A Multi-Channel Opto-Electronic Sensor to Accurately Monitor Heart Rate against Motion Artefact during Exercise
Abstract
:1. Introduction
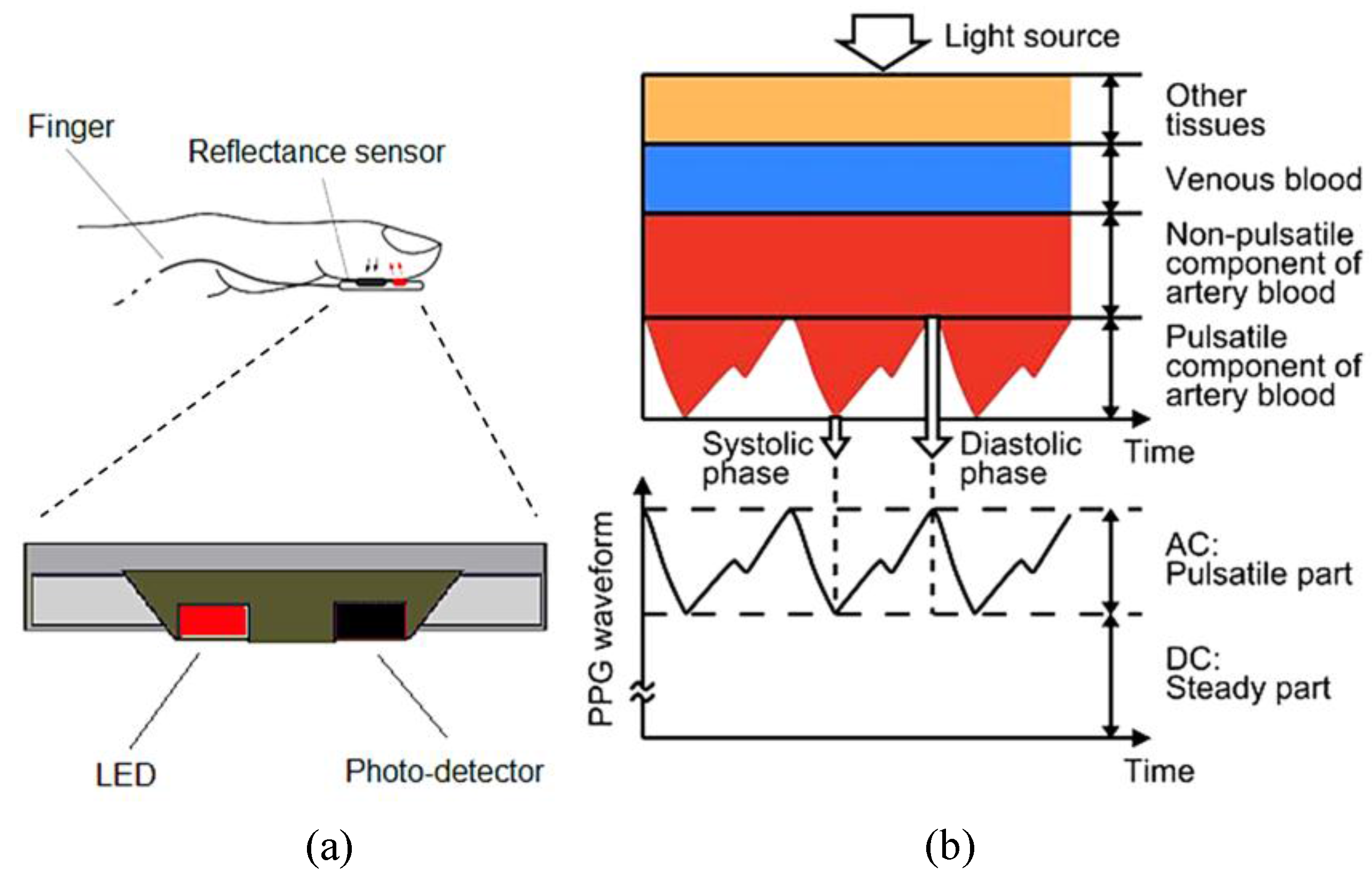
- The tissue optics properties to determine an optimal optical sensing position;
- An optimal optical layout for the OEPS operable to monitor the tissue optic properties of the tissue type; and
- Optical design involving in the options of a wavelength, intensity and an optical path length for the LED illumination sources.
2. Method
2.1. Experimental Setup of Opto-Electronic Patch Sensor (OEPS)
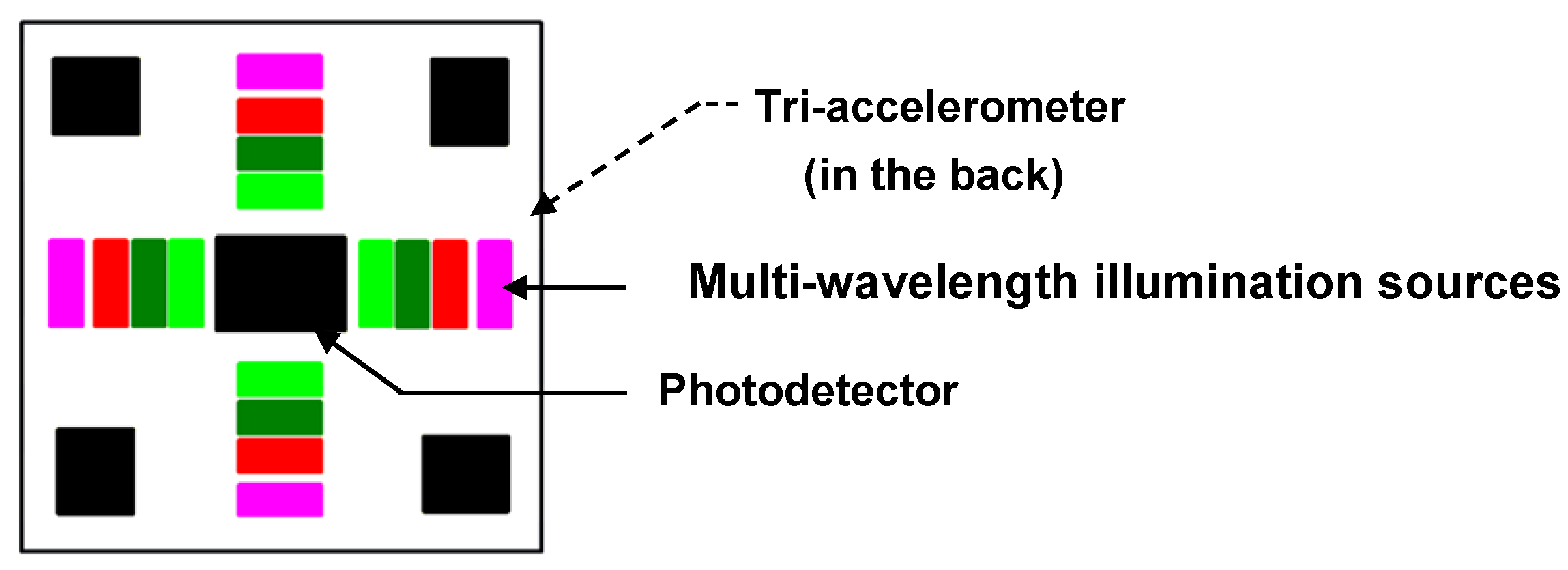
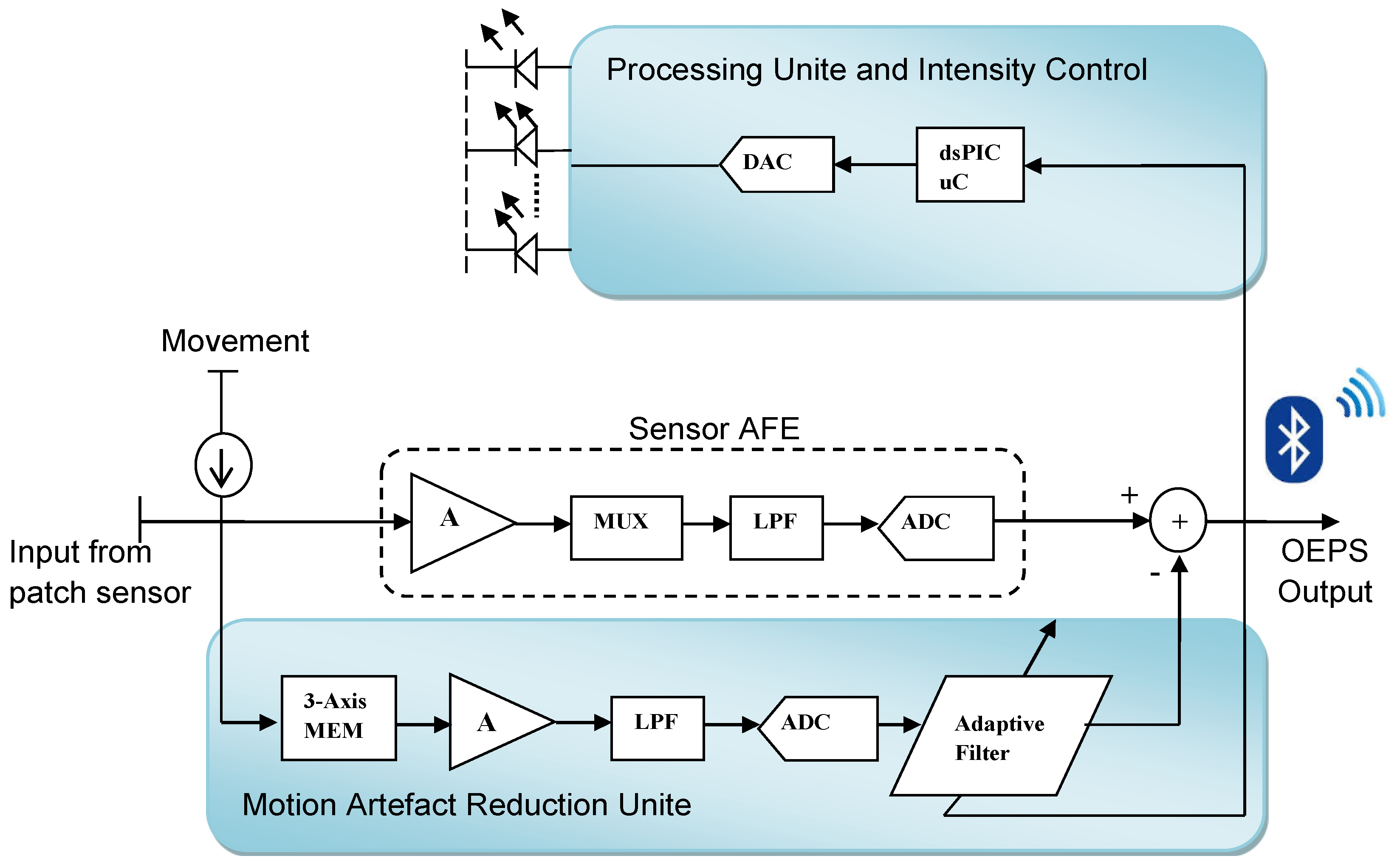
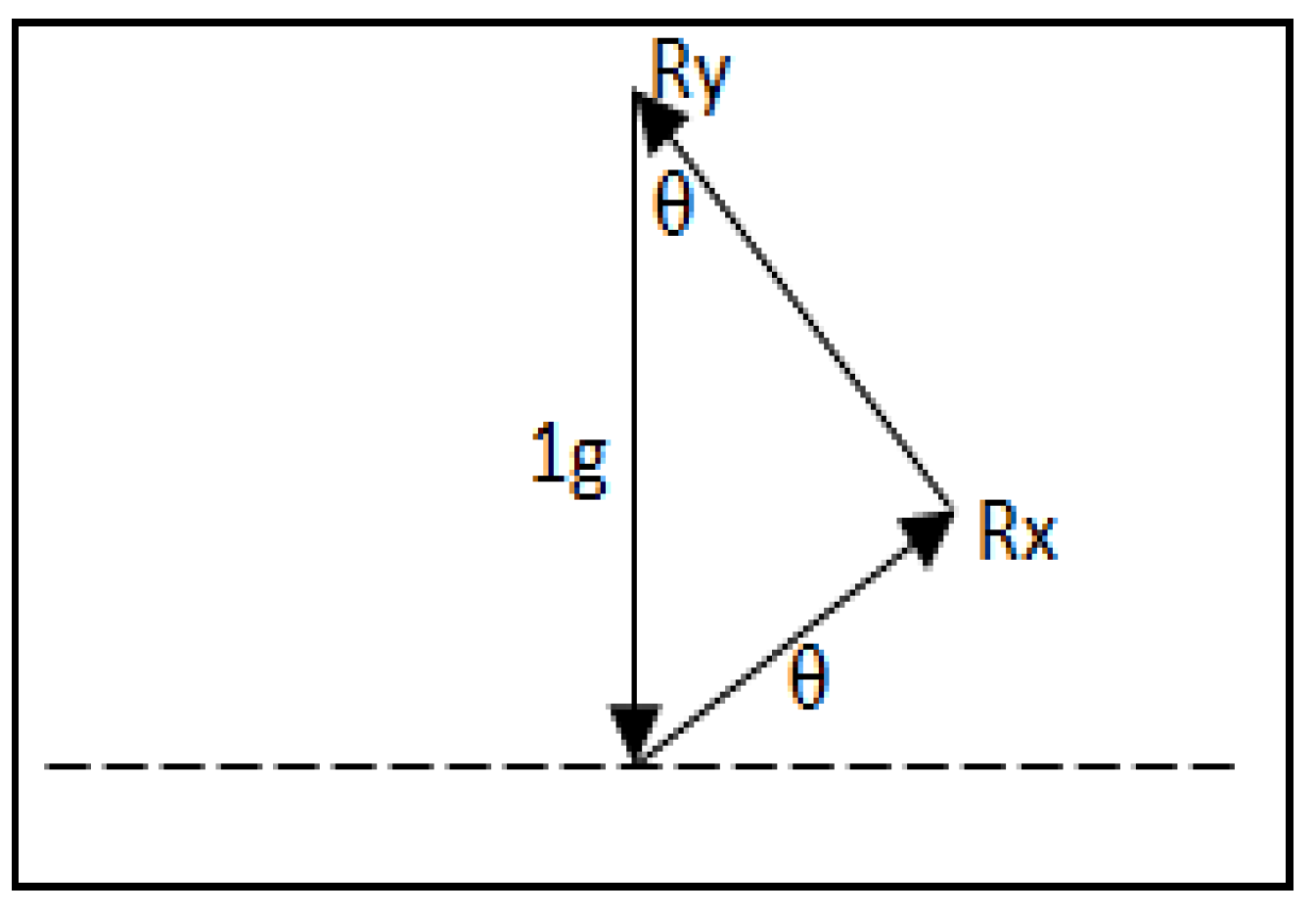


2.2. Multiple Wavelength Illumination Source

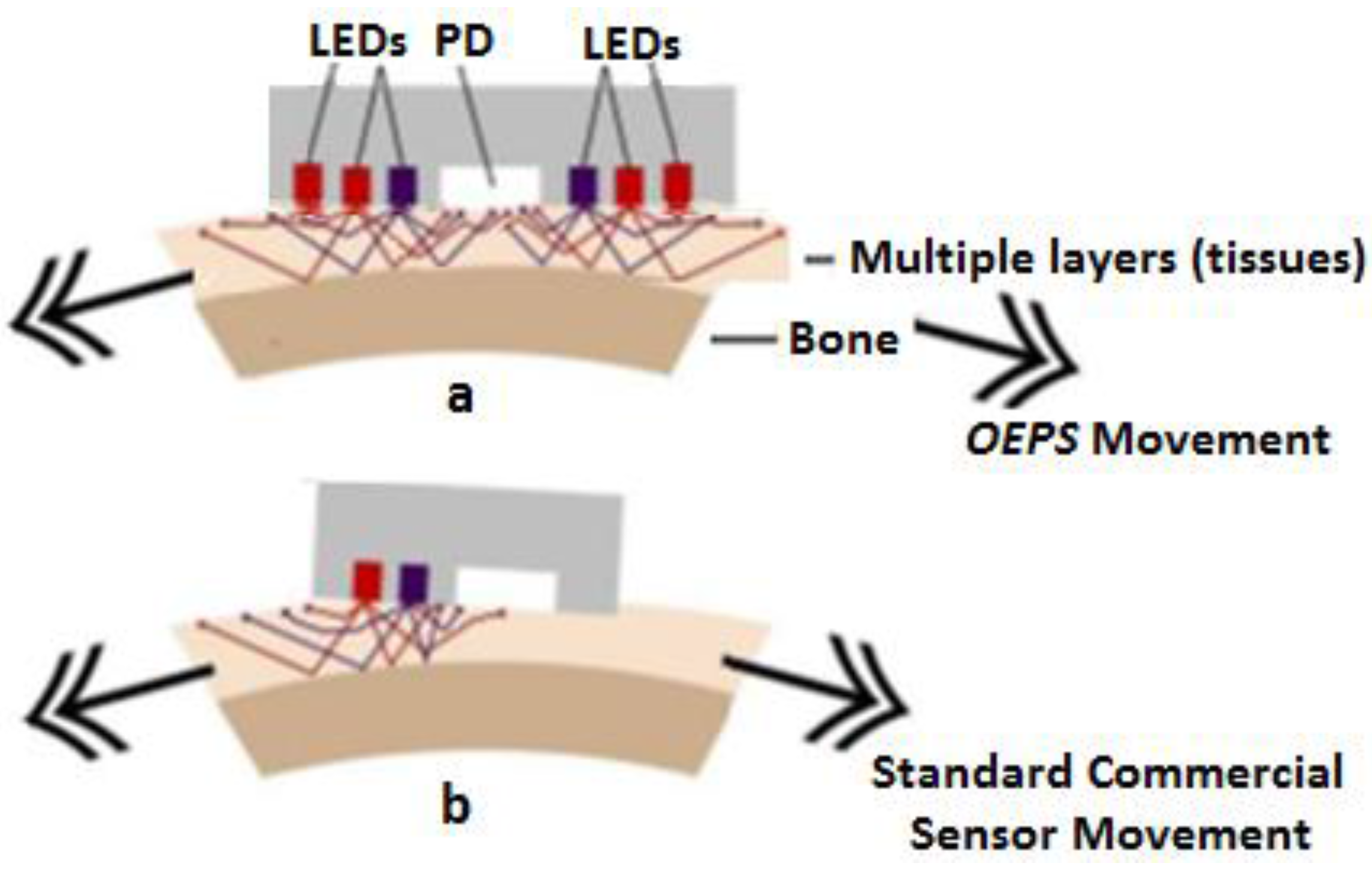
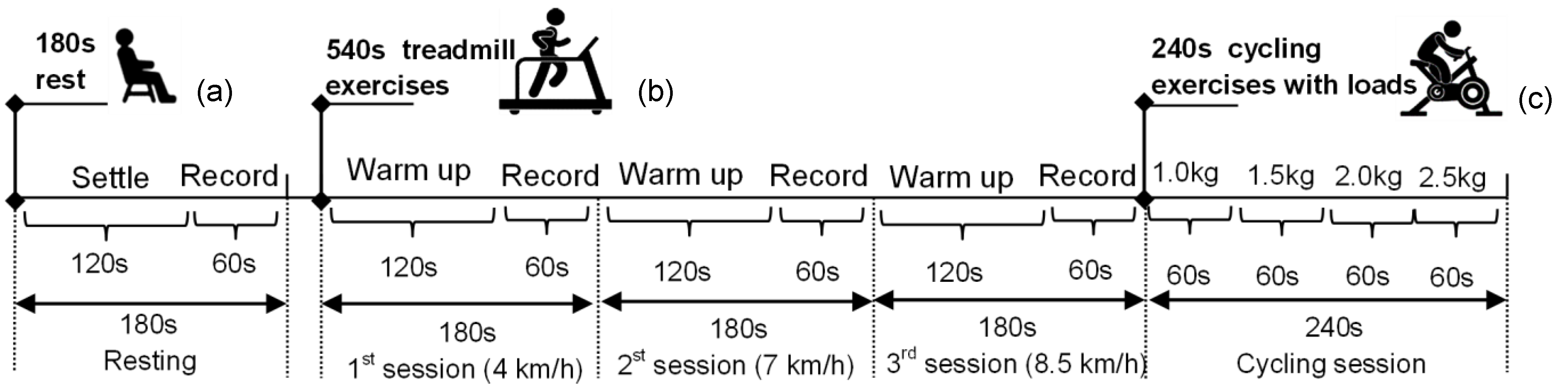
2.3. Physiological Monitoring Protocol
2.4. OEPS Measurement System

3. Results
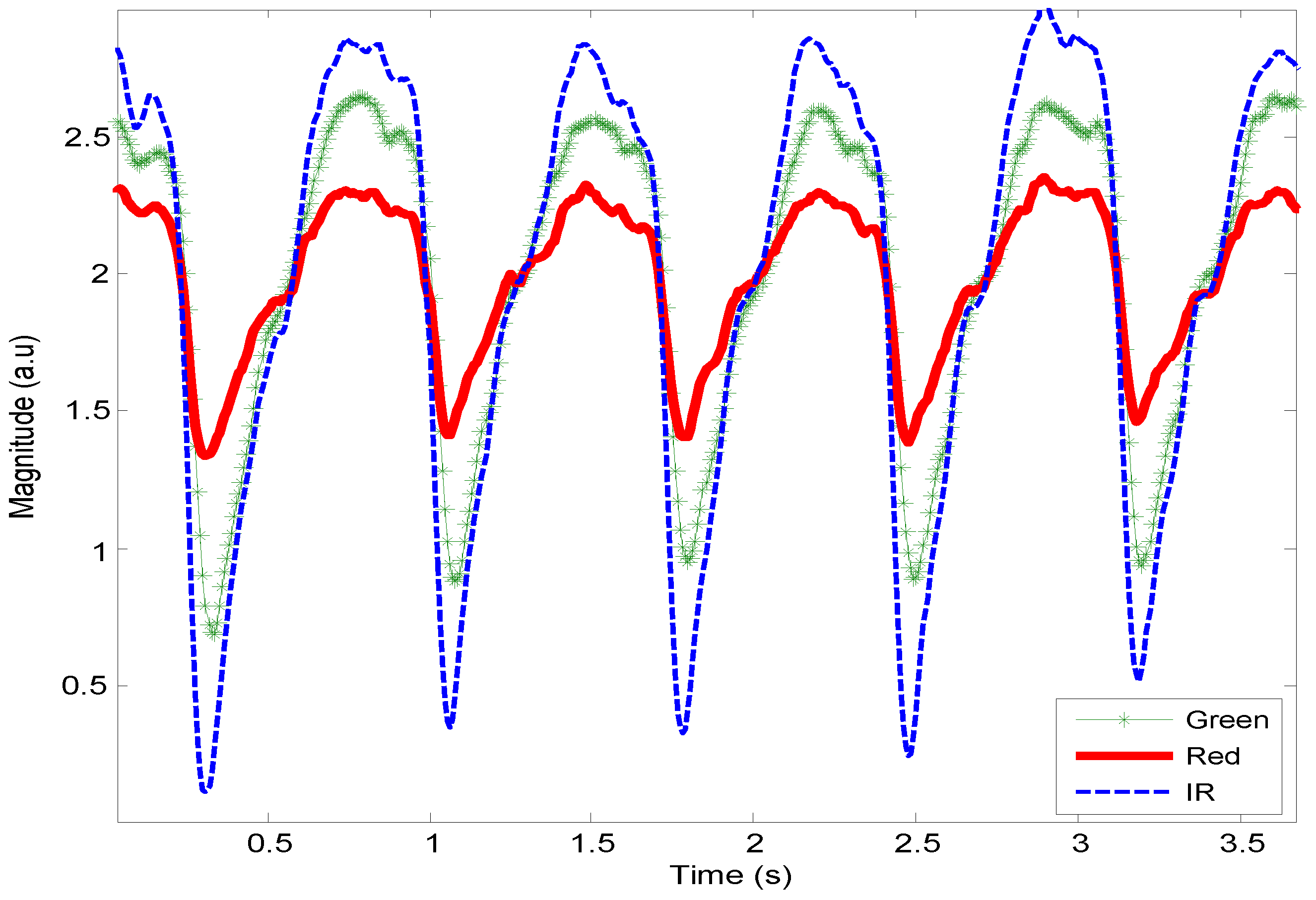
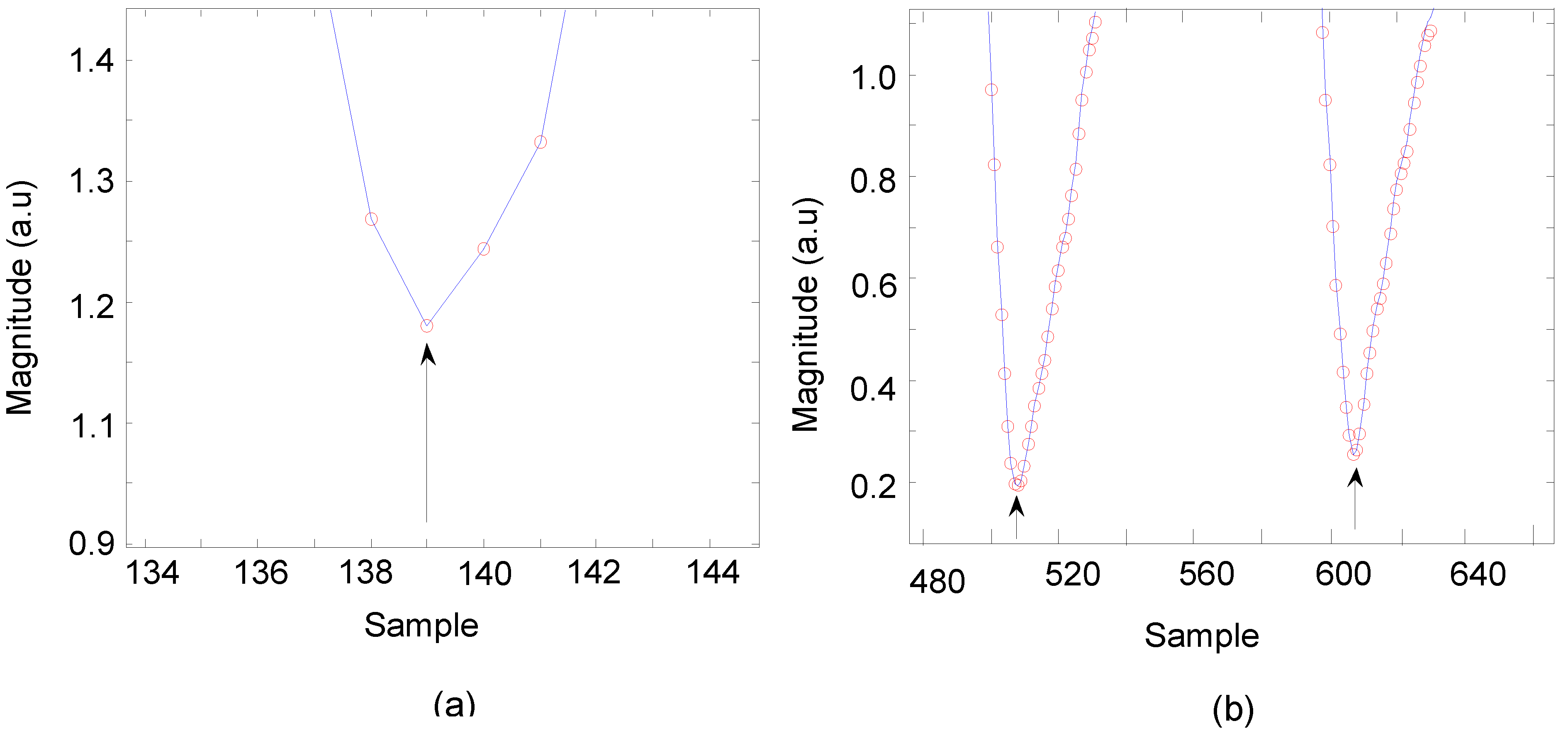
3.1. Data Analysis of HR Detection

