Fracture of Human Femur Tissue Monitored by Acoustic Emission Sensors
Abstract
:1. Introduction
2. Experimental Details
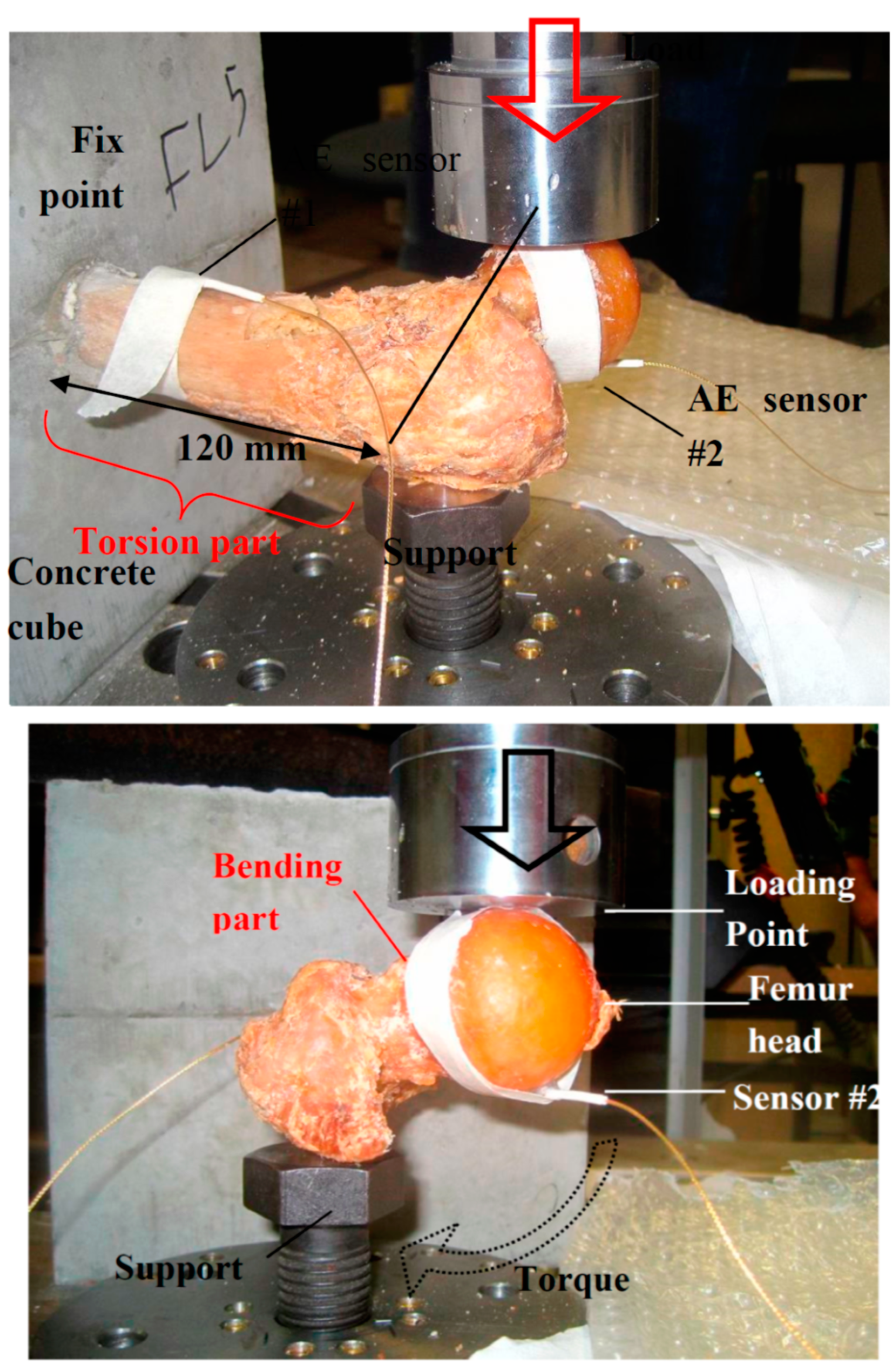
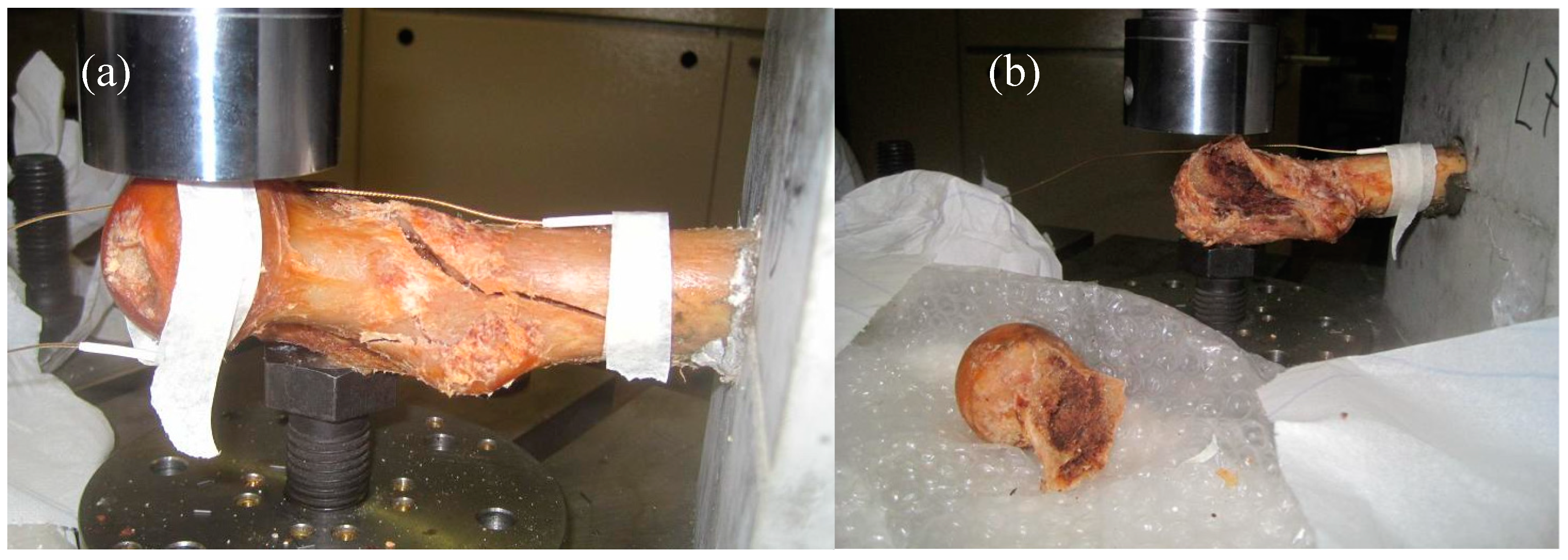
3. Results and Discussion
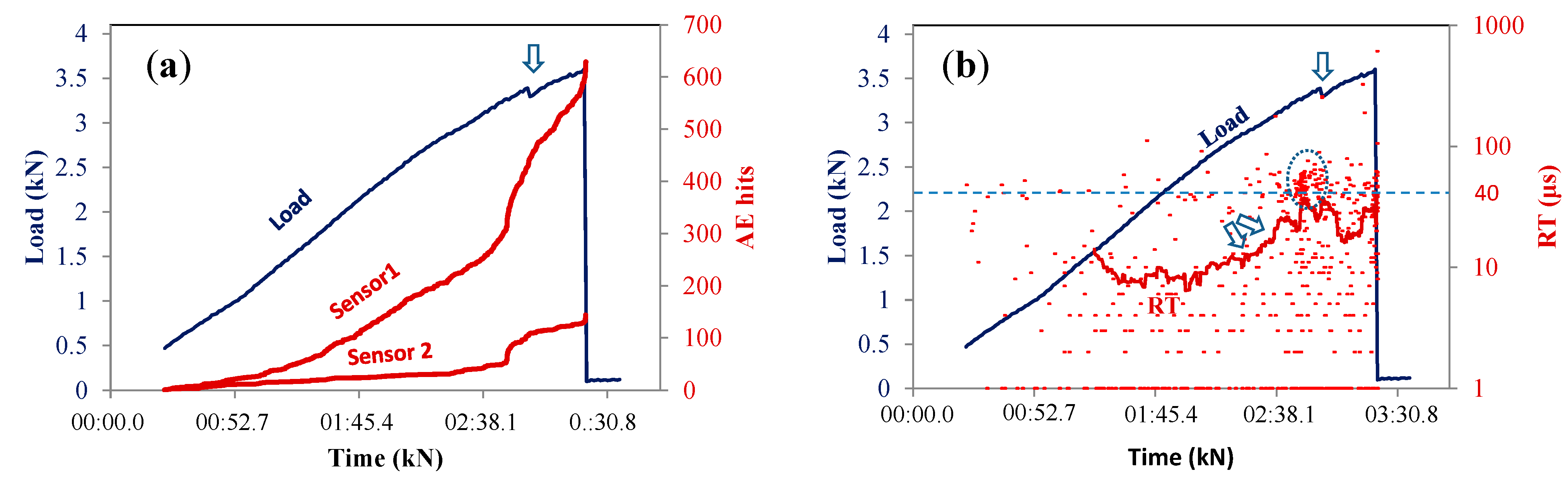
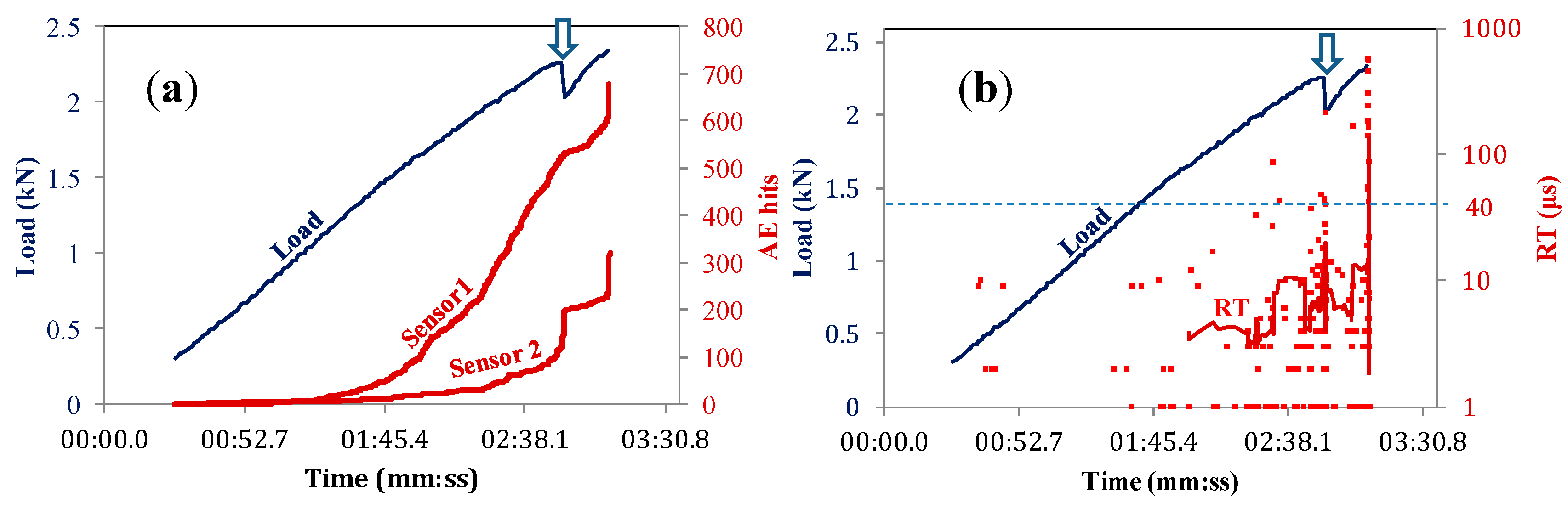
3.1. AE Activity
| Specimen # | Load (%) |
|---|---|
| 1 | 6.7 |
| 2 | 15.4 |
| 3 | 9.2 |
| 4 | 29.6 |
| 5 | 10 |
| 6 | 13.9 |
| 7 | 13.1 |
| 8 | 14.1 |
| 9 | 38.3 |
| 10 | 16.7 |
| 11 | 13.6 |
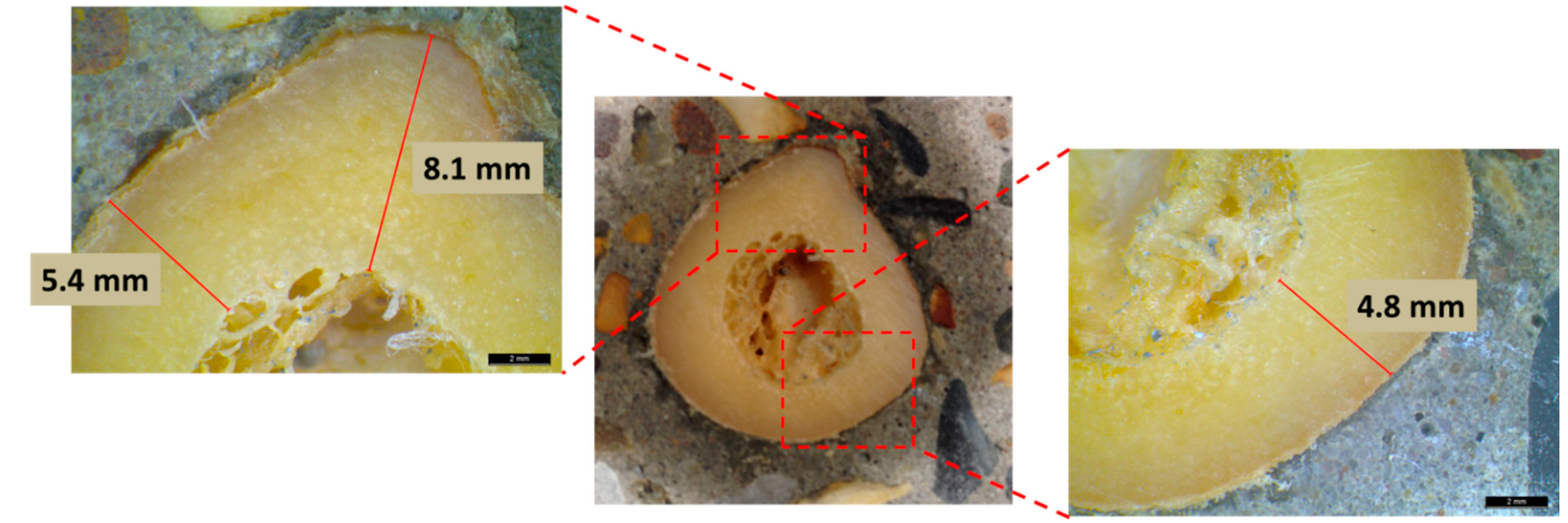
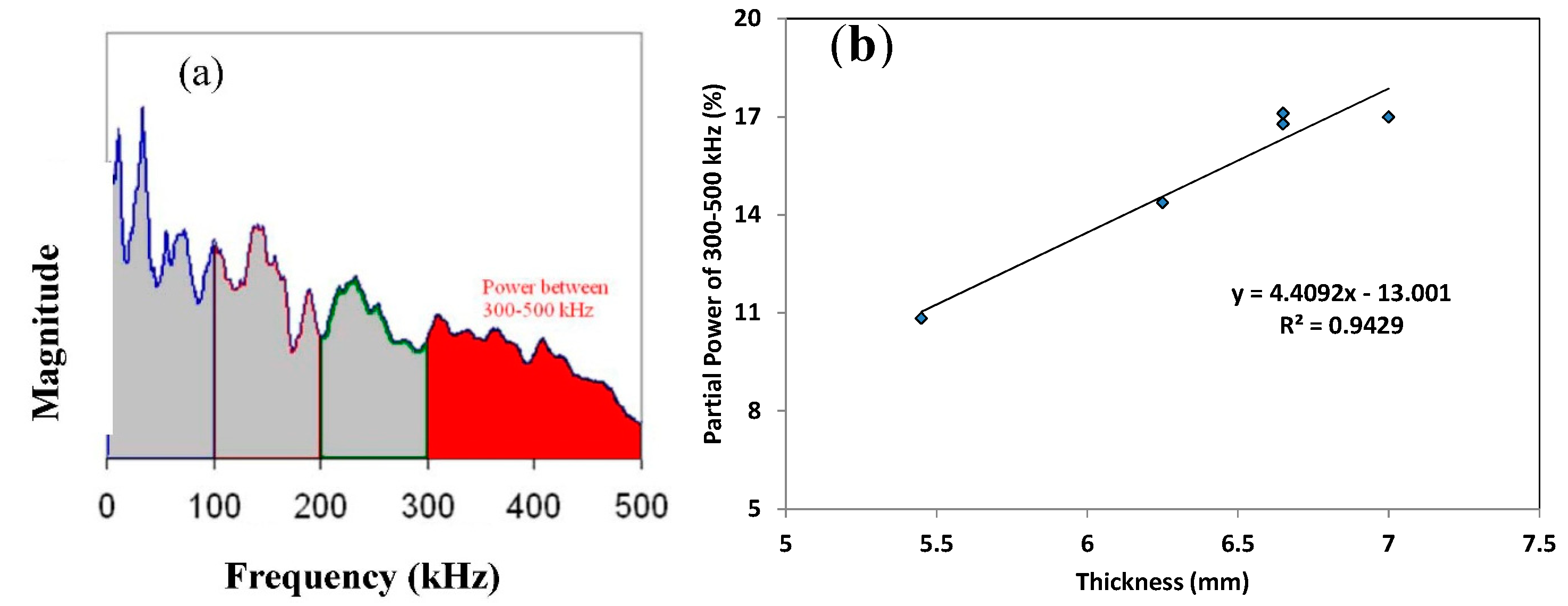
3.2. Correlation with Thickness
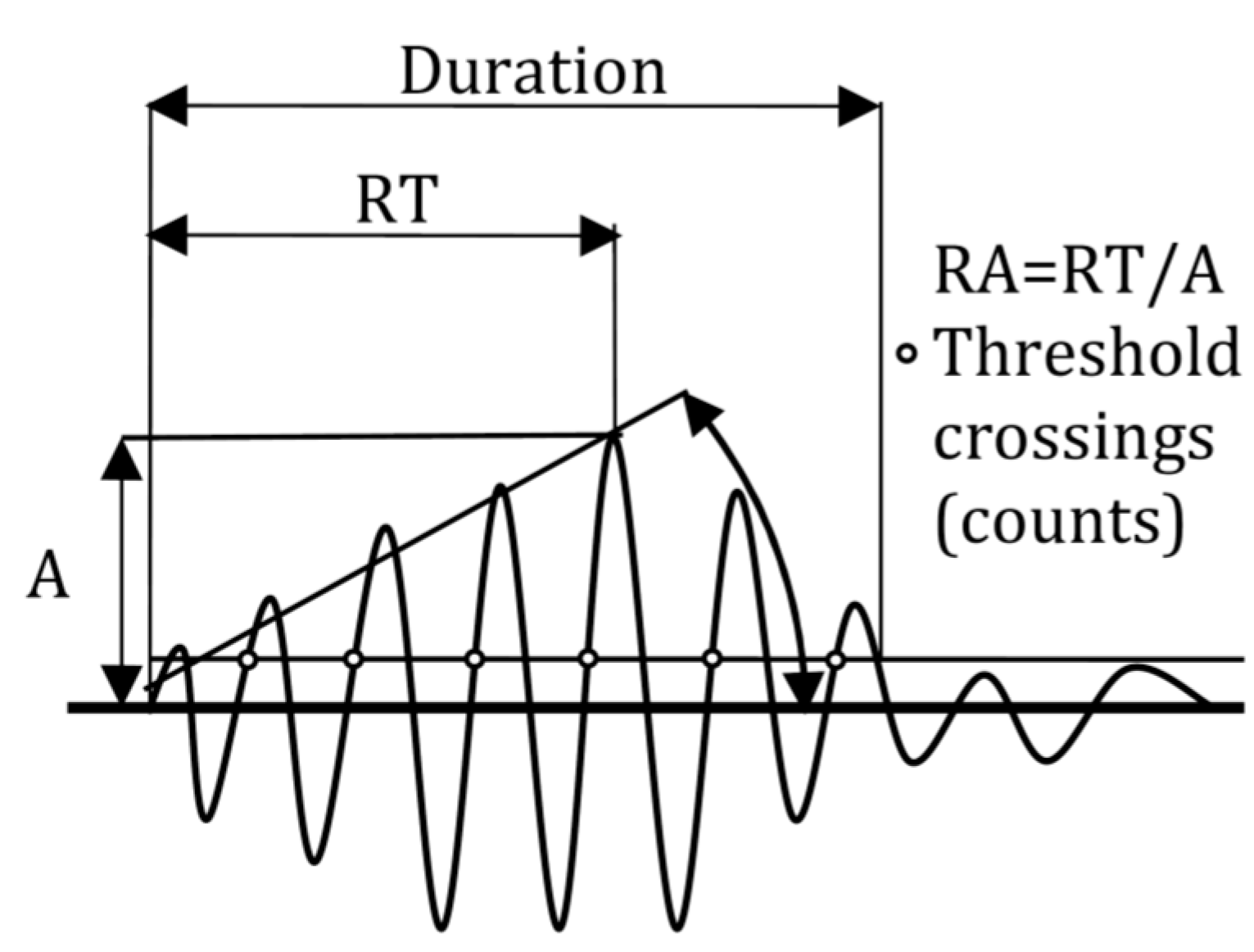
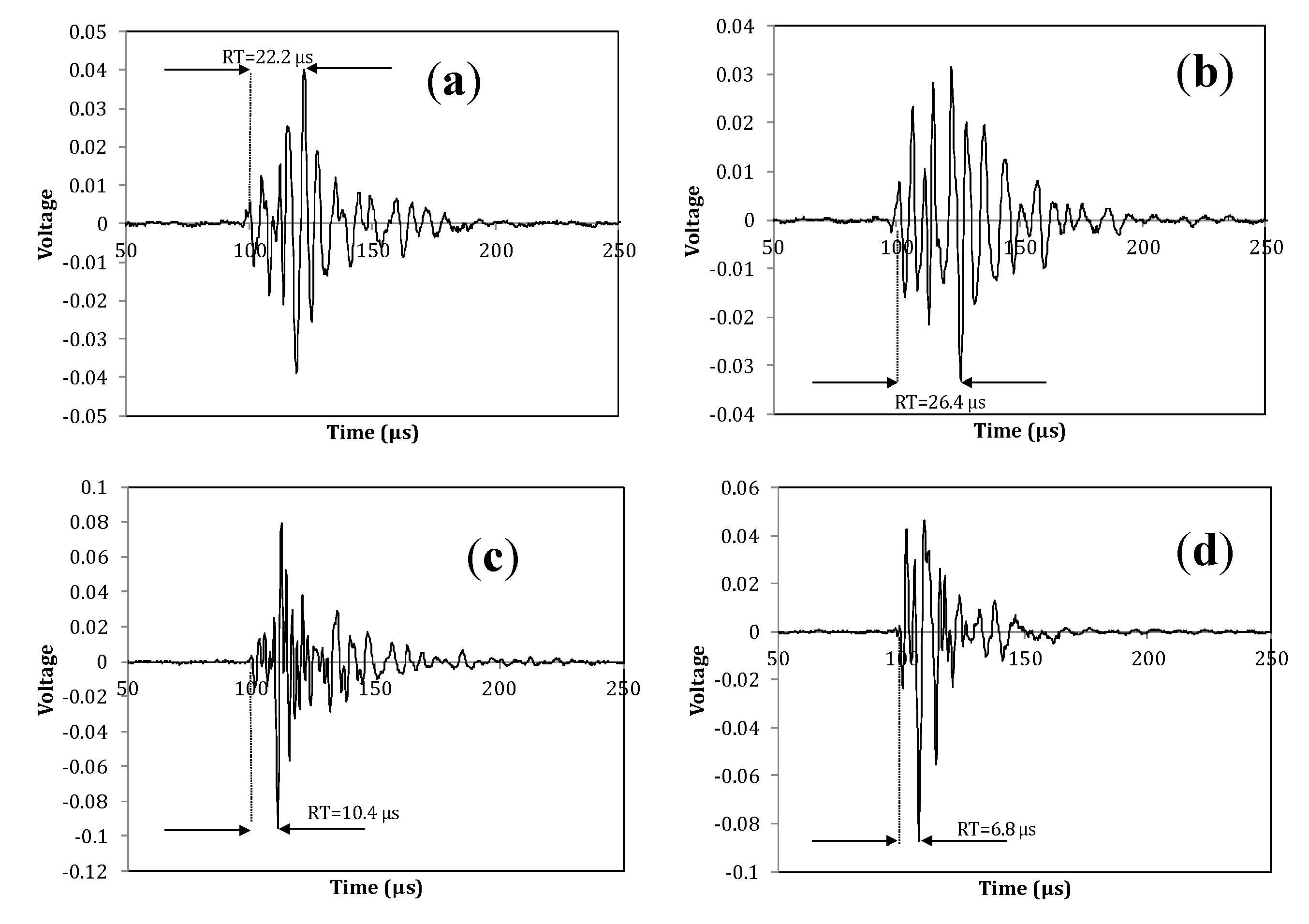
3.3. Fracture Mode Influence on AE Parameters

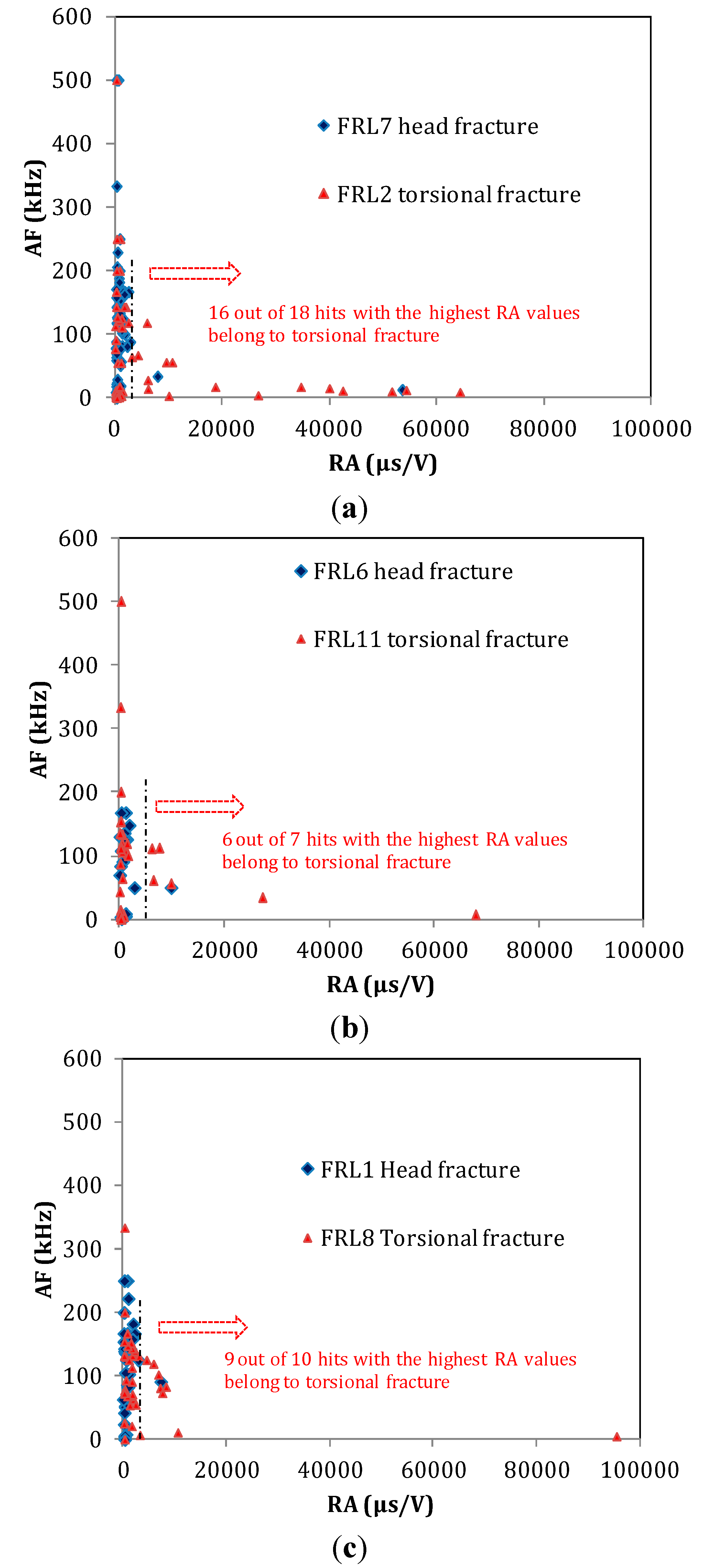
| Fracture Type | Specimen Code | AE Channel * | RISE TIME (μs) | A-FRQ (kHz) | RA (μs/V) |
|---|---|---|---|---|---|
| Torsion fracture through the body | FRL2 | 1 | 82.5 | 106.9 | 14544.9 |
