Physiological Sensing of Carbon Dioxide/Bicarbonate/pH via Cyclic Nucleotide Signaling
Abstract
: Carbon dioxide (CO2) is produced by living organisms as a byproduct of metabolism. In physiological systems, CO2 is unequivocally linked with bicarbonate (HCO3−) and pH via a ubiquitous family of carbonic anhydrases, and numerous biological processes are dependent upon a mechanism for sensing the level of CO2, HCO3, and/or pH. The discovery that soluble adenylyl cyclase (sAC) is directly regulated by bicarbonate provided a link between CO2/HCO3/pH chemosensing and signaling via the widely used second messenger cyclic AMP. This review summarizes the evidence that bicarbonate-regulated sAC, and additional, subsequently identified bicarbonate-regulate nucleotidyl cyclases, function as evolutionarily conserved CO2/HCO3/pH chemosensors in a wide variety of physiological systems.1. Introduction
Carbon dioxide (CO2) and water are the major end products of energy producing pathways in living organisms (Equation (1)). As such, in non-photosynthetic organisms, CO2 and water represent the most fundamental catabolites.
In unicellular organisms, CO2 gas can simply diffuse away, but once multicellular organisms evolved, they had to devise methods for safely dealing with CO2. In solution, CO2 combines with water to form carbonic acid (H2CO3), which dissociates to liberate a proton and a bicarbonate ion (HCO3−) (Equation (2)). CO2, bicarbonate and pH equilibrate on their own within minutes, but in biological systems, equilibrium is reached nearly instantaneously due to the ubiquitous presence of carbonic anhydrases [1]. This equilibrium is used to buffer pH inside cells and in intercellular fluids; for example, intracellular pH is regulated via an interplay between CO2 diffusion, and bicarbonate and proton transporters and/or exchangers. In mammals, and terrestrial vertebrates in general, this equilibrium is tightly controlled in two ways; the kidneys regulate the bicarbonate concentration and the breathing frequency determines the concentration of carbon dioxide. Each of these processes requires a ‘sensor,’ i.e., an exquisitely sensitive and rapid way to measure the precise concentration of either CO2 and/or bicarbonate and/or pH and elicit an appropriate response. Many other physiological processes, in addition to diuresis and breathing rate regulation, are modulated by CO2 and/or bicarbonate and/or pH (i.e., sperm activation, blood flow, aqueous humor in the eye and cerebrospinal fluid formation), and they also require a CO2/HCO3/pH sensor. For many years, the effects of CO2 and pH had been ascribed to undefined chemoreceptors, and the effects of bicarbonate were traditionally thought to be mediated by changes in cellular pH [1]. In 2000, our research group demonstrated that HCO3− directly modulates the activity of soluble adenylyl cyclase (sAC), a novel form of the enzyme generating the ubiquitous second messenger, cAMP [2], revealing that physiological CO2/HCO3/pH could be sensed via second messenger signaling.
Cyclic AMP was discovered more than 50 years ago by Earl Sutherland to act as a ‘second’ or intracellular messenger which mediated cellular responses to extracellular signals in organisms as diverse as bacteria and mammals [3]. Still, our understanding of cAMP signaling has recently undergone two transformative changes: cAMP signaling is organized into multiple, independently-regulated microdomains within a cell [4–6], and in addition to its role mediating cellular changes, cAMP can affect cellular physiology by modulating the amplitude or duration of other signaling cascades [7].
Over the decades of studying cAMP signaling in mammalian biology, this single second messenger had been implicated in a wide variety of often-contradictory physiological processes, including different aspects of metabolism, proliferation, apoptosis, differentiation, migration, development, ion transport, pH regulation, and gene expression. This seeming conundrum was finally resolved with the appreciation that cAMP acts locally within independently regulated microdomains. The microdomain model posits that cAMP is generated at distinct locations within the cell by independently regulated adenylyl cyclases [8–10], where it modulates only nearby targets, including cyclic nucleotide gated ion channels, Exchange Proteins Activated by cAMP (EPACs), or Protein Kinase A (PKA). Ultimately, the cAMP is degraded by phosphodiesterases (PDEs) which serve two functions; they act as barriers to cAMP diffusion [11,12] preventing unregulated cross-communication between microdomains [13] and the more traditionally accepted role restoring cAMP levels to their basal level terminating the signaling cascade [14]. Individual microdomains can be wholly contained within an organelle, such as the mitochondria or nucleus [9,10,15] or can be defined by A-kinase anchoring proteins (AKAPs), which tether PKA [16,17], and possibly adenylyl cyclases [18–20] and/or PDEs [21–28] to specific locations inside cells. Organization in microdomains enables this one second messenger to simultaneously mediate disparate processes throughout a cell.
