Effects of Calcination Temperature and Acid-Base Properties on Mixed Potential Ammonia Sensors Modified by Metal Oxides
Abstract
: Mixed potential sensors were fabriated using yttria-stabilized zirconia (YSZ) as a solid electrolyte and a mixture of Au and various metal oxides as a sensing electrode. The effects of calcination temperature ranging from 600 to 1,000 °C and acid-base properties of the metal oxides on the sensing properties were examined. The selective sensing of ammonia was achieved by modification of the sensing electrode using MoO3, Bi2O3 and V2O5, while the use of WO3, Nb2O5 and MgO was not effective. The melting points of the former group were below 820 °C, while those of the latter group were higher than 1,000 °C. Among the former group, the selective sensing of ammonia was strongly dependent on the calcination temperature, which was optimum around melting point of the corresponding metal oxides. The good spreading of the metal oxides on the electrode is suggested to be one of the important factors. In the former group, the relative response of ammonia to propene was in the order of MoO3 > Bi2O3 > V2O5, which agreed well with the acidity of the metal oxides. The importance of the acidic properties of metal oxides for ammonia sensing was clarified.1. Introduction
The Uera-SCR (Selective Catalytic Reduction) technique is known to be an effective technology for the removal of nitrogen oxide (NOx) emissions from heavy-duty diesel engine cars [1–4]. In this system, an aqueous solution of urea is injected into a catalytic converter, hydroxylation of urea in the converter results in the formation of NH3, and the thus formed NH3 then successfully reduces NOx to N2 over Fe-zeolite or vanadium-based catalysts in a wide range of temperatures. The urea-SCR system has been already put into practical application, however, monitoring of the NH3 concentration in the catalytic converter is required to achieve proper operation of a urea-SCR system. For the practical application of the ammonia sensors to automobile exhausts, sufficient response altitude and cross-sensitivity, quick response, and tolerance to high temperatures under hydrothermal conditions are required.
Various types of ammonia sensors have been proposed [5,6]. The ammonia sensors using surface proton-conducting metal oxides, such as zeolites [7,8] and WO3/ZrO2 [9,10], show excellent cross-sensitivity to NH3 in the presence of various interfering gases, such as hydrocarbons, CO, and NOx. However, these materials have high surface area, and consequently they should have low thermal stability. Semiconductors of n-type metal oxides such as WO3 [11], MoO3 [12–15], V2O5 [16,17], SnO2 [18,19], TiO2 [20], In2O3 [21–23] and Ru/ZnO [24] have high hydrothermal stability, and have been extensively investigated as sensing materials. They usually act at lower temperatures (below 300 °C) than those needed in the automobile industry, but show low cross-sensitivity to NH3 in the presence of various interfering gases. Consequently, it is highly desirable to develop thermally stable ammonia sensors which show high cross-sensitivity to NH3 at high temperatures.
Mixed potential sensors are thought to be one of the promising technologies for this purpose because they are used at high temperatures around 500–600 °C. They are usually applied to sensors for CO and hydrocarbons [25–38], however, selective ammonia sensors can be designed by selection of appropriate sensing materials. Wang et al. examined various metals and metal oxides as sensing electrodes for ammonia sensors, and demonstrated that V2O5, BiVO4, MoO3, and WO3 are all effective for the sensing of NH3 [39]. Especially, BiVO4 showed the best output voltage in the presence of NH3, which was far higher than those of CO, C3H6, and NO. Schönauer and co-workers developed a novel selective ammonia sensor based on the mixed potential effect using a porous V2O5-WO3-TiO2-based SCR catalyst as a sensing material [40]. The proposed sensor showed good cross-sensitivity to NH3, and they demonstrated that the sensor can detect very small NH3 slips at the downstream of a real SCR catalyst. Elumalai et al. fabricated a planar mixed-potential-type sensor using a YSZ electrolyte and NiO/Au sensing electrode [41]. The sensor exhibited good sensitivity and cross-sensitivity to NH3 at 800 °C under wet conditions, i.e., the emf response to 100 ppm NH3 was about −34 mV, while the cross-sensitivities to the other examined gases were about ±5 mV or negligible. Hibino et al. prepared a proton-conducting thin Zr1−xYxP2O7 film on a YSZ substrate by reacting with liquid H3PO4 [42]. This sensor yielded a remarkably sensitive and selective response to low concentrations of NH3. Their approach suggests a strong contribution of acidity to selective NH3 detection.
It can be expected that the acid properties of the sensing material is one of the important factors for better cross-sensitivity because NH3 is a basic molecule, while the other infering gasses, CO, HC, and NOx, are not. However, the effects of the acid-base properties of sensing materials have not been clarified. The aim of this study was to obtain knowledge for the design of a metal oxide-modified mixed potential ammonia sensor. From the relationships between sensing properties and character of the metal oxides, the important factors for the selective sensing of NH3 are clarified.
2. Experimental Section
2.1. Materials Synthesis and Sensor Setup
MnO2, MoO3, Bi2O3, WO3, Nb2O5, and MgO (99% purity) were purchased from Kishida Chemical Co., Ltd. V2O5 (99% purity) was purchased from Mituswa Chemical Co., Ltd. BiVO4 was prepared by milling V2O5 and Bi2O3 for 24 h, followed by calcination of the mixture at 900 °C.