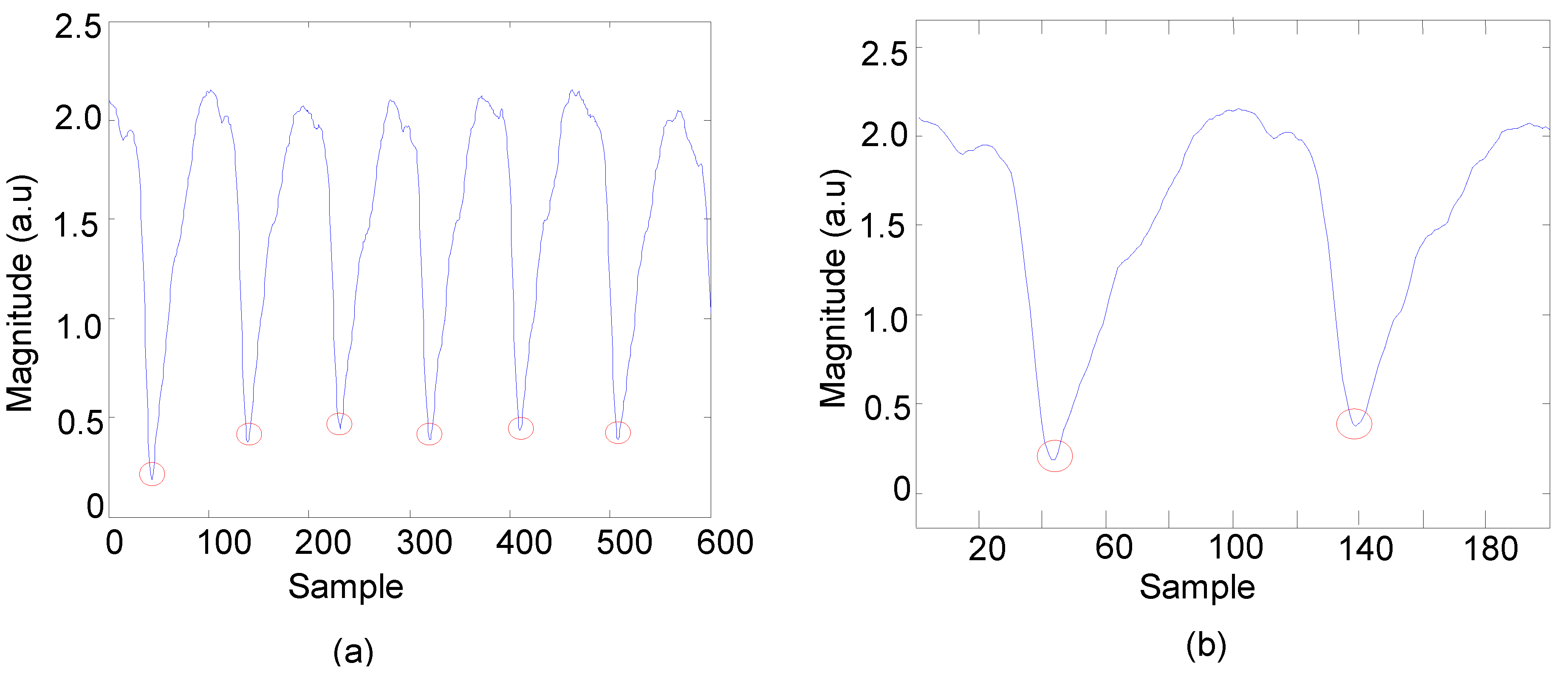
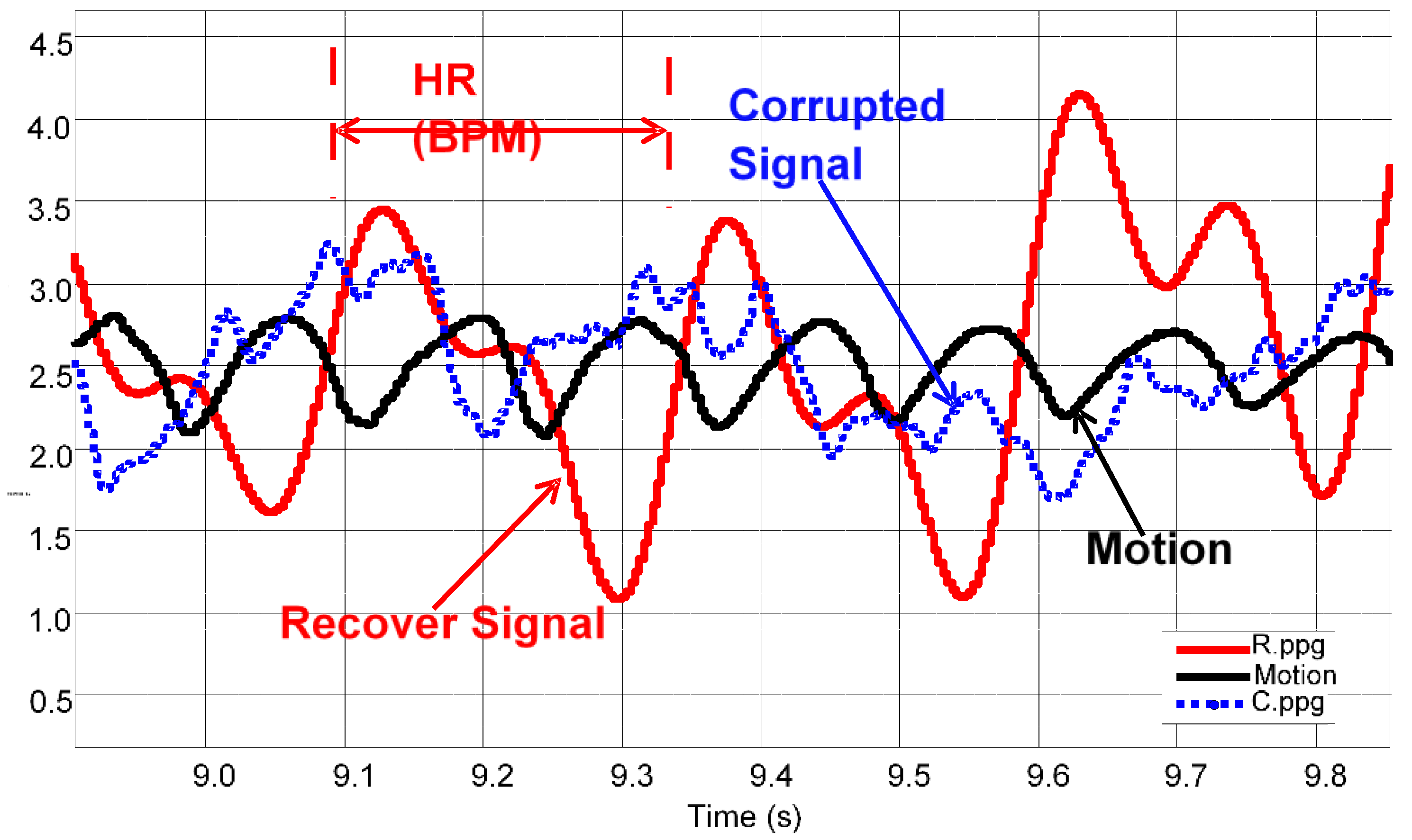

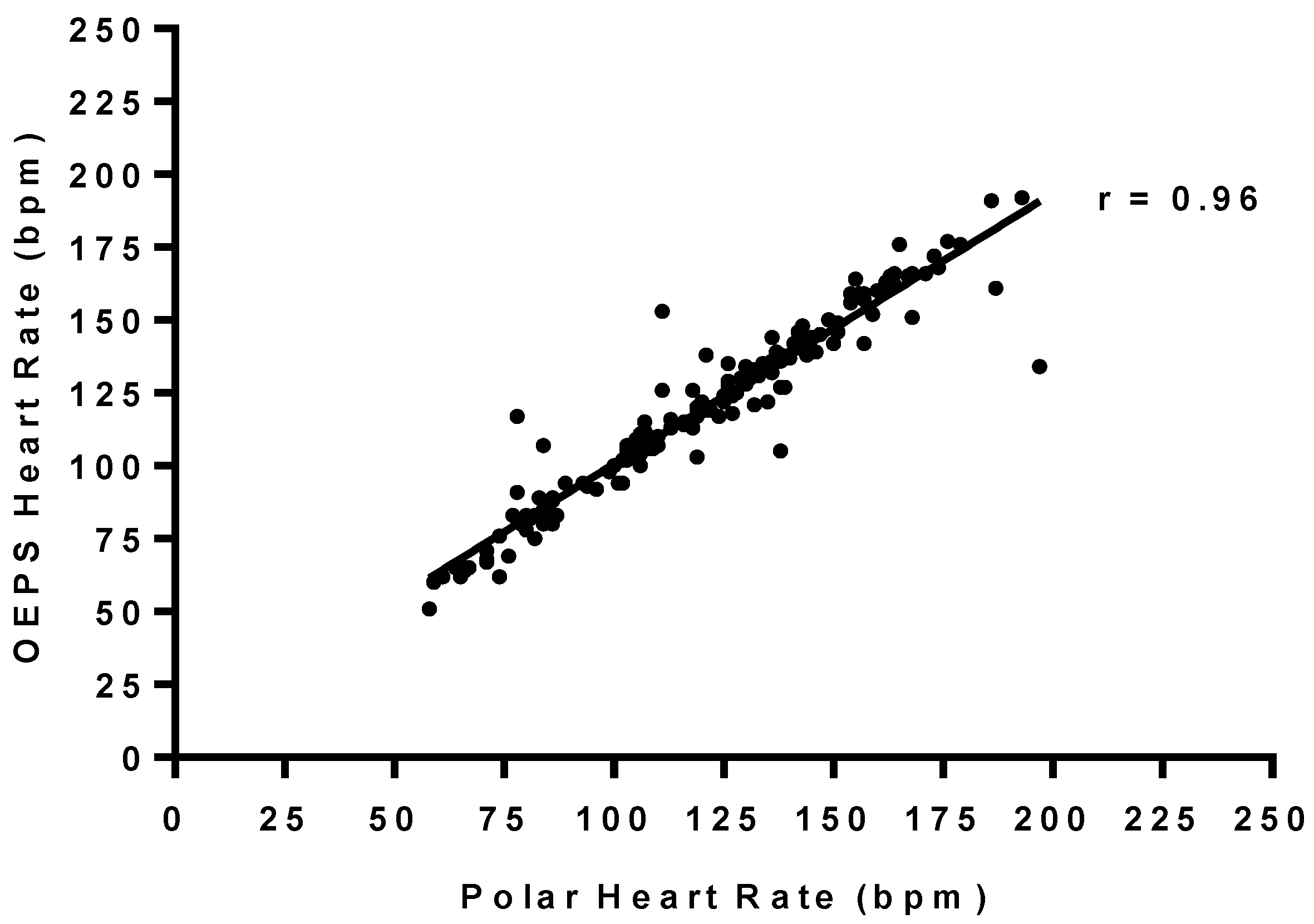
3.2. Statistical Analysis of HR between OEPS and Commercial Devices
| Polar | OEPS | Bias | LOA − | LOA + | r | Intercept | Gradient | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |||||||
| Rest | 72 | 3 | 71 | 3 | −1.13 | −6 | 3 | 0.99 | 6.50 | 0.92 |
| Treadmill (movement) | 116 | 4 | 118 | 4 | 2.48 | −14 | 19 | 0.96 | 2.98 | 0.95 |
| 4km/h a | 85 | 3 | 89 | 4 | 3.66 | −18 | 26 | 0.70 | 36.42 | 0.55 |
| 7 km/h | 117 | 3 | 118 | 3 | 1.56 | −10 | 13 | 0.89 | −4.55 | 1.03 |
| 8.5 km/h a | 144 | 4 | 148 | 4 | 2.33 | −13 | 17 | 0.89 | 23.84 | 0.82 |
| Treadmill (still) | 119 | 5 | 118 | 5 | −1.08 | −23 | 20 | 0.93 | 6.74 | 0.95 |
| 4 km/h b | 88 | 3 | 89 | 4 | 1.15 | −14 | 17 | 0.84 | 33.51 | 0.61 |
| 7 km/h c | 121 | 5 | 119 | 4 | −2.83 | −13 | 8 | 0.94 | 5.16 | 0.98 |
| 8.5 km/h c | 147 | 6 | 148 | 6 | −1.75 | −35 | 31 | 0.62 | 57.77 | 0.62 |
| Cycling | 135 | 3 | 133 | 3 | −3.11 | −21 | 15 | 0.93 | 5.64 | 0.98 |