There were early hints that cAMP signaling was compartmentalized within a cell; for example, distinct hormones which have the same effects on cAMP levels in bladder epithelial cells do not have the same effect on osmotic water flow [29]. But a need for independently-regulated cAMP microdomains was best demonstrated in cardiomyocytes, where it was observed that two hormones, which both functioned via cAMP, elicited completely different responses [30]. Modern FRET-based [31–33] and biophysical methods [34,35] that enable measuring cAMP concentrations in situ revealed that cAMP levels are not uniform within cells (reviewed in [4,5]). The microdomain organization of cAMP signaling was definitively confirmed by the demonstration of independently regulated, membrane-proximal cAMP microdomains in neurons [36] and cardiomyocytes [37]; by the demonstration of the role of AKAPs [16,17]; and by the unique functions of artificial, localized production of second messenger within distinct subcellular compartments [38–40]. Among the implications for a locally acting second messenger is the realization that changes in cAMP levels do not have to be large (or even detectable in a whole cell context) to be physiologically relevant; meaningful cAMP fluctuations within a microdomain could be insignificant compared to the total cAMP content of a cell. Thus, even for a cAMP-mediated process, measuring a cAMP rise may prove difficult. The microdomain organization of signaling seems to be true for both cAMP and the other second messenger cyclic nucleotide, cGMP; in cultured hippocampal neurons, localized cAMP was shown to be essential for axonal determination while compartmentalized cGMP defined dendrites [41].
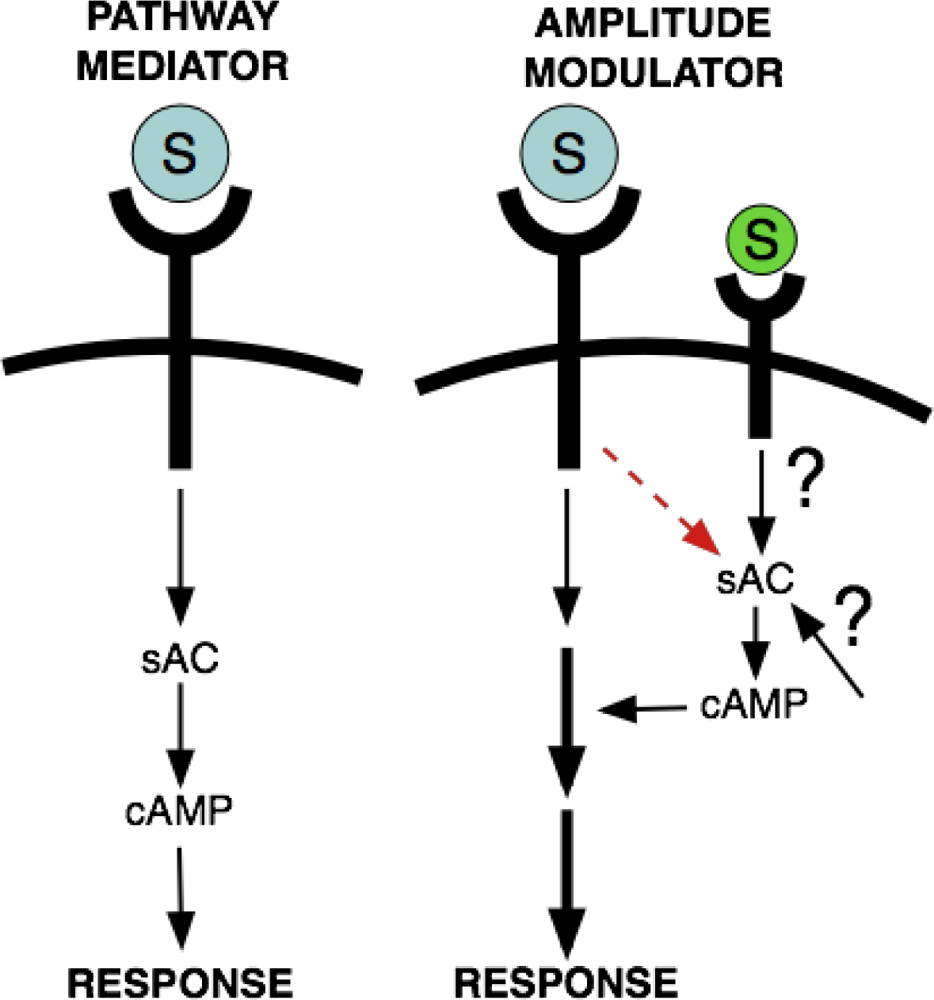
The concept of cAMP as an amplitude or frequency modulator of other signaling pathways derives from an idea posited 15 years ago by Ravi Iyengar [7]. In addition to its role as a signal mediator (Iyengar referred to this role as functioning as part of a “bucket-brigade” where cAMP is both necessary and sufficient to elicit a response), he suggested that cAMP might be functioning as a “gate” to regulate information flow through distinct signaling pathways. In his “gating” model, cAMP served a permissive role, turning a pathway on or off. Our studies of sAC have confirmed and extended this model for cAMP function; our studies identified a role for sAC-generated cAMP functioning like a rheostat, modulating intensity or frequency of a signaling pathway (Figure 1).