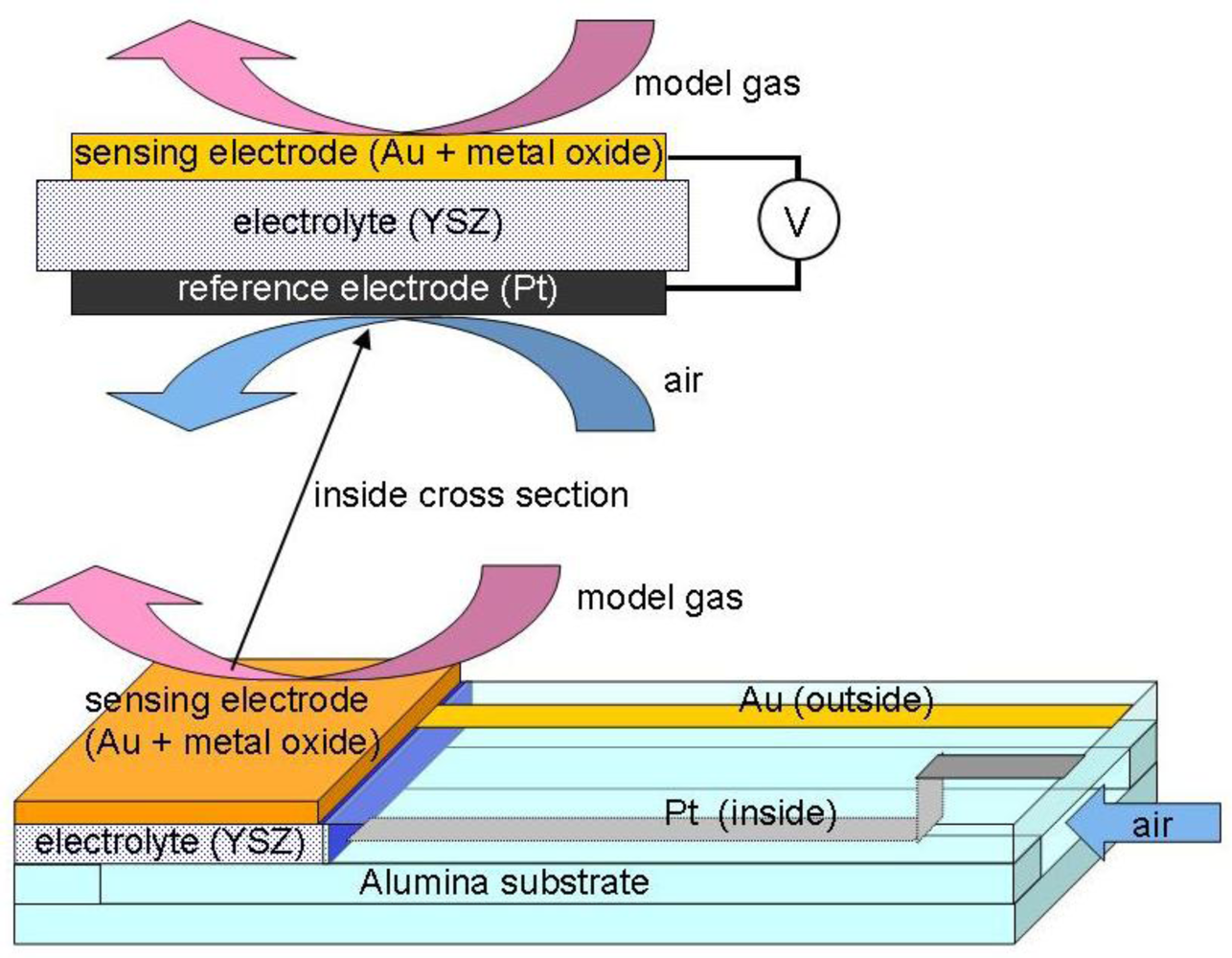
The schematic structure of the two-chamber cell constructed with a YSZ solid electrolyte on an alumina substrate is illustrated in Figure 1. One side of the YSZ solid electrolyte covered by a Pt electrode is exposed to the inside chamber with outside air. Another side of the YSZ solid electrolyte is covered with a mixture of Au and metal oxide thick film as a sensing material prepared by the screen-printing technique in the same manner reported previously [9,10,17]. For the preparation of electrodes, screen printable pastes were produced by mixing gold paste (purchased from Daiken Chemical Co., Ltd., Au100-1) with 10 wt% of the metal oxide powders. The sensor was calcined in air for 5 h at 650–1,000 °C depending on the metal oxide use, and assembled in a stainless case. Thickness of the film after the calcination step was ca. 10–20 μm. The name of each sensor electrode is abbreviated as the name of the metal oxide and calcination temperature in °C, for example, Bi2O3(850).
2.2. Gas Sensor Measurements
Sensing characteristics were evaluated by using a conventional gas-flow apparatus equipped with a furnace operating at 600 °C. The stainless flow cell was heated at operating temperatures. The reference electrode (cathode) was exposed to air, and the sensing electrode (anode) was exposed to a flow of mixture gas. The composition of the base gas is 10% O2, 3% H2O, and N2 as a balance gas at a flow rate of 150 cm3 min−1. The standard concentrations of NH3 and C3H6 are 500 ppm. The electromotive forces (EMF) value of the cell was measured by an electrometer (Hokuto Denko HZ-5000).
