Portable and Reusable Optofluidics-Based Biosensing Platform for Ultrasensitive Detection of Sulfadimidine in Dairy Products
Abstract
:1. Introduction
2. Experimental Section
2.1. Apparatus
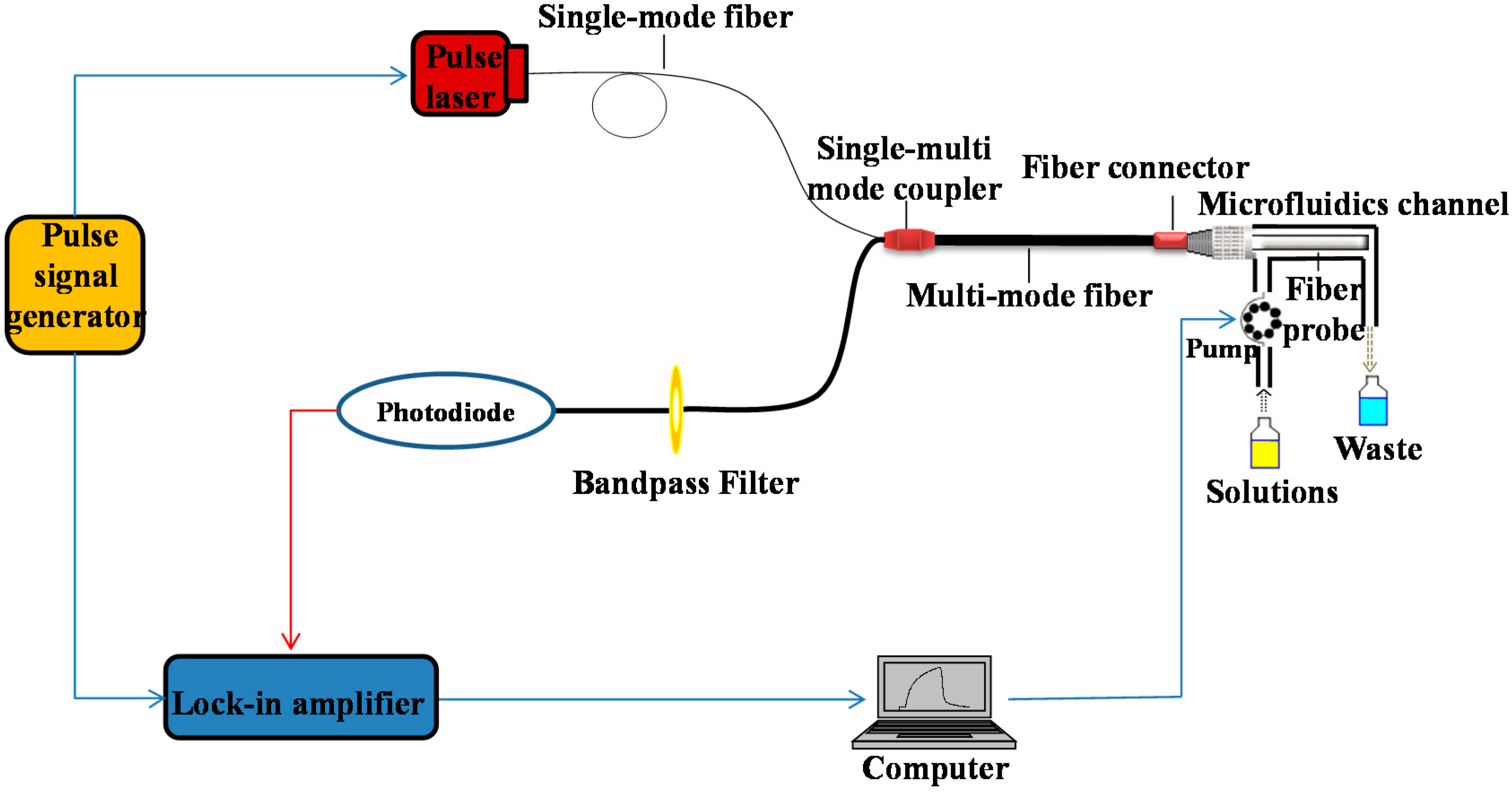
2.2. Chemicals and Reagents
2.3. Surface Chemical Modification of Optic Fiber Probe
2.4. Immunoassay Procedures and Regeneration
2.5. Recovery Experiments

2.6. Data Analysis
3. Results and Discussion
3.1. Analytical Performance

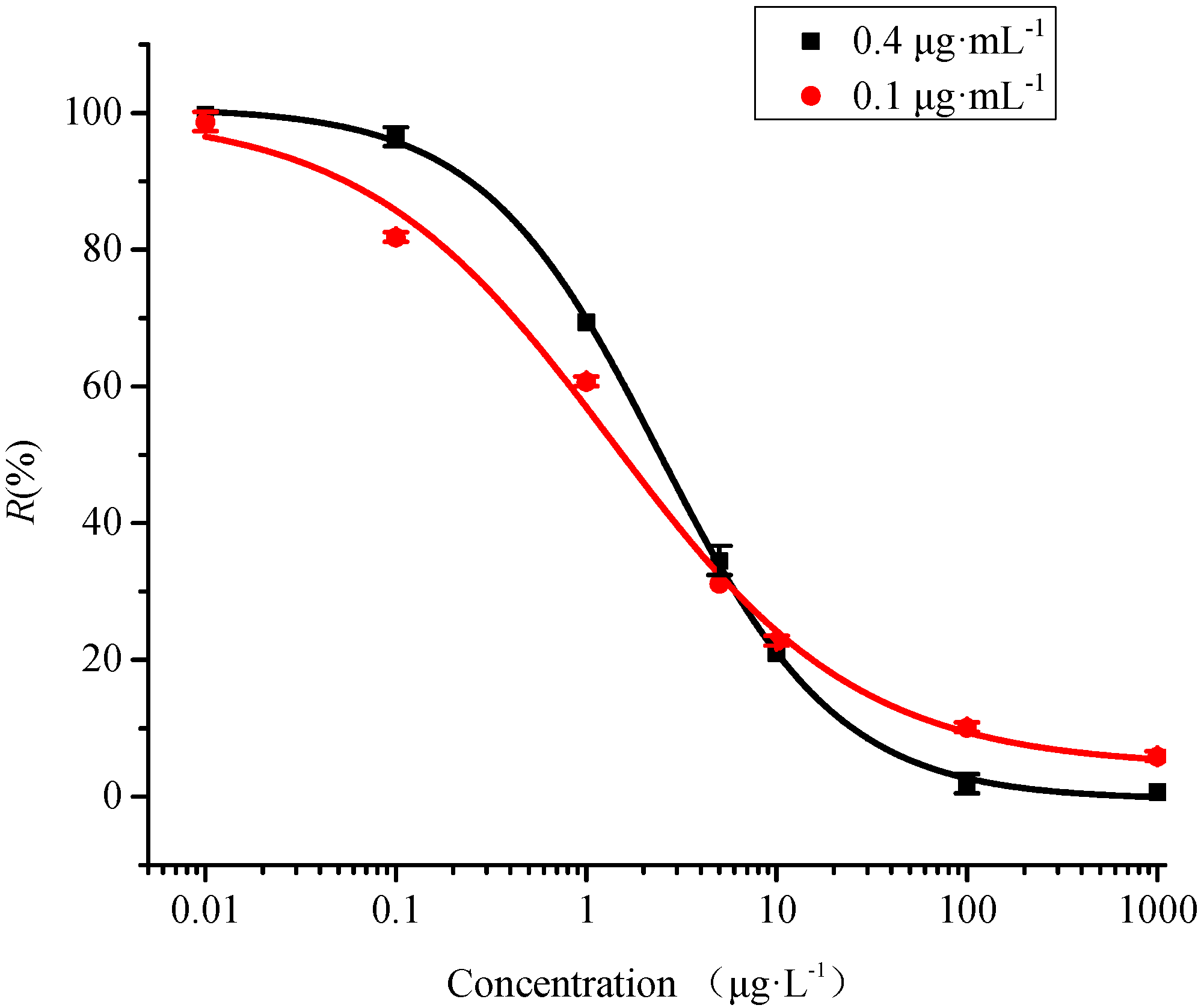
3.2. Selectivity
| Structurally Analogues | IC50 μg·L−1 | LOD μg·L−1 | CR % |
|---|---|---|---|
| SM2 | 0.05 | 0.05 | 100 |
| SMR | 60.08 | 14.41 | <0.08 |
| SMX | >10000 | - | <10−5 |
| SDZ | >10000 | - | <10−5 |
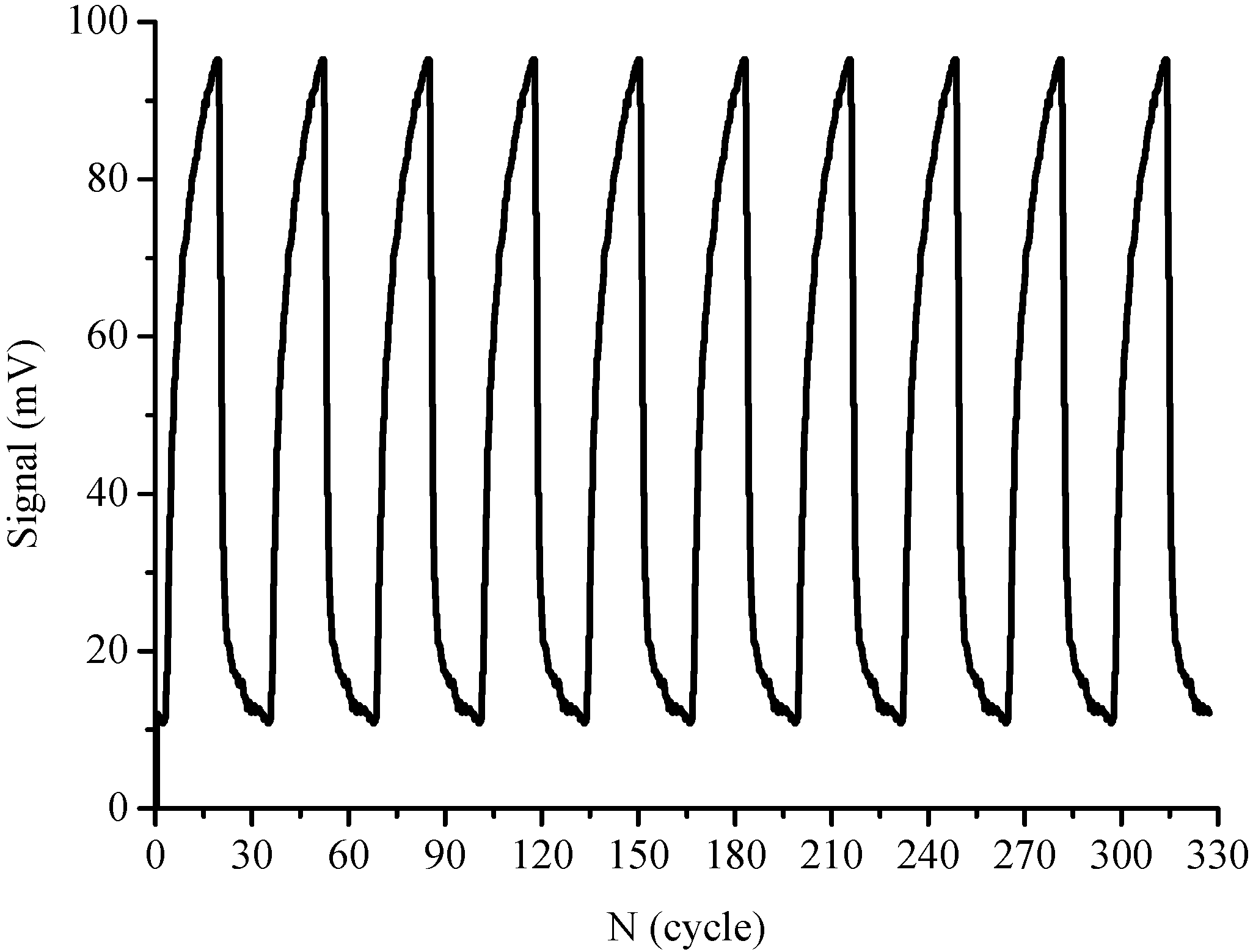
3.3. Reproducibility and Stability
3.4. Detection of SM2 in Real Milk and Other Dairy Samples
| Sample | Spiked μg·L−1 | Measured μg·L−1 | RSD (%) | Recovery (%) |
|---|---|---|---|---|
| Milk | 0.50 | 0.49 | 0.35 | 97.82 |
| 1.00 | 1.00 | 0.71 | 99.78 | |
| Yoghurt | 0.50 | 0.53 | 0.35 | 105.44 |
| 1.00 | 1.16 | 0.71 | 115.94 | |
| Baby formula | 0.50 | 0.55 | 0.71 | 109.47 |
| 1.00 | 1.08 | 0.35 | 107.56 |
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Reference
- Msagati, T.A.; Nindi, M.M. Multiresidue determination of sulfonamides in a variety of biological matrices by supported liquid membrane with high pressure liquid chromatography-electrospray mass spectrometry detection. Talanta 2004, 64, 87–100. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.J.; Qiu, N.N.; Yuan, D.X. Simple and sensitive monitoring of sulfonamide veterinary residues in milk by stir bar sorptive extraction based on monolithic material and high performance liquid chromatography analysis. J. Chromatogr. A 2009, 1216, 8240–8245. [Google Scholar] [CrossRef] [PubMed]