| 1 kg | 116 | 4 | 113 | 4 | −2.81 | −9 | 3 | 0.98 | 3.40 | 0.99 |
| 1.5 kg | 129 | 5 | 126 | 5 | −2.38 | −12 | 7 | 0.97 | 10.33 | 0.94 |
| 2 kg a | 144 | 6 | 141 | 5 | −2.53 | −16 | 11 | 0.96 | −15.86 | 1.13 |
| 2.5 kg a | 158 | 6 | 153 | 5 | −4.80 | −38 | 28 | 0.69 | 44.59 | 0.74 |

| Mio-Alpha | OEPS | Bias | LOA − | LOA + | r | Intercept | Gradient | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |||||||
| Rest | 71 | 3 | 71 | 3 | 0.18 | −4 | 5 | 0.98 | −8.63 | 0.90 |
| Treadmill (movement) | 119 | 4 | 118 | 4 | 2.48 | −14 | 19 | 0.96 | 6.98 | 0.92 |
| 4 km/h | 91 | 4 | 89 | 4 | 0.68 | −8 | 9 | 0.96 | −24.06 | 0.91 |
| 7 km/h | 116 | 3 | 118 | 3 | −2.37 | −19 | 15 | 0.74 | 8.42 | 0.30 |
| 8.5 km/h a | 150 | 4 | 148 | 4 | 2.33 | −13 | 17 | 0.89 | −23.93 | 0.59 |
| Treadmill (still) | 120 | 5 | 118 | 5 | 1.39 | −23 | 20 | 0.93 | 9.74 | 0.94 |
| 4 km/h b | 89 | 3 | 89 | 4 | 0.42 | −13 | 14 | 0.89 | −1.77 | 0.51 |
| 7 km/h d | 122 | 5 | 119 | 4 | 3.25 | −10 | 17 | 0.92 | −9.12 | 0.55 |