3. Results and Discussion
3.1. Effect of Calcination
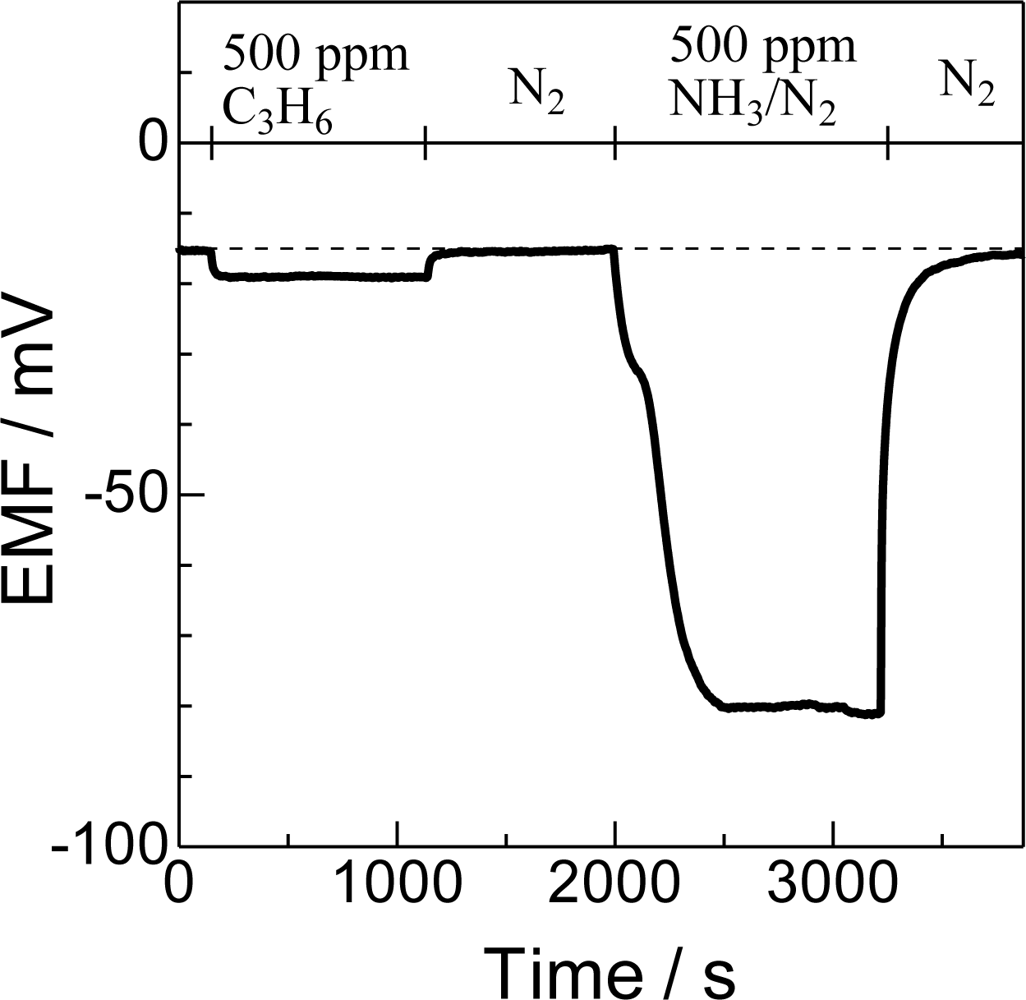
Figure 2 shows an example of transient response of Bi2O3(850) to the injection of ammonia and C3H6. Due to the difference in the oxygen concentration (20% at the reference electrode and 10% at the sensing electrode), the EMF of the sensor electrode is −15.5 mV in the absence of reducing gases. In the presence of 500 ppm C3H6, EMF decreased to −19.1 mV. The difference in EMF between those in the presence and absence of a probe molecule is determined as ΔEMF, 3.6 mV in this case, and used as an indicator of response height. On the other hand, the ΔEMF in a flow of the same amount of NH3 was −68.2 mV, which is one order higher than that in C3H6, indicating the very high selective sensing of the Bi2O3(850) electrode. After the evacuation of NH3, the EMF value quickly recovered to the original level. The ΔEMF of NH3 relative to that of the same concentration of C3H6 (ΔEMFNH3/ΔEMFC3H6) is used as a measure of cross-sensitivity.
