6130–6139. [Google Scholar] [CrossRef] [PubMed]
- Tyrakowski, C.M.; Snee, P.T. Ratiometric CdSe/ZnS quantum dot protein sensor. Anal. Chem. 2014, 86, 2380–2386. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Feng, G.; Guo, Y.; Zhou, D. Robust and specific ratiometric biosensing using a copper-free clicked quantum dot-DNA aptamer sensor. Nanoscale 2013, 5, 10307–10315. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, T.; Yamaguchi, Y.; Hosaka, M.; Takeuchi, T.; Tobita, S. Ratiometric molecular sensor for monitoring oxygen levels in living cells. Angew. Chem. Int. Ed. Engl. 2012, 51, 4148–4151. [Google Scholar] [CrossRef] [PubMed]
- Coogan, M.P.; Court, J.B.; Gray, V.L.; Hayes, A.J.; Lloyd, S.H.; Millet, C.O.; Pope, S.J.; Lloyd, D. Probing intracellular oxygen by quenched phosphorescence lifetimes of nanoparticles containing polyacrylamide-embedded [Ru(dpp(SO3Na)2)3]Cl2. Photochem. Photobiol. Sci. 2010, 9, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.A.; Ferraro, F.; Roussakis, E.; Klein, A.; Wu, J.; Runnels, J.M.; Zaher, W.; Mortensen, L.J.; Alt, C.; Turcotte, R.; et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 2014, 508, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Scheicher, S.R.; Kainz, B.; Kostler, S.; Suppan, M.; Bizzarri, A.; Pum, D.; Sleytr, U.B.; Ribitsch, V. Optical oxygen sensors based on Pt(II) porphyrin dye immobilized on s-layer protein matrices. Biosens. Bioelectron. 2009, 25, 797–802. [Google Scholar] [CrossRef] [PubMed]
- Amelia, M.; Lavie-Cambot, A.; McClenaghan, N.D.; Credi, A. A ratiometric luminescent oxygen sensor based on a chemically functionalized quantum dot. Chem. Commun. Camb. 2011, 47, 325–327. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Carrero, S.; de la Guardia, M.; Galian, R.E.; Perez-Prieto, J. Pyrene-capped CdSe@ZnS nanoparticles as sensitive flexible oxygen sensors in non-aqueous media. ChemistryOpen 2014, 3, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Ethirajan, M.; Chen, Y.; Joshi, P.; Pandey, R.K. The role of porphyrin chemistry in tumor imaging and photodynamic therapy. Chem. Soc. Rev. 2011, 40, 340–362. [Google Scholar] [CrossRef] [PubMed]
- Di Corato, R.; Bealle, G.; Kolosnjaj-Tabi, J.; Espinosa, A.; Clement, O.; Silva, A.K.; Menager, C.; Wilhelm, C. Combining magnetic hyperthermia and photodynamic therapy for tumor ablation with photoresponsive magnetic liposomes. ACS Nano 2015, 9, 2904–2916. [Google Scholar] [CrossRef] [PubMed]
- Samia, A.C.; Chen, X.; Burda, C. Semiconductor quantum dots for photodynamic therapy. J. Am. Chem. Soc. 2003, 125, 15736–15737. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.-M.; Sun, X.-J.; Wang, L.-L.; Fei, M.-Y.; Yan, Z.-Y. Singlet oxygen-generating from fluorescence probes based on denatured bovine serum albumin-conjugated CdTe quantum dots and photosensitizer chlorin e6. J. Nanopart. Res. 2014, 16, 1–9. [Google Scholar] [CrossRef]
- Li, F.; He, Z.; Li, M.; Zhang, J.; Han, J.; Lu, P. Photoinduced energy transfer in a CdTe quantum dot–copper phthalocyanine system via two-photon excitation. Mater. Lett. 2014, 132, 263–266. [Google Scholar] [CrossRef]
- Yaghini, E.; Giuntini, F.; Eggleston, I.M.; Suhling, K.; Seifalian, A.M.; MacRobert, A.J. Fluorescence lifetime imaging and FRET-induced intracellular redistribution of TAT-conjugated quantum dot nanoparticles through interaction with a phthalocyanine photosensitiser. Small 2014, 10, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Tsay, J.M.; Trzoss, M.; Shi, L.; Kong, X.; Selke, M.; Jung, M.E.; Weiss, S. Singlet oxygen production by peptide-coated quantum dot-photosensitizer conjugates. J. Am. Chem. Soc. 2007, 129, 6865–6871. [Google Scholar] [CrossRef] [PubMed]
- McLaurin, E.J.; Greytak, A.B.; Bawendi, M.G.; Nocera, D.G. Two-photon absorbing nanocrystal sensors for ratiometric detection of oxygen. J. Am. Chem. Soc. 2009, 131, 12994–13001. [Google Scholar] [CrossRef] [PubMed]
- Lemon, C.M.; Karnas, E.; Bawendi, M.G.; Nocera, D.G. Two-photon oxygen sensing with quantum dot-porphyrin conjugates. Inorg. Chem. 2013, 52, 10394–10406. [Google Scholar] [CrossRef] [PubMed]
- Ingram, J.M.; Zhang, C.; Xu, J.; Schiff, S.J. FRET excited ratiometric oxygen sensing in living tissue. J. Neurosci. Methods 2013, 214, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Jana, N.R.; Chen, Y.F.; Peng, X.G. Size- and shape-controlled magnetic (Cr, Mn, Fe, Co, Ni) oxide nanocrystals via a simple and general approach. Chem. Mater. 2004, 16, 3931–3935. [Google Scholar] [CrossRef]
- Ravindran, S.; Snee, P.T.; Ramachandran, A.; George, A. Acidic domain in dentin phosphophoryn facilitates cellular uptake implications in targeted protein delivery. J. Biol. Chem. 2013, 288, 16098–16109. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Yong, K.-T.; Liu, L.; Roy, I.; Hu, R.; Zhu, J.; Cai, H.; Law, W.-C.; Liu, J.; Wang, K.; et al. A pilot study in non-human primates shows no adverse response to intravenous injection of quantum dots. Nat. Nanotechnol. 2012, 7, 453–458. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shamirian, A.; Ghai, A.; Snee, P.T. QD-Based FRET Probes at a Glance. Sensors 2015, 15, 13028-13051. https://doi.org/10.3390/s150613028
Shamirian A, Ghai A, Snee PT. QD-Based FRET Probes at a Glance. Sensors. 2015; 15(6):13028-13051. https://doi.org/10.3390/s150613028
Chicago/Turabian StyleShamirian, Armen, Aashima Ghai, and Preston T. Snee. 2015. "QD-Based FRET Probes at a Glance" Sensors 15, no. 6: 13028-13051. https://doi.org/10.3390/s150613028