| 8.5 km/h d | 150 | 6 | 148 | 6 | 4.91 | −21 | 31 | 0.76 | −33.84 | 0.29 |
| Cycling | 135 | 3 | 133 | 3 | 2.47 | −21 | 15 | 0.93 | 4.64 | 0.98 |
| 1 kg c | 113 | 3 | 113 | 4 | 1.00 | −9 | 11 | 0.95 | −7.20 | 0.68 |
| 1.5 kg a | 128 | 5 | 126 | 5 | 2.86 | −7 | 13 | 0.96 | −14.00 | 0.77 |
| 2 kg a | 142 | 5 | 141 | 5 | −1.21 | −5 | 8 | 0.98 | −2.02 | 0.80 |
| 2.5 kg a | 157 | 6 | 153 | 5 | −4.66 | −29 | 39 | 0.63 | −22.56 | 0.17 |


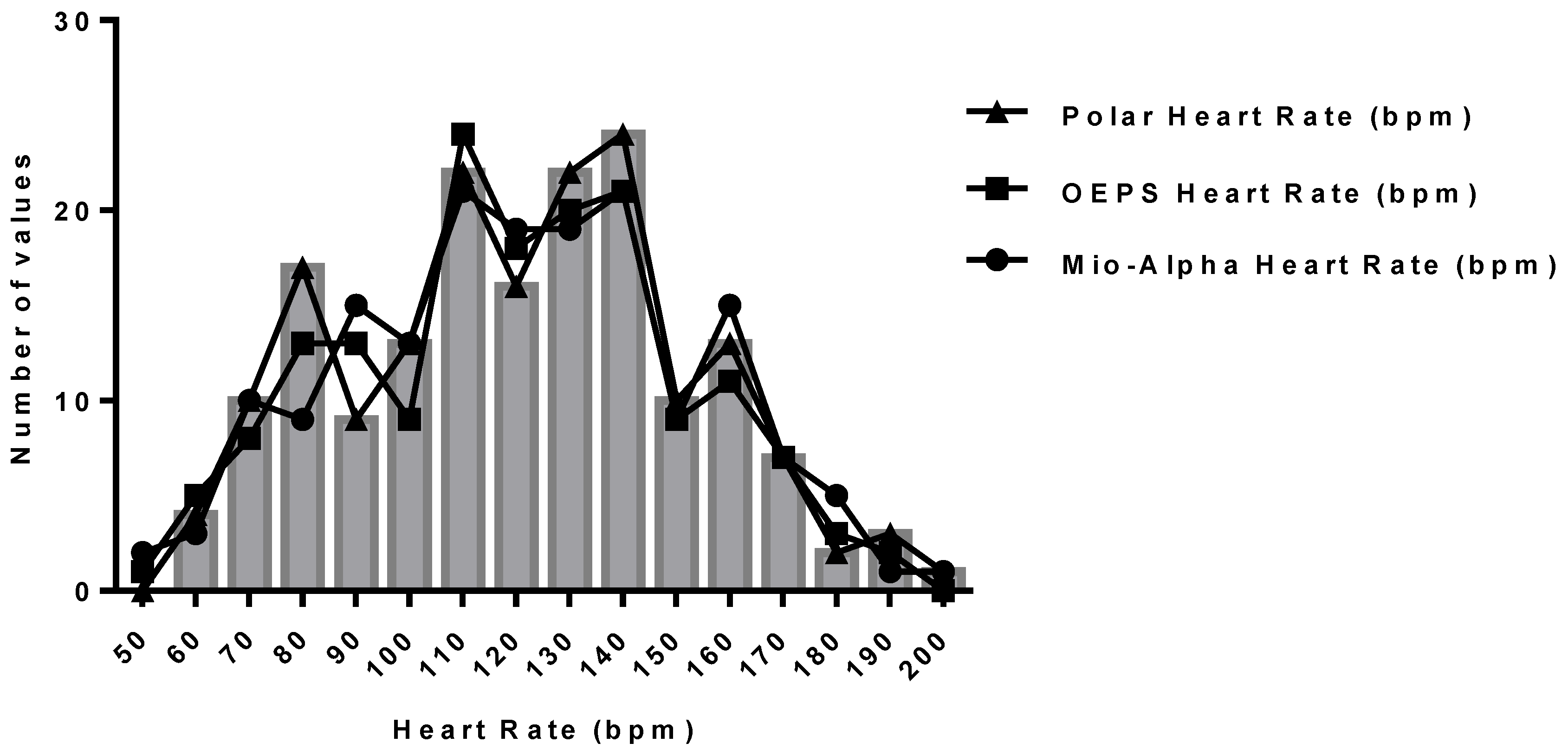
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bonato, P. Clinical applications of wearable technology. IEEE Proc. Eng. Med. Biol. 2009, 3, 6580–6583. [Google Scholar]
- Teichmann, D.; Matteis, D.; Bartelt, T.; Walter, M.; Leonhardt, S. A Bendable and Wearable Cardiorespiratory Monitoring Device Fusing Two Noncontact Sensor Principles. IEEE J. Biomed. Health Inform. Heal. Inf. 2015, 19, 784–793. [Google Scholar] [CrossRef] [PubMed]
- Perk, J.; Backer, G.D.; Gohlke, H. European Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2012, 33, 1635–1701. [Google Scholar] [PubMed]