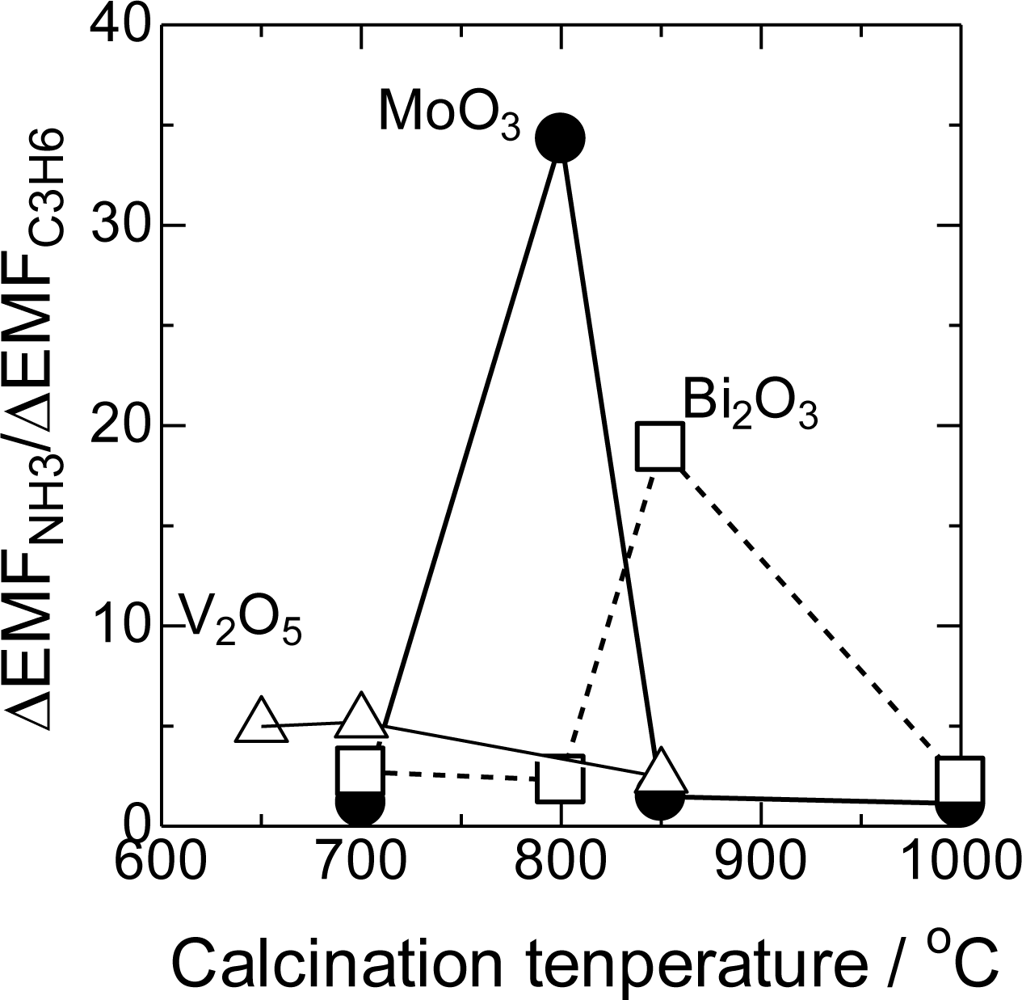
The cross-sensitivity strongly depended on the calcination temperatures of the electrodes. In Figure 3, the ΔEMFNH3/ΔEMFC3H6 ratios of the selected sensors as a function of the calcination temperature are shown. V2O5, MoO3, and Bi2O3 showed the maximum ΔEMFNH3/ΔEMFC3H6 at 700, 800, and 850 °C, respectively. Since the melting points of these metal oxides are 695, 790, and 820 °C, the maximum cross-sensitivity was observed around their melting points.
Various kinds of the sensing electrodes listed in Table 1were prepared using the corresponding metal oxides. Due to the strong dependence of the cross-sensitivity on calcination temperatures as shown in Figure 3, the sensing performances were compared after the calcination at the optimum temperatures ranging from 700 to 1,000 °C. For WO3(1000), Nb2O5(1000), and MgO(1000) electrodes, the ΔEMFNH3/ΔEMFC3H6 ratios were comparable to that of the electrode without modification (Au only). This implies a negligible chemical interaction between Au and metal oxides, which results in the ΔEMFNH3/ΔEMFC3H6 ratio of around unity and thus, non-selective NH3 detection. Melting points of these metal oxides were higher than the calcination temperature of 1,000 °C.
On the other hand, very high ΔEMFNH3 and low ΔEMFC3H6 were obtained when MoO3 was used as a sensing material. As a result, the ΔEMFNH3/ΔEMFC3H6 ratio was the highest for MoO3(800). MnO2, V2O5, and Bi2O3 also showed high ΔEMFNH3/ΔEMFC3H6 ratios and sufficient ΔEMF values in the presence of NH3. In these metal oxides melting points’ were below 820 °C, and the calcination temperatures were optimized around their melting points. The results show that the melting point of the sensing material is one of the important factors for the preparation of the selective sensors.
The maximum cross-sensitivity at around the melting points of the metal oxides suggests that the good mixing of the metal oxides, Au electrode, and YSZ is essential for selective NH3 detection. It can be speculated that the acidic metal oxides are well spread on Au and YSZ surface and achieved the successful modification of Au and YSZ. Above the melting points, too much spreading of the metal oxide results in the undesired covering of Au and YSZ electrode. The strong dependence of the selective sensing of NH3 on the calcination temperature suggests that the spreading of metal oxide is important for the successful preparation of the sensor. As shown in Table 1, MoO3(850) showed the highest response altitude and ΔEMFNH3/ΔEMFC3H6 ratio. However, owing to sublimation of MoO3 at 1,155 °C [43], MoO3 is not suitable for the application of ammonia sensing in automobile exhaust systems. Therefore, further investigations have been done by using Bi2O3(850) which showed the second highest ΔEMFNH3/ΔEMFC3H6 and sufficient response altitude.