As described in more detail below, sAC is responsible for CO2-dependent regulation of oxidative phosphorylation in mitochondria [42]. In performing this role, sAC-generated cAMP does not elicit a response on its own, but functions as an ‘amplitude modulator;’ it alters the rate of ATP production dependent upon the amount of metabolically generated CO2. sAC also has modulatory functions in the CO2-dependent regulation of beat frequency of cilia in airway epithelia [43] and the HCO3-induced activation of sperm motility [44–46]; in both cases, sAC-generated cAMP alters the frequency of an already existing beating response (in cilia or flagella, respectively). Interestingly, sAC in sperm exhibits both types of functionalities; sAC-generated cAMP acts as a ‘frequency modulator’ to control the rate of flagellar beating for hyperactivated motility, but it also acts as an “on-off” pathway mediator, initiating swimming and the process of capacitation, the developmental program needed to enable sperm to penetrate and fertilize an egg [44,45,47]. Other examples where cAMP seems to serve a “gating” function included growth factor activation of the MAP Kinase pathway [48], long-range patterning induced by the diffusible morphogen, Sonic Hedgehog [49–51], long-term potentiation evoked by repeated stimulation in hippocampal CA1 region [52], and neurotrophin-dependent survival and growth of neurons [53]. Interestingly, these processes may also involve sAC [54–58].
‘Amplitude or frequency modulation’ provides a mechanism for cells to fine tune responses to a signal such that more (or less) signal is required to provide a consistent or maintained response. This property is particularly useful for a gradient morphogen or any diffusible signal that induces directional movement, such as a neuronal guidance cue, where a cell responds by moving up (or down) a concentration gradient.
2. Discovery of sAC and Regulation by Bicarbonate
G protein regulated, transmembrane adenylyl cyclases (tmACs) mediate intracellular changes due to extracellular signals such as hormones and neurotransmitters binding to G protein coupled receptors (GPCRs); for a long time, these were thought to be the predominant (if not only) sources of cAMP in higher eukaryotes. In 1999, our laboratory purified and cloned mammalian soluble adenylyl cyclase (sAC) [59] defining a unique signaling enzyme (Table 1; Reviewed in [60]). sAC is more closely related to (cyano)bacterial ACs than to tmACs or other metazoan cyclases providing a link between prokaryotic and eukaryotic signal transduction mechanisms. Isoform diversity for tmACs is generated via nine distinct genes; whereas for mammalian sAC, a single gene is alternatively spliced [61,62] and uses multiple promoters [63]. Unlike tmACs, sACs are not transmembrane proteins and are found distributed throughout the cytoplasm and in specific organelles [9,10,15] where they are thought to be the source of second messenger mediating the intracellular functions of cAMP [8,15]. As stated above, tmACs are directly modulated by heterotrimeric G proteins which transduce extracellular signals into intracellular cAMP changes. In contrast, sAC isoforms are insensitive to heterotrimeric G proteins [59] but are instead regulated by intracellular signals, including bicarbonate [2,64–67], calcium [68,69], and ATP [69].
Structurally, sAC and tmACs are quite similar [70]; both sAC [70] and tmACs [71] are active as dimers of two catalytic (C) units (Reviewed in [60,72]). However, structures (to a resolution of 1.9 Å) of various complexes of a bicarbonate- and calcium-regulated bacterial sAC-like cyclase with different substrate analogs provide a rationale for sAC-like cyclases’ insensitivity to heterotrimeric G proteins and their lower affinity for substrate ATP. These structures also reveal how calcium increases sAC-like cyclases’ affinity for ATP, and how bicarbonate stimulates catalytic rate. Bicarbonate regulation is conserved in sAC-like cyclases throughout evolution [2,73–76] as well as in yeast adenylyl cyclases [77–79] and a number of transmembrane (i.e., receptor-type) guanylyl cyclases [80–83]; thus, bicarbonate regulation of cyclic nucleotide synthesis is poised to be an evolutionarily conserved mechanism for physiological sensing CO2/HCO3/pH. In this review, we focus specifically on the functions of sAC (and other bicarbonate-regulated cyclases) where it functions as a physiological CO2/HCO3/pH chemosensor. Broader reviews, describing the various functions of mammalian sAC [84] and the variety of physiological CO2/HCO3/pH chemosensors [58], have recently been published.