| 2 | 67.7 | 132.3 | 6339.3 | ||
| FRL11 | 1 | 34.5 | 176.6 | 6339.4 | |
| 2 | 39.1 | 91.3 | 4014.4 | ||
| FRL8 | 1 | 35.1 | 129.7 | 1744.8 | |
| 2 | 31.5 | 105.0 | 5342.2 | ||
| Bending fracture of the head | FRL7 | 1 | 3.6 | 62.9 | 720.1 |
| 2 | 10.5 | 168.3 | 1503.9 | ||
| FRL6 | 1 | 18.9 | 150.8 | 1659.0 | |
| 2 | 12.6 | 117.5 | 1140.2 | ||
| FRL1 | 1 | 28.4 | 132.7 | 2396.6 | |
| 2 | 7.3 | 226.8 | 746.3 |

4. Conclusions
Author Contributions
Conflicts of Interest
References
- Grosse, C.U.; Ohtsu, M. Acoustic Emission Testing; Springer: Heidelberg, Germany, 2008. [Google Scholar]
- Han, Z.; Luo, H.; Cao, J.; Wang, H. Acoustic emission during fatigue crack propagation in a micro-alloyed steel and welds. Mater. Sci. Eng. A 2011, 528, 7751–7756. [Google Scholar] [CrossRef]
- Carpinteri, A.; Corrado, M.; Lacidogna, G. Heterogeneous materials in compression: Correlations between absorbed, released and acoustic emission energies. Eng. Fail. Anal. 2013, 33, 236–250. [Google Scholar] [CrossRef]