3.2. Effect of Acid-Base Property
Figure 4 shows ΔEMFNH3/ΔEMFC3H6 ratio of MoO3(800), Bi2O3(850) and V2O5(700) as a function of electronegativity of the metals in the metal oxides. As we have reported in a previous paper [17], the electronegativity can be used as an indicator of the acid-base properties of metal oxides: the higher electronegativity implies higher acidity of the oxides. Clearly, the ΔEMFNH3/ΔEMFC3H6 ratio increased with the electronegativity. This correlation shows that the acidity of metal oxide is very essential for the cross-sensitivity to NH3.
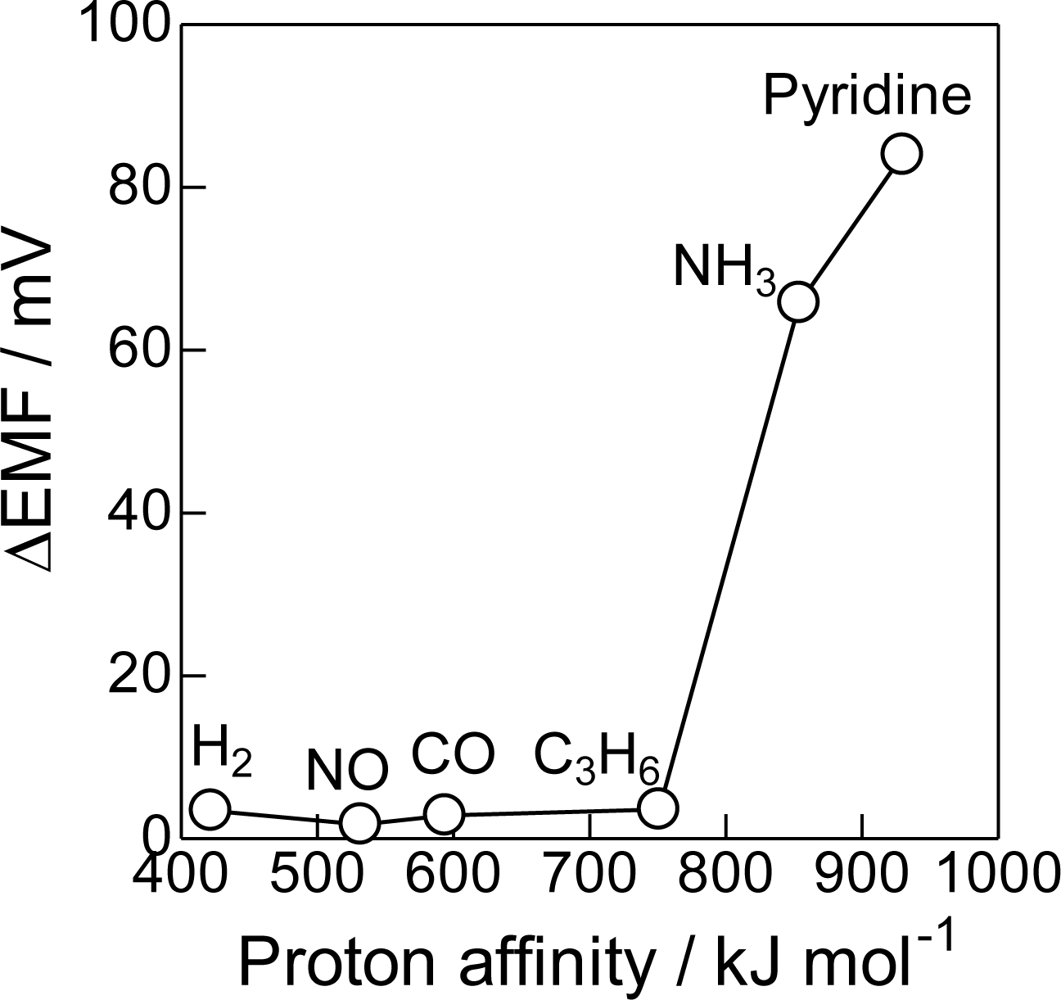
The importance of acid-base properties also can be seen in the effect of the acid-base properties of probe molecules shown in Figure 5. The proton affinity is a good indicator for the acid-base properties of molecules [44]. The Bi2O3(850) electrode was not sensitive to H2, NO, CO, C3H6 having lower proton affinity. However, ΔEMF became very high to NH3 and pyridine which have high proton affinity, i.e., are highly basic molecules. These results clearly indicate that the sensing response of the prepared electrodes strongly depends on the acid-base interaction of the sensing electrodes and probe molecules. A highly acidic property is required for the selective detection of NH3.
3.3. Performance of Bi2O3(850) as an Ammonia Sensor
The sensing performance of Bi2O3(850) as an ammonia sensor was investigated. The response of the Bi2O3(850) electrode was examined by stepwise change in the NH3 concentration up to 1,000 ppm. The measured ΔEMF was plotted as a function of NH3 concentration in Figure 6. The sensor characteristic was semi-logarithmic to the NH3 concentration. This means that the sensor has a high sensitivity, even at lower ammonia concentrations.