3. Physiological CO2/HCO3/pH Chemosensing via sAC
3.1. Bicarbonate Activation of Sperm
Morphologically mature epididymal sperm do not have the “capacity” to fertilize an egg [85]. They acquire fertilization-competence during ejaculation and transit through the female reproductive tract. Upon ejaculation, sperm acquire flagellar motility (i.e., swim) and begin a poorly defined maturation process called capacitation. Capacitation continues inside the female reproductive tract, where it includes hyperactivation of flagellar motility and attaining the ability to perforate the egg’s zona pellucida via the acrosome reaction. These events lead to binding and fusion to the egg’s plasma membrane and fertilization. At least two of these stages, motility and capacitation, are induced by bicarbonate [86–89] and dependent upon cAMP signaling [89–92].
We originally purified sAC from testis [59] and sAC mRNA is highly expressed in male germ cells [93]. At least two isoforms of sAC are present in male germ cells [44]: a 187 kDa protein (“full length”, or sACfl) and a shorter, 53 kDa variant (“truncated”, or sACt) [59]. sACt has an approximately ten times higher specific activity than sACfl [94], and while both are found in testis and sperm [44,95,96], sACt appears to be responsible for the majority of cAMP production in mature sperm [44,45,47,97]. We (and others) demonstrated that the effects of bicarbonate on sperm are directly mediated by sAC [44,45,47,97]. Specifically, both motility [44,47,97] and capacitation [44,45] are abrogated in sAC knockout mice and by the sAC-specific pharmacological inhibitor, KH7 [44].
3.2. pH Sensing
Prior to ejaculation, sperm are stored in the cauda epididymis where they are maintained in a quiescent state by an acidic pH of 6.5–6.8 and a low bicarbonate concentration of 2–7 mM (compared to 25 mM in serum, prostate and other bodily fluids) [98]. In 2003, we demonstrated that sAC functions as a pH sensor in the clear cells of the epididymis to ensure that the luminal pH and bicarbonate concentration remain low [99]. sAC is highly expressed in clear cells, and apical membrane accumulation of the proton pumping vacuolar ATPase (V-ATPase) is triggered by a sAC-dependent rise in cAMP in response to alkaline luminal pH. The apical mobilization of the V-ATPase is also dependent upon carbonic anhydrase (CA), the enzyme responsible for the nearly instantaneous equilibration of pH and HCO3−, presumably facilitating sAC activation by bicarbonate in response to elevated pH.
sAC [76], CA [100], and V-ATPase [101,102] are also instrumental in regulating the recovery from alkalotic challenge in the dogfish shark. In the shark gill, which is the main acid-base sensing organ of this ancient vertebrate, alkalotic stress induces a sAC- and CA-dependent translocation of V-ATPase into the basolateral membrane of the gill. The V-ATPase then pumps protons back into the body to counter the systemic alkalosis. Additionally, sAC forms a complex with the V-ATPase in acid-base transporting intercalated cells in mammalian kidney [103], and sAC, CA and V-ATPase are postulated to mediate proton secretion from acid (A-type) secreting cells into the renal collecting duct [104]. Thus, sAC, CA and V-ATPase seem to form a functional unit for sensing, and responding to, alterations in pH [105]. Interestingly, the sAC-CA-V-ATPase mechanism is capable of moving the proton transporter to wherever it is needed; i.e., the V-ATPase translocates to the apical membranes in clear cells of the epididymis and A-type cells of the renal collecting duct while it moves to the basolateral membrane in the shark gill. Because sAC, CA, and V-ATPase are evolutionarily ancient, it is tempting to hypothesize that this functional unit for sensing pH and moving protons to correct pH imbalances will be found widely utilized throughout biology.
3.3. CO2 Regulation of Beating Frequency of Cilia on Airway Epithelia
Airway epithelial cells express motile cilia that are important for innate host defense; the beat of the cilia removes the mucous layer clearing toxins, pathogens, allergens, and debris [106]. To accomplish this feat, cilia beat faster during exhalation relative to inhalation. Exhaled breath has higher CO2 than inspired air. sAC ‘senses’ this elevated CO2, and sAC-generated cAMP activates PKA which increases the frequency of ciliary beating during exhalation [43]. This represents an example where sAC-generated cAMP acts as a pathway modulator; CO2 chemosensing via sAC controls the rate of ciliary beating, not whether or not the cilia beat.