- Teichmann, D.; Kuhn, A.; Leonhardt, S.; Walter, M. The MAIN Shirt: A textile-integrated magnetic induction sensor array. Sensors 2014, 14, 1039–1056. [Google Scholar] [CrossRef] [PubMed]
- Venema, B.; Blazek, V.; Blanik, N.; Schiefer, J.; Leonhardt, S. Evaluating innovative in-ear pulse oximetry for unobtrusive cardiovascular and pulmonary monitoring during sleep. IEEE J. Trans. Eng. Heal. Med. 2013, 1, 2168–2372. [Google Scholar] [CrossRef]
- Achten, J.; Jeukendrup, E. Heart rate monitoring, applications and limitations. Sports Med. 2003, 33, 517–538. [Google Scholar] [CrossRef]
- Keytel, R.; Goedecke, H.; Noakes, D.; Hiiloskorpi, H.; Laukkanen, R.; Merwe, L.; Lambert, V. Prediction of energy expenditure from heart rate monitoring during submaximal exercise. J. Sport Sci. 2005, 23, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Venema, B.; Gehring, H.; Michelsen, I.; Blanik, N.; Blazek, V.; Leonhardt, S. Robustness, Specificity, and Reliability of an In-Ear Pulse Oximetric Sensor in Surgical Patients. IEEE J. Biomed. Health Inform. Heal. Inf. 2014, 18, 1178–1185. [Google Scholar] [CrossRef]