The mixed potential mechanism was evaluated for the Bi2O3(850) electrode by modified polarization curves [25–27] in 10% O2 and in 500 ppm NH3 at 600 °C, in the same manner proposed by Miura [25]. The intersection of the polarization curves was observed at the same ΔEMF value in the presence of the same concentrations of NH3 and O2. When the following Equation (1) at the cathode and Equation (2) at the anode proceed at an equal rate, an electrode potential shows the mixed potential:
It is important to examine the effect of interfering gaseous species on the response of the sensor. Figure 7 shows the influence of the concentration of O2 and water vapor on the responses of the Bi2O3(850) electrode to 500 ppm NH3. It should be noted that a different lot of the Bi2O3(850) sensor was used in this section, and the sensing responses were compared with those of BiVO4(750) electrode, which is known as one of the excellent electrode for NH3 sensing [39], prepared by the same manner reported by Wang et al. [39]. The concentration of water vapor was varied from 1 to 10%. The responses of both the sensors were not much affected by water vapor. While, the higher concentration of O2 slightly decreased the ΔEMF vale of the Bi2O3(850) electrode, though the ΔEMF of the BiVO4(750) electrode significantly decreased when the O2 concentration is 15%. The Bi2O3(850) electrode is less interfered by the concentration of O2 than the BiVO4(750) electrode.
4. Conclusions
The effect of calcination temperature and acid-base properties of metal oxides used as a sensing material for an ammonia sensor have been investigated. The sensor electrode was prepared by the two-chamber cell constructed from a YSZ solid electrolyte, on which one side is covered by a Pt electrode and another side is covered with a mixture of Au and a metal oxide thick film as a sensing material. When MoO3, Bi2O3 and V2O5, having melting points below 820 °C, are used as the sensing material, the sensors exhibited very high cross-sensitivity to NH3. The use of WO3, Nb2O5 and MgO having melting points above 1,000 °C as sensing materials was not effective. The good spreading of the metal oxide on the sensing electrode was suggested to be one of the important factors. The sensing selectivity to ammonia was in the order of MoO3 > Bi2O3 > V2O5, which was in agreement with the corresponding acidity of the metal oxides. It was clarified that the acidity of metal oxides is the determining factor for the selective sensing of NH3.
References
- Koebel, M.; Elsener, M.; Kleemann, M. Urea-SCR: A promising technique to reduce NOx emissions from automotive diesel engines. Catal. Today 2000, 59, 335–345. [Google Scholar]
- Burch, R. Knowledge and know-how in emission control for mobile. Catal. Rev 2004, 46, 271–334. [Google Scholar]
- Matsumoto, S. Recent advances in automobile exhaust catalysts. Catal. Today 2004, 90, 183–190. [Google Scholar]
- Klingstedt, F.; Arve, K.; Eränen, K.; Murzin, D.Y. Toward improved catalytic low-temperature NOx removal in diesel-powered vehicles. Acc. Chem. Res 2006, 39, 273–282. [Google Scholar]
- Moos, R. A brief overview on automotive exhaust gas sensors based on electroceramics. Int. J. Appl. Ceram. Technol 2005, 2, 401–413. [Google Scholar]
- Moos, R. Automotive exhaust gas sensors. In Encyclopedia of Sensors; Grimes, C.A., Dickey, E.C., Pishko, M.V., Eds.; American Scientific Publishers: Valencia, CA, USA, 2006; Volume 1, pp. 295–312. [Google Scholar]
- Moos, R.; Muller, R.; Plog, C.; Knezevic, A.; Leye, H.; Irion, E.; Braun, T.; Marquardt, K.-J.; Binder, K. Selective ammonia exhaust gas sensor for automotive applications. Sens. Actuat. B 2002, 83, 181–189. [Google Scholar]
- Kubinsk, D.J.; Visser, J.H. Sensor and method for determining the ammonia loading of a zeolite SCR catalyst. Sens. Actuat. B 2008, 130, 425–429. [Google Scholar]
- Satsuma, A.; Shimizu, K.; Hattori, T.; Nishiyama, H.; Kakimoto, S.; Sugaya, S.; Yokoi, H. Polytungstate clusters on zirconia as a sensing material for a selective ammonia gas sensor. Sens. Actuat. B 2007, 123, 757–762. [Google Scholar]