- Maillet, E.; Godin, N.; R’Mili, M.; Reynaud, P.; Fantozzi, G.; Lamon, J. Damage monitoring and identification in SiC/SiC minicomposites using combined acousto-ultrasonics and acoustic emission. Composites 2014, 57, 8–15. [Google Scholar] [CrossRef]
- Philippidis, T.P.; Assimakopoulou, T.T. Strength degradation due to fatigue-induced matrix cracking in FRP composites: An acoustic emission predictive model. Compos. Sci. Technol. 2008, 68, 3272–3277. [Google Scholar] [CrossRef]
- Dongsheng, L.; Jinping, O.; Chengming, L.; Hui, L. Monitoring and Failure Analysis of Corroded Bridge Cables under Fatigue Loading Using Acoustic Emission Sensors. Sensors 2012, 12, 3901–3915. [Google Scholar] [CrossRef] [PubMed]
- Kurz, J.H.; Finck, F.; Grosse, C.U.; Reinhardt, H.W. Stress drop and stress redistribution in concrete quantified over time by the b-value analysis. Struct. Health Monit. 2006, 5, 69–81. [Google Scholar] [CrossRef]
- Shiotani, T.; Ohtsu, M.; Ikeda, K. Detection and evaluation of AE waves due to rock deformation. Constr. Build. Mater. 2001, 15, 235–246. [Google Scholar] [CrossRef]
- Browne, M.; Roques, A.; Taylor, A. The acoustic emission technique in orthopaedics-a review. J. Strain Anal. Eng. Des. 2005, 40, 59–79. [Google Scholar] [CrossRef]
- Shrivastava, S.; Prakash, R. Assessment of bone condition by acoustic emission technique: A review. J. Biomed. Sci. Eng. 2009, 2, 144–154. [Google Scholar] [CrossRef]
- Ossi, Z.; Abdou, W.; Reuben, R.L.; Ibbetson, R.J. Transmission of acoustic emission in bones, implants and dental materials. Proc. Inst. Mech. Eng. H 2013, 227, 1237–1245. [Google Scholar] [CrossRef] [PubMed]
- Agcaoglu, S.; Akkus, O. Acoustic emission based monitoring of the microdamage evolution during fatigue of human cortical bone. J. Biomech. Eng. 2013, 135, 81005. [Google Scholar] [CrossRef] [PubMed]