- Raviolo, M.A.; Rambla-Alegre, M.; Clausell-Tormos, J.; Capella-Peiró, M.-E.; Carda-Broch, S.; Esteve-Romero, J. Determination of sulfonamides in milk after precolumn derivatisation by micellar liquid chromatography. Anal. Chim. Acta 2007, 593, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Gentili, A.; Perret, D.; Marchese, S. Liquid chromatography-tandem mass spectrometry for performing confirmatory analysis of veterinary drugs in animal-food products. Trends Anal. Chem. 2005, 24, 704–733. [Google Scholar] [CrossRef]
- Koesukwiwat, U.; Jayanta, S.; Leepipatpiboon, N. Validation of a liquid chromatography–mass spectrometry multi-residue method for the simultaneous determination of sulfonamides, tetracyclines, and pyrimethamine in milk. J. Chromatogr. A 2007, 1140, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Establishment of Maximum Residue Levels of Veterinary Medical Products in Foodstuffs and Animal Origin; Council Regulation No. 2377/90 of EEC. Publications Office of the European Union: Luxembourg, Luxembourg, 26 June 1990. Available online: https://www.fsai.ie/uploadedFiles/Legislation/Food_Legisation_Links/Veterinary_Medicines,_Animal_Remedies,_Control_of_Illegal_Substances_and_Po/Consol_Reg2377_90.pdf (accessed on 8 April 2015).
- Gao, Q.; Luo, D.; Ding, J.; Feng, Y.Q. Rapid magnetic solid-phase extraction based on magnetite/silica/poly(methacrylic acid–co–ethylene glycol dimethacrylate) composite microspheres for the determination of sulfonamide in milk samples. J. Chromatogr. A 2010, 1217, 5602–5609. [Google Scholar] [CrossRef]
- Zhou, Q.; Peng, D.; Wang, Y.; Pan, Y.; Wan, D.; Zhang, X.; Yuan, Z. A novel hapten and monoclonal-based enzyme-linke immunosorbent assay for sulfonamides in edible animal tissues. Food Chem. 2014, 154, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Fursuawa, N. Simplified determining procedure for routine residue monitoring of sulphamethazine and sulphadimethoxine in milk. J. Chromatogr. A 2000, 898, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Lopes, R.P.; Reyes, R.C.; Romero-González, R.; Frenicha, A.G.; Vidal, J.L.M. Development and validation of a multiclass method for the determination of veterinary drug residues in chicken by ultra high performance liquid chromatography–tandem mass spectrometry. Talanta 2012, 89, 201–208. [Google Scholar] [CrossRef]
- Yu, H.; Tao, Y.F.; Chen, D.; Wang, Y.; Huang, L.; Peng, D.; Dai, M.; Liu, Z.; Wang, X.; Yuan, Z. Development of a high performance liquid chromatography method and a liquid chromatography–tandem mass spectrometry method with the pressurized liquid extraction for the quantification and confirmation of sulfonamides in the foods of animal origin. J. Chromatogr. B 2011, 879, 2653–2662. [Google Scholar] [CrossRef]
- Díaz-Cruz, M.S.; García-Galán, M.J.; Barceló, D. Highly sensitive simultaneous determination of sulfonamide antibiotics and one metabolite in environmental waters by liquid chromatography–quadrupole linear ion trap–mass spectrometry. J. Chromatogr. A 2008, 1193, 50–59. [Google Scholar] [CrossRef]
- García-Galán, M.J.; Garrido, T.; Fraile, J.; Ginebreda·M, A.; Ginebreda, A.; Díaz-Cruz, S.; Barcelo, D. Application of fully automated online solid phase extraction-liquid chromatography-electrospray-tandem mass spectrometry for the determination of sulfonamides and their acetylated metabolites in groundwater. Anal. Bioanal. Chem. 2011, 399, 795–806. [Google Scholar] [CrossRef]
- Nebot, C.; Regal, P.; Miranda, J.; Cepeda, A.; Fente, C. Simultaneous Determination of Sulfonamides, Penicillins and Coccidiostats in Pork by High-Performance Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. Sci. 2012, 50, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Tso, J.; Dutta, S.; Inamdar, S.; Aga, D.S. Simultaneous Analysis of Free and Conjugated Estrogens, Sulfonamides, and Tetracyclines in Runoff Water and Soils Using Solid-Phase Extraction and Liquid Chromatography-Tandem Mass Spectrometry. J Agric. Food Chem. 2011, 59, 2213–2222. [Google Scholar] [CrossRef] [PubMed]