3.4. Krebs Cycle Generated CO2 Regulates the Rate of Oxidative Phosphorylation
sAC resides inside mitochondria [9,15,107] where it coordinates the rate of ATP production via oxidative phosphorylation (OXPHOS) with nutritional availability. Mitochondrial sAC activity is stimulated by Krebs Cycle-generated CO2 in a carbonic anhydrase dependent manner [15]. CO2/HCO3− stimulation of sAC activates intramitochondrial PKA which phosphorylates Complex IV of the electron transport chain, increasing its rate and capacity to handle electrons. Because the electrons feeding the electron transport chain also originate from the Krebs Cycle, this mitochondrial CO2-sAC-cAMP-PKA pathway couples nutrient utilization (i.e., Krebs Cycle activity) to ATP production. Once again, this pathway does not turn on or off the electron transport chain, it simply modulates the rate of ATP generation to ensure optimal utilization of electrons.
3.5. Physiological Processes Dependent upon CO2/HCO3/pH which May Involve sAC
There are a number of physiological processes where CO2/HCO3/pH chemosensing is known to play a role, but the chemosensor is not yet identified. Some are even thought to employ cAMP as a second messenger, but involvement of sAC has yet been demonstrated. For example, bone resorption by osteoclasts is thought to be mediated via V-ATPase dependent proton pumping [108]. And while sAC seems to regulate growth and differentiation of osteoclasts [109], there is only circumstantial evidence that sAC plays a role in bone formation. Human sAC was identified as a locus for absorptive hypercalciuria (AH), a kidney stone-forming condition frequently complicated by bone loss [110], and polymorphisms in the human sAC locus are associated with phenotypic variations in bone mineral density [111].
Cerebrospinal fluid formation (CSF) by the choroid plexus and aqueous humor formation by ocular ciliary processes are dependent on bicarbonate [112,113]. In ciliary processes and choroid plexus transport systems, carbonic anhydrase inhibitors decrease fluid secretion [114], and carbonic anhydrase inhibitors can be used to treat glaucoma, a fluid secretion defect in the eye. sAC seems to be present in choroid plexus [2] and in ciliary processes [115], but as yet, there have been no functional studies linking sAC to either process.
Partial CO2 pressure (PCO2) is the main determinant of ventilation rate [1]. Elevations of PCO2 increase breathing frequency, while decreased PCO2 slows breathing frequency. These rate changes are mediated by peripheral and central chemoreceptors which monitor changes in arterial PO2 and PCO2 blood gases. The peripheral chemoreceptors are in the carotid and aortic bodies, and their actions have long been thought to be due to alterations in intracellular pH (pHi). However, studies in the chemosensitive (glomus) cells of the carotid body reveal a direct role for CO2, independent of pHi [116]. These studies also demonstrated that elevations in PCO2 elicited an increase in glomus cell cAMP leading the authors to suggest involvement of sAC. PCO2 also plays a role in regulating blood flow. Blood flow is tightly coupled to tissue metabolism [1]; cerebral arterioles dilate in response to increases in metabolic activity, and CO2, protons, and adenosine function as vasodilators by relaxing smooth muscles. Cerebral arterioles are exquisitely sensitive to the vasodilatory action of PCO2; however, the molecular nature of the vascular PCO2 receptor is unknown. Interestingly, cAMP was postulated to be downstream of the CO2 signal [117], but once again, functional studies assessing sAC’s role in sensing circulating PCO2 have not yet been performed.
4. Evolutionary Conservation of Physiological CO2/HCO3/pH Chemosensing via Nucleotidyl Cyclases
4.1. Fungal Adenylyl Cyclases Integrate CO2 Sensing with cAMP Signaling and Virulence
The CO2 concentration in mammals (5%) is more than 150 fold higher than in atmospheric air (0.033%). We identified this difference in CO2 as a physiological signal inducing the yeast-to-hyphal transition essential for virulence of the fungal pathogen, Candida albicans [77]. The C. albicans adenylyl cyclase (AC) is directly stimulated by HCO3, and it is responsible for ‘sensing’ in a carbonic anhydrase dependent manner, the elevated CO2 inside infected hosts. CO2/HCO3 regulation of cAMP synthesis is conserved in other fungi. In the fungal pathogen Cryptococcus neoformans, capsule formation is essential for evading host immune detection. Once again, the signal inducing capsule formation is the higher CO2 concentration inside the infected host, and the C. neoformans cyclase serves as the pathogen’s CO2/HCO3 chemosensor [78].