- Satsuma, A.; Shimizu, K.; Kashiwagi, K.; Endo, T.; Nishiyama, H.; Kakimoto, S.; Sugaya, S.; Yokoi, H. Ammonia sensing mechanism of tungstated-zirconia thick film sensor. J. Phys. Chem. C 2007, 111, 12080–12085. [Google Scholar]
- Ando, M.; Tsuchida, T.; Suto, S.; Suzuki, T.; Nakayama, C.; Miura, N.; Yamazoe, N. Ammonia gas sensor using thick film of Au-loaded tungsten trioxide. J. Ceram. Soc. Jpn 1996, 104, 1112–1116. [Google Scholar]
- Xu, C.N.; Miura, N.; Ishida, Y.; Matsuda, K.; Yamazoe, N. Selective detection of NH3 over NO in combustion exhausts by using Au and MoO3 doubly promoted WO3 element. Sens. Actuat. B 2000, 65, 163–165. [Google Scholar]
- Mutschall, D.; Holzner, K.; Obermeier, E. Sputtered molybedeum oxide thin films for NH3 detection. Sens. Actuat. B 1996, 36, 320–324. [Google Scholar]
- Prasad, A.K.; Kubinski, D.J.; Gouma, P.I. Comparison of sol-gel and ion beam deposited MoO3 thin film gas sensors for selective ammonia detection. Sens. Actuat. B 2003, 93, 25–30. [Google Scholar]
- Raju, A.R.; Rae, C.N.R. MoO3/TiO2 and Bi2MoO6 as ammonia sensors. Sens. Actuat. B-Chem 2003, 93, 25–30. [Google Scholar]
- Meixner, H.; Gerblinger, J.; Lampe, U.; Fleischer, M. Thin-film gas sensors based on semiconducting metal oxides. Sens. Actuat. B 1994, 23, 23–26. [Google Scholar]
- Shimizu, K.; Chinzei, I.; Nishiyama, H.; Kakimoto, S.; Sugaya, S.; Matsutani, M.; Satsuma, A. Doped-vanadium oxides as sensing materials for high temperature operative selective ammonia gas sensors. Sens. Actuat. B 2009, 41, 410–416. [Google Scholar]
- Ivanov, P.; Hubalek, J.; Malysz, K.; Prášek, J.; Vilanova, X.; Llobet, E.; Correig, X. A route toward more selective and less humidity sensitive screen-printed SnO2 and WO3 gas sensitive layers. Sens. Actuat. B 2004, 100, 221–227. [Google Scholar]
- Teeramongkonrasmee, A.; Sriyudthsak, M. Methanol and ammonia sensing characteristics of sol-gel derived thin film gas sensor. Sens. Actuat. B 2000, 66, 256–259. [Google Scholar]
- Shimizu, Y.; Okamoto, T.; Takao, Y.; Egashira, M. Desorption behavior of ammonia from TiO2-based specimens—ammonia sensing mechanism of double-layer sensors with TiO2-based catalyst layers. J. Mol. Catal. A 2000, 155, 183–191. [Google Scholar]
- Romanovskaya, V.; Ivanovskaya, M.; Bogdanov, P. A study of sensing properties of Pt- and Au-loaded In2O3 ceramics. Sens. Actuat. B 1999, 56, 31–36. [Google Scholar]
- Li, C.; Zhang, D.; Lei, B.; Han, S.; Liu, X.; Zhou, C. Surface treatment and doping dependence of In2O3 nanowires as ammonia sensors. J. Phys. Chem. B 2003, 107, 12451–12455. [Google Scholar]
- Guo, P.; Pan, H. Selectivity of Ti-doped In2O3 ceramics as an ammonia sensor. Sens. Actuat. B 2006, 114, 762–767. [Google Scholar]
- Aslam, M.; Chaudhary, V.A.; Mulla, I.S.; Sainkar, S.R.; Mandale, A.B.; Belhekar, A.A.; Vijayamohanan, K. A highly selective ammonia gas sensor using surface ruthenated zinc oxide. Sens. Actuat. B 1999, 75, 162–167. [Google Scholar]
- Miura, N.; Raisen, T.; Lu, G.; Yamazoe, N. Highly selective CO sensor using stabilized zirconia and a couple of oxide electrodes. Sens. Actuat. B 1998, 47, 84–91. [Google Scholar]
- Hibino, T.; Kakimoto, S.; Sano, M. Non-Nernstian behavior at modified Au electrodes for hydrocarbon gas sensing. J. Electrochem. Soc 1999, 146, 3361–3366. [Google Scholar]
- Garzon, F.H.; Mukundan, R.; Brosha, E.L. Solid-state mixed potential gas sensors: Theory, experiments and challenges. Solid State Ionics 2000, 136–137, 633–638. [Google Scholar]
- Brosha, E.L.; Mukundan, R.; Brown, D.R.; Garzon, F.H.; Visser, J.H.; Zanini, M.; Zhou, Z.; Logothetis, E.M. CO/HC sensors based on thin films of LaCoO3 and La0.8Sr0.2CoO3-d metal oxides. Sens. Actuat. B 2000, 69, 171–182. [Google Scholar]
- Mukundan, R.; Brosha, E.L.; Brown, D.R.; Garzon, F.H. A mixed-potential sensor based on a Ce0.8Gd0.2O1.9 Electrolyte and platinum and gold Electrodes. J. Electrochem. Soc 2000, 147, 1583–1588. [Google Scholar]
- Brosha, E.L.; Mukundan, R.; Brown, D.R.; Garzon, F.H. Mixed potential sensors using lanthanum manganate and terbium yttrium zirconium oxide electrodes. Sens. Actuat. B 2002, 87, 47–57. [Google Scholar]