- Van Toen, C.; Street, J.; Oxland, T.R.; Cripton, P.A. Acoustic emission signals can discriminate between compressive bone fractures and tensile ligament injuries in the spine during dynamic loading. J. Biomech. 2012, 45, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Aggelis, D.G.; Paschos, N.K.; Barkoula, N.M.; Paipetis, A.S.; Matikas, T.E.; Georgoulis, A.D. Rupture of anterior cruciate ligament monitored by acoustic emission. J. Acoust. Soc. Am. 2011, 129, EL217–EL222. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.G.; Rawlins, R.D. Acoustic emission and mechanical properties of trabecular bone. Biomaterials 1985, 6, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Shark, L.-K.; Chen, H.; Goodacre, J. Acoustic emission: A potential biomarker for quantitative assessment of joint ageing and degeneration. Med. Eng. Phys. 2011, 33, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Schwalbe, H.-J.; Bamfaste, G.; Franke, R.-P. Non-destructive and non-invasive observation of friction and wear of human joints and of fracture initiation by acoustic emission. Proc. Inst. Mech. Eng. 1999, 213, 41–48. [Google Scholar] [CrossRef]
- Rodgers, G.W.; Young, J.; Fields, A.V.; Shearer, R.Z.; Woodfield, T.B.F.; Hooper, G.J.; Chase, J.G. Acoustic Emission Monitoring of Total Hip Arthroplasty Implants. In Proceedings of the 19th World Congress of the International Federation of Control and Automation (IFAC), Cape Town, South Africa, 24–29 August 2014.
- Qi, G.; Li, J. Experimental–computational solution to fatigue induced microcrack source localization in cemented hip arthroplasty models. NDT&E Int. 2007, 40, 378–389. [Google Scholar] [CrossRef]
- Laugier. Available online: http://www.ultrasonics.org/Proceedings_2007_UIA/Laugier_2007_UIA.pdf (accessed on 5 January 2015).
- Devendra, K.D.; Vikas, T. Microstructure dependent dynamic fracture analyses of trabecular bone based on nascent bone atomistic simulations. Mech. Res. Commun. 2008, 35 P, 24–31. [Google Scholar] [CrossRef]
- Rahmoun, J.; Auperrin, A.; Delille, R.; Naceur, H.; Drazetic, P. Characterization and micromechanical modeling of the human cranial bone elastic properties. Mech. Res. Commun. 2014, 60, 7–14. [Google Scholar] [CrossRef]
- Florio, C.S.; Narh, K.A. Effect of modeling method on prediction of cortical bone strength adaptation under various loading conditions. Meccanica 2013, 48, 393–413. [Google Scholar] [CrossRef]
- Vavva, M.G.; Protopappas, V.C.; Gergidis, L.N.; Charalambopoulos, A.; Fotiadis, D.I.; Polyzos, D. Velocity dispersion of guided waves propagating in a free gradient elastic plate: Application to cortical bone. J. Acoust. Soc. Am. 2009, 125, 3414–3427. [Google Scholar] [CrossRef] [PubMed]
- Strantza, M.; Louis, O.; Polyzos, D.; Boulpaep, F.; van Hemelrijck, D.; Aggelis, D.G. Measurement of Elastic Wave Propagation on Human Femur Tissue. In Proceedings of Smart Sensor Phenomena, Technology, Networks, and Systems Integration 2014, San Diego, CA, USA, 8 March 2014.