- Hu, S.; Azorin-Peris, V.; Zheng, J. Opto-physiological modeling applied to photoplethysmographic cardiovascular assessment. J. Healthc. Eng. 2009, 4, 505–528. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Sun, Y.; Azorin-Peris, V.; Greenwald, S.; Chambers, J.; Zhu, Y. Motion-compensated noncontact imaging photoplethysmography to monitor cardiorespiratory status during exercise. J. Biomed. Opt. 2011, 16, 1–9. [Google Scholar]
- Anliker, U.; Ward, A.; Lukowicz, P.; Tröster, G.; Dolveck, F.; Baer, M.; Keita, F.; Schenker, B.; Catarsi, F.; Coluccini, L.; et al. AMON: A wearable multiparameter medical monitoring and alert system. IEEE Trans. Inf. Technol. Biomed. 2004, 8, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Nonin Medical Inc. NoninWristOx 3100. Medical Product Service GmbH Nonin; Nonin Medical Inc.: Plymouth, MN, USA, 2006. [Google Scholar]
- Fruin, L.; Rankin, W. Validity of a multi-sensor armband in estimating rest and exercise energy expenditure. Med. Sci. Sports Exerc. 2004, 36, 1063–1069. [Google Scholar] [CrossRef]
- Park, S.; Jayaraman, S. E-Health and quality of life: The role of the Wearable Motherboard. Stud. Health Technol. Inform. 2004, 108, 239–252. [Google Scholar] [PubMed]
- Grossman, P. The Life Shirt: A multi-function ambulatory system monitoring health, disease, and medical intervention in the real world. Stud. Health Technol. Inform. 2004, 108, 133–141. [Google Scholar] [PubMed]
- Pandian, S.; Mohanavelu, K.; Safeer, P.; Kotresh, M.; Shakunthala, T.; Parvati, G.; Padaki, C. Smart Vest: Wearable multi-parameter remote physiological monitoring system. Med. Eng. Phys. 2008, 30, 466–477. [Google Scholar] [CrossRef]
- Lukowicz, P.; Anliker, U.; Ward, J.; Troster, G. AMON: A Wearable Medical Computer for High Risk Patients. In Proceedings of the 6th International Symposium on Wearable Computers (ISWC 02), Seattle, WA, USA, 7–10 October 2002; Volume 8, pp. 7695–1816.