- Brosha, E.L.; Mukundan, R.; Brown, D.R.; Garzon, F.H.; Visser, J.H. Development of ceramic mixed potential sensors for automotive applications. Solid State Ionics 2002, 148, 61–69. [Google Scholar]
- Mukundan, R.; Brosha, E.L.; Garzon, F.H. Mixed potential hydrocarbon sensors based on a YSZ electrolyte and oxide electrodes. J. Electrochem. Soc 2003, 150, H279–H284. [Google Scholar]
- Li, X.; Kale, G.M. (BaxLa1−x)2In2O5+x (0.4 ≤ x ≤ 0.6) Electrolyte-Supported Mixed-Potential CO Sensors. Anal. Chem 2007, 79, 8940–8946. [Google Scholar]
- Li, X.; Kale, G.M. Influence of sensing electrode and electrolyte on performance of potentiometric mixed-potential gas sensors. Sens. Actuat. B 2007, 123, 254–261. [Google Scholar]
- Zosel, J.; Ahlborn, K.; Müller, R.; Westphal, D.; Vashook, V.; Gutha, U. Selectivity of HC-sensitive electrode materials for mixed potential gas sensors. Solid State Ionics 2004, 169, 115–119. [Google Scholar]
- Yoo, J.; Chatterjee, S.; Van Assche, F.M.; Wachsman, E.D. Influence of adsorption and catalytic reaction on sensing properties of a potentiometric La2CuO4/YSZ/Pt sensor. J. Electrochem. Soc 2007, 154, J190–J195. [Google Scholar]
- Fergus, J.W. Solid electrolyte based sensors for the measurement of CO and hydrocarbon gases. Sens. Actuat. B 2007, 122, 683–693. [Google Scholar]
- Morata, A.; Viricelle, J.P.; Tarancóon, A.; Dezanneau, G.; Pijolat, C.; Peiro, F.; Morante, J.R. Development and characterisation of a screen-printed mixed potential gas sensor. Sens. Actuat. B 2008, 130, 561–566. [Google Scholar]
- Wang, D.Y.; Symons, W.T.; Farhat, R.J.; Valdes, C.A.; Briggs, E.M.; Polikarpus, K.K.; Kupe, J. Ammonia Gas Sensors. U. S. Patent 7,074,319 B2,. 11 July 2006. [Google Scholar]
- Schönauer, D.; Wiesner, K.; Fleischer, M.; Moos, R. Selective mixed potential ammonia exhaust gas sensor. Sens. Actuat. B 2009, 140, 585–590. [Google Scholar]
- Elumalai, P.; Plashnitsa, V.V.; Fujio, Y.; Miura, A. stabilized zirconia-based sensor attached with NiO/Au sensing electrode aiming for highly selective detection of ammonia in automobile exhausts. Electrochem. Solid-State Lett 2008, 11, J79–J81. [Google Scholar]
- Teranishi, S.; Kondo, K.; Nishida, M.; Kanematsu, W.; Hibino, T. Proton-conducting thin film grown on yttria-stabilized zirconia surface for ammonia gas sensing technologies. Electrochem. Solid-State Lett 2009, 12, J73–J76. [Google Scholar]
- Budavari, S.; O’Neil, M.J.; Smith, A.; Heckelman, P.E.; Kinneary, J.F. The Merck Index, 5th ed; Merck Research Laboratories: Whitehouse Station, NJ, USA, 1996; p. 6312. [Google Scholar]
- Hunter, E.P.; Lias, S.G. Proton affinity evaluation. In NIST Chemistry WebBook, NIST Standard Reference Database Number 69; Linstrom, P.J., Mallard, W.G., Eds.; National Institute of Standards and Technology: Gaithersburg, MD, USA; Available online: http://webbook.nist.gov (accessed on 24 December 2010).



| Electrode | m.p. of MOx | Calcination/°C | ΔEMFNH3 | ΔEMFC3H6 | ΔEMFNH3/ΔEMFC3H6 |
|---|---|---|---|---|---|
| MnO2(700) | 535 | 700 | 176 | 19.6 | 9.0 |
| V2O5(700) | 690 | 700 | 188 | 33.2 | 5.7 |
| MoO3(800) | 795 | 800 | 336 | 9.5 | 35.5 |
| Bi2O3(850) | 820 | 850 | 68.2 | 3.6 | 18.9 |
| WO3(1000) | 1,470 | 1,000 | 50.9 | 30.5 | 1.7 |
| Nb2O5(1000) | 1,520 | 1,000 | 224 | 173 | 1.3 |
| MgO(1000) | 2,800 | 1,000 | 190 | 146 | 1.3 |
| Au only | (1,064) | 1,000 | 89.4 | 71.3 | 1.3 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Satsuma, A.; Katagiri, M.; Kakimoto, S.; Sugaya, S.; Shimizu, K. Effects of Calcination Temperature and Acid-Base Properties on Mixed Potential Ammonia Sensors Modified by Metal Oxides. Sensors 2011, 11, 2155-2165. https://doi.org/10.3390/s110202155
Satsuma A, Katagiri M, Kakimoto S, Sugaya S, Shimizu K. Effects of Calcination Temperature and Acid-Base Properties on Mixed Potential Ammonia Sensors Modified by Metal Oxides. Sensors. 2011; 11(2):2155-2165. https://doi.org/10.3390/s110202155
Chicago/Turabian StyleSatsuma, Atsushi, Makoto Katagiri, Shiro Kakimoto, Satoshi Sugaya, and Kenichi Shimizu. 2011. "Effects of Calcination Temperature and Acid-Base Properties on Mixed Potential Ammonia Sensors Modified by Metal Oxides" Sensors 11, no. 2: 2155-2165. https://doi.org/10.3390/s110202155