- Määttä, M. Assessment of Osteoporosis and Fracture Risk, Axial Transmission Ultrasound and Lifestyle-Related Risk Factors. Ph.D. Thesis, University of Oulu, Oulu, Finland, January 2013. [Google Scholar]
- Sarkalkan, N.; Loeve, A.J.; van Dongen, K.W.A.; Tuijthof, G.J.M.; Zadpoor, A.A. A novel ultrasound technique for detection of osteochondral defects in the ankle joint: A parametric and feasibility study. Sensors 2015, 15, 148–165. [Google Scholar] [CrossRef]
- Bhalla, S.; Suresh, R. Condition monitoring of bones using piezo-transducers. Meccanica 2013, 48, 2233–2244. [Google Scholar] [CrossRef]
- Li, S.; Abdel-Wahab, A.; Silberschmidt, V.V. Analysis of fracture processes in cortical bone tissue. Eng. Fract. Mech. 2013, 110, 448–458. [Google Scholar] [CrossRef]
- Strantza, M.; Louis, O.; Polyzos, D.; Boulpaep, F.; van Hemelrijck, D.; Aggelis, D.G. Wave dispersion and attenuation on human femur tissue. Sensors 2014, 14, 15067–15083. [Google Scholar] [CrossRef] [PubMed]
- Aggelis, D.G. Classification of cracking mode in concrete by acoustic emission parameters. Mech. Res. Commun. 2011, 38, 153–157. [Google Scholar] [CrossRef]
- Ohno, K.; Ohtsu, M. Crack classification in concrete based on acoustic emission. Constr. Build. Mater. 2010, 24, 2339–2346. [Google Scholar] [CrossRef]
- Watanabe, T.; Nishibata, S.; Hashimoto, C.; Ohtsu, M. Compressive failure in concrete of recycled aggregate by acoustic emission. Constr. Build. Mater. 2007, 21, 470–476. [Google Scholar] [CrossRef]
- Feik, S.A.; Thomas, C.D.L.; Clement, J.G. Age-related changes in cortical porosity of the midshaft of the human femur. J. Anat. 2002, 191, 407–416. [Google Scholar] [CrossRef]
- Stein, M.S.; Thomas, C.D.L.; Feik, S.A.; Wark, J.D.; Clement, J.G. Bone size and mechanics at the femoral diaphysis across age and sex. J. Biomech. 1988, 31, 1101–1110. [Google Scholar] [CrossRef]
- Polyzos, D.; Papacharalampopoulos, A.; Shiotani, T.; Aggelis, D.G. Dependence of AE parameters on the propagation distance. J. Acoust. Emiss. 2011, 29, 57–67. [Google Scholar]
- Eaton, M.; May, M.; Featherston, C.; Holford, K.; Hallet, S.; Pullin, R. Characterisation of Damage in Composite Structures Using Acoustic Emission. J. Phys. 2011, 305, 012086. [Google Scholar]
- Aggelis, D.G.; Verbruggen, S.; Tsangouri, E.; Tysmans, T.; van Hemelrijck, D. Characterization of mechanical performance of concrete beams with external reinforcement by acoustic emission and digital image correlation. Constr. Build. Mater. 2013, 47, 1037–1045. [Google Scholar] [CrossRef]
- Farhidzadeh, A.; Salamone, S.; Singla, P. A probabilistic approach for damage identification and crack mode classification in reinforced concrete structures. J. Intell. Mater. Syst. Struct. 2013, 24, 1722–1735. [Google Scholar] [CrossRef]
- Blom, J.; el Kadi, M.; Wastiels, J.; Aggelis, D.G. Bending fracture of textile reinforced cement laminates monitored by acoustic emission: Influence of aspect ratio. Constr. Build. Mater. 2014, 70, 370–378. [Google Scholar] [CrossRef]
- Ohtsu, M.; Mori, K.; Kawasaki, Y. Corrosion process and mechanisms of corrosion induced cracks in reinforced concrete identified by AE analysis. Strain 2011, 47, 179–186. [Google Scholar] [CrossRef]
- Shiotani, T.; Oshima, Y.; Goto, M.; Momoki, S. Temporal and spatial evaluation of grout failure process with PC cable breakage by means of acoustic emission. Constr. Build. Mater. 2013, 48, 1286–1292. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Z.; Liao, W.I. Damage monitoring of reinforced concrete frames under seismic loading using cement-based piezoelectric sensor. Mater. Struct. 2011, 44, 1273–1285. [Google Scholar] [CrossRef]
- Aggelis, D.G.; Kordatos, E.Z.; Matikas, T.E. Acoustic emission for fatigue damage characterization in metal plates. Mech. Res. Commun. 2011, 38, 106–110. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aggelis, D.G.; Strantza, M.; Louis, O.; Boulpaep, F.; Polyzos, D.; Van Hemelrijck, D. Fracture of Human Femur Tissue Monitored by Acoustic Emission Sensors. Sensors 2015, 15, 5803-5819. https://doi.org/10.3390/s150305803
Aggelis DG, Strantza M, Louis O, Boulpaep F, Polyzos D, Van Hemelrijck D. Fracture of Human Femur Tissue Monitored by Acoustic Emission Sensors. Sensors. 2015; 15(3):5803-5819. https://doi.org/10.3390/s150305803
Chicago/Turabian StyleAggelis, Dimitrios. G., Maria Strantza, Olivia Louis, Frans Boulpaep, Demosthenes Polyzos, and Danny Van Hemelrijck. 2015. "Fracture of Human Femur Tissue Monitored by Acoustic Emission Sensors" Sensors 15, no. 3: 5803-5819. https://doi.org/10.3390/s150305803