- Lee, W.; Jung, G.; Lee, K. The periodic moving average filter for removing motion artifacts from PPG signals. Int. J. Control Autom. Syst. 2007, 6, 701–706. [Google Scholar]
- Tamura, T.; Maeda, Y.; Sekine, M.; Yoshida, M. Wearable Photoplethysmographic Sensors—Past and Present. J. Electron. 2014, 3, 282–302. [Google Scholar] [CrossRef]
- Yoo, K.; Kim, S. Motion artifact reduction in photoplethysmography using independent component analysis. IEEE Trans. Biomed. Eng. 2006, 53, 566–568. [Google Scholar]
- Natarajan, B.; Krishnan, R.; Warren, S. Two-stage approach for detection and reduction of motion artifacts in photoplethysmographic data. IEEE Trans. Biomed. Eng. 2010, 57, 1867–1876. [Google Scholar]
- Yao, J.; Warren, S. A short study to assess the potential of independent component analysis for motion artifact separation in wearable pulse oximeter signal. In Proceedings of the 27th Annual International Conference of the Engineering in Medicine and Biology Society (EMBS), Shanghai, China, 1–4 September 2005; pp. 3585–3588.
- Stoica, P.; Moses, L. Spectral Analysis of Signals; Prentice Hall: Upper Saddle River, NJ, USA, 2005. [Google Scholar]
- Ram, M.; Madhav, V.; Krishna, H.; Komalla, R.; Reddy, A. A novel approach for motion artifact reduction in PPG signals based on AS-LMS adaptive filter. IEEE Trans. Instr. Meas. 2012, 61, 1445–1457. [Google Scholar] [CrossRef]
- Yousefi, R.; Nourani, M.; Ostadabbas, S.; Panahi, I. A motion-tolerant adaptive algorithm for wearable photoplethysmographic biosensors. J. Biomed. Health Inform. 2014, 18, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, H.; Kawanaka, H.; Bhuiyan, S.; Oguri, K. Estimating heart rate using wrist-type photoplethysmography and acceleration sensor while running. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), San Diego, CA, USA, 28 August–1 September 2012; pp. 2901–2904.
- Lee, B.; Han, J.; Baek, J.; Shin; Park, S.; Yi, J. Improved elimination of motion artifacts from a photoplethysmographic signal using a kalman smoother with simultaneous accelerometry. Physiol. Meas. 2010, 31, 1585–1603. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Yang, P.; Li, Y.; Gao, Z.; Zhang, Y. Robust heart beat detection from photoplethysmography interlaced with motion artifacts based on empirical mode decomposition. In Proceedings of the IEEE-EMBS International Conference on Biomedical and Health Informatics (BHI), Hong Kong, China, 5–7 January 2012; pp. 775–778.
- Hayes, J.; Smith, R. A new method for pulse oximetry possessing inherent insensitivity to artifact. IEEE Trans. Biol. Med. Eng. 2001, 48, 452–461. [Google Scholar] [CrossRef] [PubMed]
- Raghuram, M.; Madhavi, V.; Krishna, H.; Redd, A. Evaluation of wavelets for reduction of motion artifacts in photoplethysmographic signals. IEEE Inf. Sci. 2010, 2, 460–463. [Google Scholar]
- Yan, Y.; Poon, C.; Zhang, Y. Reduction of motion artifact in pulse oximetry by smoothed pseudo wigner-ville distribution. J. NeuroEng. Rehabil. 2005, 2, 1186–1743. [Google Scholar]
- Peng, F.; Zhang, Z.; Gou, X.; Liu, H.; Wang, W. Motion artifact removal from photoplethysmographic signals by combining temporally constrained independent component analysis and adaptive filter. Biomed. Eng. 2014, 13, 1186–1475. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.; George, B.; Kumar, J. Use of fourier series analysis for motion artifact reduction and data compression of photoplethysmographic signals. IEEE Trans. Instrum. Meas. 2009, 58, 1706–1711. [Google Scholar] [CrossRef]
- Poh, Z.; Swenson, C.; Picard, W. Motion-tolerant magnetic earring sensor and wireless earpiece for wearable photoplethysmography. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Giannetti, R.; Dotor, L.; Silveira, P.; Golmayo, D.; Miguel, F.; Bilbao, A.; Canales, M.; Pilar, M. Heuristic algorithm for photoplethysmographic heart rate tracking during maximal exercise test. J. Med. Biol. Eng. 2012, 32, 181–188. [Google Scholar] [CrossRef]
- Hu, S.; Azorin-Peris, V. Opto-Physiological Sensor and Method of Design. GB Patent Application GB2, 519,335, 22 April 2014. [Google Scholar]
- Hu, S.; Azorin Peris, V.; Zheng, J. A study of opto-physiological modelling to quantify tissue absorbance in imaging photoplethysmography. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Buenos Aires, Argentina, 31 August–4 September 2010; pp. 5776–5779.
- Alzahrani, A; Hu, S.; Azorin-Peris, V. A Comparative Study of Physiological Monitoring with a Wearable Opto-Electronic Patch Sensor (OEPS) for Motion Reduction. J. Biosens. 2015, 5, 288–307. [Google Scholar]
- Hu, S.; Shi, P.; Azorin-Peris, V.; Echiadis, A.; Zheng, J.; Zhu, Y.; Cheang, Y. Non-Contact Reflection Photoplethysmography towards Effective Human Physiological Monitoring. J. Med. Biol. Eng. 2010, 30, 161–167. [Google Scholar]
- Hu, S.; Azorin Peris, V.; Echiadis, A.; Zheng, J.; Shi, P. Development of effective photoplethysmographic measurement techniques: From contact to non-contact and from point to imaging. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Minneapolis, MN, USA, 3–6 September 2009; pp. 6550–6553.
- Belkin, M.; Schwartz, M. New Biological Phenomena Associated with Laser Radiation. Health Phys. 1989, 56, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Matsumura, K.; Yamakoshi, K.; Rolfe, P.; Tanaka, S.; Yamakoshi, T. Comparison between Red, Green and Blue Light Reflection Photoplethysmography for Heart Rate Monitoring during Motion. In Proceedings of the 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 1724–1727.
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzahrani, A.; Hu, S.; Azorin-Peris, V.; Barrett, L.; Esliger, D.; Hayes, M.; Akbare, S.; Achart, J.; Kuoch, S. A Multi-Channel Opto-Electronic Sensor to Accurately Monitor Heart Rate against Motion Artefact during Exercise. Sensors 2015, 15, 25681-25702. https://doi.org/10.3390/s151025681
Alzahrani A, Hu S, Azorin-Peris V, Barrett L, Esliger D, Hayes M, Akbare S, Achart J, Kuoch S. A Multi-Channel Opto-Electronic Sensor to Accurately Monitor Heart Rate against Motion Artefact during Exercise. Sensors. 2015; 15(10):25681-25702. https://doi.org/10.3390/s151025681
Chicago/Turabian StyleAlzahrani, Abdullah, Sijung Hu, Vicente Azorin-Peris, Laura Barrett, Dale Esliger, Matthew Hayes, Shafique Akbare, Jérôme Achart, and Sylvain Kuoch. 2015. "A Multi-Channel Opto-Electronic Sensor to Accurately Monitor Heart Rate against Motion Artefact during Exercise" Sensors 15, no. 10: 25681-25702. https://doi.org/10.3390/s151025681
APA StyleAlzahrani, A., Hu, S., Azorin-Peris, V., Barrett, L., Esliger, D., Hayes, M., Akbare, S., Achart, J., & Kuoch, S. (2015). A Multi-Channel Opto-Electronic Sensor to Accurately Monitor Heart Rate against Motion Artefact during Exercise. Sensors, 15(10), 25681-25702. https://doi.org/10.3390/s151025681