- Verheijen, R.; Stouten, P.; Cazemier, G. Development of a one step strip test for the detection of sulfadimidine residues. Analyst 1998, 123, 2437–2441. [Google Scholar] [CrossRef] [PubMed]
- Fránek, M.; Kolář, V.; Deng, A.; Crooks, S. Determination of Sulphadimidine (Sulfamethazine) Residues in Milk, Plasma, Urine and Edible Tissues by Sensitive ELISA. Food Agric. Immunol. 1999, 11, 339–349. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Radiative Decay Engineering: Biophysical and Biomedical Applications. Anal. Biochem. 2001, 298, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Sun, J.D.; Wu, L.Y.; Ji, J.; Sun, X.; Qian, Y. Surface-enhancedfluorescence immunosensor using Au nano-crosses for the detection of microcystin-LR. Biosens. Bioelectron. 2014, 62, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Hedström, L.L.M. Capacitive sensing of microcystin variants of Microcystis aeruginosa using a gold immunoelectrode modified with antibodies, gold nanoparticles and polytyramine. Microchim. Acta 2014, 181, 1009–1017. [Google Scholar] [CrossRef]
- Herranz, S.; Marazuela, M.D.; Moreno-Bondi, M.C. Automated portable array biosensor for multisample microcystin analysis in freshwater samples. Biosens. Bioelectron. 2012, 33, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Zhu, A.N.; Zhou, X.H.; Wang, H.C.; Zhao, Z.; Liu, L.H.; Shi, H.C. Highly sensitive and selective optofluidics-based immunosensor for rapid assessment of Bisphenol A leaching risk. Biosens. Bioelectron. 2014, 56, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.D.; White, L.M. Optofluidic microsystems for chemical and biological analysis. Nat. Photonics 2011, 5, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.H.; Liu, L.H.; Xu, W.Q.; Song, B.D.; Sheng, J.W.; He, M.; Shi, H.C. A reusable evanescent wave immunosensor for highly sensitive detection of bisphenol A in water samples. Sci. Rep. 2014, 4, 4572. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; He, M.; Shi, H.C.; Zhu, A.N. Development of evanescent wave all-fiber immunosensor for environmental water analysis. Biosens. Bioelectron. 2008, 23, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.J.; Zhou, X.H.; Zhang, Y.; Liu, L.H.; Long, F.; Song, L.; Shi, H.C. Melamine detection in dairy products by using a reusable evanescent wave biosensor. Sens. Actuators B: Chem. 2014, 204, 682–687. [Google Scholar] [CrossRef]
- Mujumdar, S.R.; Mujumdar, R.B.; Grant, C.M. Cyanine-Labeling Reagents: Sulfobenzindocyanine Succinimidyl Esters. Bioconjug. Chem. 1996, 7, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, S.K.; Shriver-lake, L.C.; Prior, K.J. Use of thiol-terminal silanes and heterobifunctional crosslinkers for immobilization of antibodies on silica surfaces. Anal. Biochem. 1989, 178, 408–413. [Google Scholar] [CrossRef] [PubMed]
- Mallat, E.; Barzen, C.; Abuknesha, R.; Gauglitz, G.; Barceló, D. Part per trillion level determination of isoproturon in certified and estuarine water samples with a direct optical immunosensor. Anal. Chim. Acta. 2001, 426, 209–216. [Google Scholar] [CrossRef]
- Mauriz, E.; Calle, A.; Abad, A.; Montoya, A.; Hildebrandt, A.; Barceló, D.; Lechuga, L.M. Determination of carbaryl in natural water samples by a surface plasmon resonance flow-through immunosensor. Biosens. Bioelectron. 2006, 21, 2129–2136. [Google Scholar] [CrossRef]
- Sheng, J.W.; He, M.; Shi, H.C.; Qian, Y. A comprehensive immunoassay for the detection of microcystins in waters based on polyclonal antibodies. Anal. Chim. Acta 2006, 572, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.T.; Su, R.; Haughey, S.A.; Wang, Q.; Xu, Z.; Yang, J.; Shen, Y.; Wang, H.; Jiang, Y.; Sun, Y. Development of a Specifically Enhanced Enzyme-Linked Immunosorbent Assay for the Detection of Melamine in Milk. Molecules 2011, 16, 5591–5603. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Shankaran, D.R.; Kim, S.J.; Gobi, K.V.; Matsumoto, K.; Toko, K.; Miura, N. Fabrication of a novel immunosensor using functionalized self-assembled monolayer for trace level detection of TNT by surface plasmon resonance. Talanta 2007, 72, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.W.; Liu, J.T.; Zhang, T.C.; Li, W.; Liu, W.; Meng, M.; He, F.; Wan, Y.; Feng, C.; Wang, S.; et al. Preparation of Monoclonal Antibody for Melamine and Development of an Indirect Competitive ELISA for Melamine Detection in Raw Milk, Milk Powder, and Animal Feeds. J. Agric. Food. Chem. 2010, 58, 8152–8157. [Google Scholar] [CrossRef]
- Hofstetter, O.; Hofstetter, H.; Wilchek, M.; Schurig, V.; Green, B.S. Chiral discrimination using an immunosensor. Nat. Biotechnol. 1999, 17, 371–374. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Present and future of surface plasmon resonance biosensors. Anal. Bioanal. Chem. 2003, 377, 528–539. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, X.-J.; Zhou, X.-H.; Zhang, Y.; Long, F.; Song, L.; Shi, H.-C. Portable and Reusable Optofluidics-Based Biosensing Platform for Ultrasensitive Detection of Sulfadimidine in Dairy Products. Sensors 2015, 15, 8302-8313. https://doi.org/10.3390/s150408302
Hao X-J, Zhou X-H, Zhang Y, Long F, Song L, Shi H-C. Portable and Reusable Optofluidics-Based Biosensing Platform for Ultrasensitive Detection of Sulfadimidine in Dairy Products. Sensors. 2015; 15(4):8302-8313. https://doi.org/10.3390/s150408302
Chicago/Turabian StyleHao, Xiu-Juan, Xiao-Hong Zhou, Yan Zhang, Feng Long, Lei Song, and Han-Chang Shi. 2015. "Portable and Reusable Optofluidics-Based Biosensing Platform for Ultrasensitive Detection of Sulfadimidine in Dairy Products" Sensors 15, no. 4: 8302-8313. https://doi.org/10.3390/s150408302