4.2. CO2 Chemosensing via cGMP Signaling
The nematode Caenorhabditis elegans also senses environmental CO2. In contrast to many parasitic nematodes, the free-living C. elegans avoids CO2 [118,119], and this response is dependent upon expression of the GCY-9 receptor-type guanylyl cyclase (along with cyclic nucleotide gated ion channels) in the CO2 chemosensing (BAG) sensory neurons [120]. Interestingly, C. elegans also avoid high levels (in excess of 12%) of oxygen; this response is mediated by a distinct subset of sensory neurons, but it also involves a receptor-type guanylyl cyclase (GCY-35) and cyclic nucleotide gated channels [121].
The fruit fly Drosophila melanogaster also avoids environmental CO2, and while this response requires two GPCR-like olfactory receptors [122], involvement of a cyclic nucleotide second messenger remains unclear [123]. In mammals, the question of sensing environmental CO2 via cyclic nucleotides also remains unresolved. A particular subset of olfactory neurons in mice seemed to be capable of sensing concentrations of CO2 approaching environmental levels [124]. These neurons express a transmembrane guanylyl cyclase, GC-D, which was subsequently demonstrated to be bicarbonate regulated [82,83]. A second transmembrane guanylyl cyclase, GC-G, which is also found in the olfactory system, has also been demonstrated to be directly modulated by bicarbonate [80]. Sensory detection of environmental CO2 in a number of organisms was recently reviewed in [125]. While these findings cement the linkage between CO2/HCO3/pH chemosensing and cyclic nucleotide signal transduction, their physiological significance remains unknown.
5. Summary and Future Trends
In physiological systems, CO2, HCO3−, and pH are intimately linked via carbonic anhydrases, and a variety of biological processes, in mammals and throughout evolution, depend upon a CO2/HCO3/pH chemosensor. Bicarbonate-regulated sAC, which links intracellular CO2, HCO3−, and/or pH levels with cAMP signal transduction, serves as the CO2/HCO3/pH chemosensor in at least a subset of these processes. The future will reveal whether other CO2/HCO3/pH chemosensing functions are also mediated by sAC. Bicarbonate regulation is observed in other mammalian nucleotidyl cyclases and in adenylyl cyclases across evolution implying that cyclic nucleotide signaling is an evolutionarily conserved mechanism for CO2/HCO3/pH chemosensing.
References
- Johnson, L.R. Essential Medical Physiology, 2nd ed; Lippincott-Raven: Philadelphia, PA, USA, 1998. [Google Scholar]
- Chen, Y.; Cann, M.J.; Litvin, T.N.; Iourgenko, V.; Sinclair, M.L.; Levin, L.R.; Buck, J. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 2000, 289, 625–628. [Google Scholar]
- Robison, G.A.; Butcher, R.W.; Sutherland, E.W. Cyclic AMP. Ann. Rev. Biochem 1968, 37, 149–174. [Google Scholar]
- Berrera, M.; Dodoni, G.; Monterisi, S.; Pertegato, V.; Zamparo, I.; Zaccolo, M. A toolkit for real-time detection of cAMP: Insights into compartmentalized signaling. Handb. Exp. Pharmacol 2008, 186, 186285–186298. [Google Scholar]
- Willoughby, D.; Cooper, D.M. Live-cell imaging of cAMP dynamics. Nat. Methods 2008, 5, 29–36. [Google Scholar]
- Zaccolo, M. cAMP signal transduction in the heart: Understanding spatial control for the development of novel therapeutic strategies. Br. J. Pharmacol 2009, 158, 50–60. [Google Scholar]
- Iyengar, R. Gating by cyclic AMP: Expanded role for an old signaling pathway. Science 1996, 271, 461–463. [Google Scholar]
- Bundey, R.A.; Insel, P.A. Discrete intracellular signaling domains of soluble adenylyl cyclase: Camps of cAMP? Sci. STKE 2004, 2004, pe19. [Google Scholar]
- Zippin, J.H.; Chen, Y.; Nahirney, P.; Kamenetsky, M.; Wuttke, M.S.; Fischman, D.A.; Levin, L.R.; Buck, J. Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. Faseb. J 2003, 17, 82–84. [Google Scholar]
- Zippin, J.H.; Farrell, J.; Huron, D.; Kamenetsky, M.; Hess, K.C.; Fischman, D.A.; Levin, L.R.; Buck, J. Bicarbonate-responsive “soluble” adenylyl cyclase defines a nuclear cAMP microdomain. J. Cell Biol 2004, 164, 527–534. [Google Scholar]
- Terrin, A.; Di Benedetto, G.; Pertegato, V.; Cheung, Y.F.; Baillie, G.; Lynch, M.J.; Elvassore, N.; Prinz, A.; Herberg, F.W.; Houslay, M.D.; Zaccolo, M. PGE(1) stimulation of HEK293 cells generates multiple contiguous domains with different [cAMP]: Role of compartmentalized phosphodiesterases. J. Cell Biol 2006, 175, 441–451. [Google Scholar]
- Gervasi, N.; TchÈnio, P.; Preat, T. PKA dynamics in a drosophila learning center: Coincidence detection by rutabaga adenylyl cyclase and spatial regulation by dunce phosphodiesterase. 2010, 65, 516–529. [Google Scholar]
- Houslay, M.D. Underpinning compartmentalised cAMP signalling through targeted cAMP breakdown. Trends Biochem. Sci 2010, 35, 91–100. [Google Scholar]
- Mongillo, M.; McSorley, T.; Evellin, S.; Sood, A.; Lissandron, V.; Terrin, A.; Huston, E.; Hannawacker, A.; Lohse, M.J.; Pozzan, T.; Houslay, M.D.; Zaccolo, M. Fluorescence resonance energy transfer-based analysis of cAMP dynamics in live neonatal rat cardiac myocytes reveals distinct functions of compartmentalized phosphodiesterases. Circ. Res 2004, 95, 67–75. [Google Scholar]
- Acin-Perez, R.; Salazar, E.; Kamenetsky, M.; Buck, J.; Levin, L.R.; Manfredi, G. Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab 2009, 9, 265–276. [Google Scholar]
- Beene, D.L.; Scott, J.D. A-kinase anchoring proteins take shape. Curr. Opin. Cell Biol 2007, 19, 192–198. [Google Scholar]
- Carnegie, G.K.; Means, C.K.; Scott, J.D. A-kinase anchoring proteins: From protein complexes to physiology and disease. IUBMB Life 2009, 61, 394–406. [Google Scholar]
- Bauman, A.L.; Soughayer, J.; Nguyen, B.T.; Willoughby, D.; Carnegie, G.K.; Wong, W.; Hoshi, N.; Langeberg, L.K.; Cooper, D.M.; Dessauer, C.W.; Scott, J.D. Dynamic regulation of cAMP synthesis through anchored PKA-adenylyl cyclase V/VI complexes. Mol. Cell 2006, 23, 925–931. [Google Scholar]
- Efendiev, R.; Samelson, B.K.; Nguyen, B.T.; Phatarpekar, P.V.; Baameur, F.; Scott, J.D.; Dessauer, C.W. AKAP79 interacts with multiple adenylyl cyclase (AC) isoforms and scaffolds AC5 and -6 to alpha-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate (AMPA) receptors. J. Biol. Chem 2010, 285, 14450–14458. [Google Scholar]
- Piggott, L.A.; Bauman, A.L.; Scott, J.D.; Dessauer, C.W. The A-kinase anchoring protein Yotiao binds and regulates adenylyl cyclase in brain. Proc. Natl. Acad. Sci. USA 2008, 105, 13835–13840. [Google Scholar]
- Dodge, K.L.; Khouangsathiene, S.; Kapiloff, M.S.; Mouton, R.; Hill, E.V.; Houslay, M.D.; Langeberg, L.K.; Scott, J.D. mAKAP assembles a protein kinase A/PDE4 phosphodiesterase cAMP signaling module. Embo J 2001, 20, 1921–1930. [Google Scholar]
- Tasken, K.A.; Collas, P.; Kemmner, W.A.; Witczak, O.; Conti, M.; Tasken, K. Phosphodiesterase 4D and protein kinase a type II constitute a signaling unit in the centrosomal area. J. Biol. Chem 2001, 276, 21999–22002. [Google Scholar]
- Baillie, G.S.; Houslay, M.D. Arrestin times for compartmentalised cAMP signalling and phosphodiesterase-4 enzymes. Curr. Opin. Cell Biol 2005, 17, 129–134. [Google Scholar]
- Bajpai, M.; Fiedler, S.E.; Huang, Z.; Vijayaraghavan, S.; Olson, G.E.; Livera, G.; Conti, M.; Carr, D.W. AKAP3 selectively binds PDE4A isoforms in bovine spermatozoa. Biol. Reprod 2006, 74, 109–118. [Google Scholar]
- McSorley, T.; Stefan, E.; Henn, V.; Wiesner, B.; Baillie, G.S.; Houslay, M.D.; Rosenthal, W.; Klussmann, E. Spatial organisation of AKAP18 and PDE4 isoforms in renal collecting duct principal cells. Eur. J. Cell Biol 2006, 85, 673–678. [Google Scholar]
- Willoughby, D.; Wong, W.; Schaack, J.; Scott, J.D.; Cooper, D.M. An anchored PKA and PDE4 complex regulates subplasmalemmal cAMP dynamics. Embo J 2006, 25, 2051–2061. [Google Scholar]
- Paulucci-Holthauzen, A.A.; Vergara, L.A.; Bellot, L.J.; Canton, D.; Scott, J.D.; O’Connor, K.L. Spatial distribution of protein kinase A activity during cell migration is mediated by A-kinase anchoring protein AKAP Lbc. J. Biol. Chem 2009, 284, 5956–5967. [Google Scholar]
- Raymond, D.R.; Carter, R.L.; Ward, C.A.; Maurice, D.H. Distinct phosphodiesterase-4D variants integrate into protein kinase A-based signaling complexes in cardiac and vascular myocytes. Am. J. Physiol. Heart Circ. Physiol 2009, 296, 263–271. [Google Scholar]
- Flores, J.; Witkum, P.A.; Beckman, B.; Sharp, G.W. Stimulation of osmotic water flow in toad bladder by prostaglandin E1. Evidence for different compartments of cyclic AMP. J. Clin. Invest 1975, 56, 256–262. [Google Scholar]
- Buxton, I.L.; Brunton, L.L. Compartments of cyclic AMP and protein kinase in mammalian cardiomyocytes. J. Biol. Chem 1983, 258, 10233–10239. [Google Scholar]
- DiPilato, L.M.; Cheng, X.; Zhang, J. Fluorescent indicators of cAMP and Epac activation reveal differential dynamics of cAMP signaling within discrete subcellular compartments. Proc. Natl. Acad. Sci. USA 2004, 101, 16513–16518. [Google Scholar]
- Ponsioen, B.; Zhao, J.; Riedl, J.; Zwartkruis, F.; van der Krogt, G.; Zaccolo, M.; Moolenaar, W.H.; Bos, J.L.; Jalink, K. Detecting cAMP-induced Epac activation by fluorescence resonance energy transfer: Epac as a novel cAMP indicator. Embo Rep 2004, 5, 1176–1180. [Google Scholar]
- Zaccolo, M.; Cesetti, T.; Di Benedetto, G.; Mongillo, M.; Lissandron, V.; Terrin, A.; Zamparo, I. Imaging the cAMP-dependent signal transduction pathway. Biochem. Soc. Trans 2005, 33, 1323–1326. [Google Scholar]
- Karpen, J.W.; Rich, T.C. High-resolution measurements of cyclic adenosine monophosphate signals in 3D microdomains. Meth. Mol. Biol 2005, 307, 15–26. [Google Scholar]
- Rich, T.C.; Fagan, K.A.; Nakata, H.; Schaack, J.; Cooper, D.M.; Karpen, J.W. Cyclic nucleotide-gated channels colocalize with adenylyl cyclase in regions of restricted cAMP diffusion. J. Gen. Physiol 2000, 116, 147–161. [Google Scholar]
- Davare, M.A.; Avdonin, V.; Hall, D.D.; Peden, E.M.; Burette, A.; Weinberg, R.J.; Horne, M.C.; Hoshi, T.; Hell, J.W. A beta2 adrenergic receptor signaling complex assembled with the Ca2+ channel Cav1.2. Science 2001, 293, 98–101. [Google Scholar]
- Marx, S.O.; Kurokawa, J.; Reiken, S.; Motoike, H.; D’Armiento, J.; Marks, A.R.; Kass, R.S. Requirement of a macromolecular signaling complex for beta adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science 2002, 295, 496–499. [Google Scholar]
- Creighton, J.; Zhu, B.; Alexeyev, M.; Stevens, T. Spectrin-anchored phosphodiesterase 4D4 restricts cAMP from disrupting microtubules and inducing endothelial cell gap formation. J. Cell Sci 2008, 121, 110–119. [Google Scholar]
- Sayner, S.; Stevens, T. Soluble adenylate cyclase reveals the significance of compartmentalized cAMP on endothelial cell barrier function. Biochem. Soc. Trans 2006, 34, 492–494. [Google Scholar]
- Sayner, S.L.; Alexeyev, M.; Dessauer, C.W.; Stevens, T. Soluble adenylyl cyclase reveals the significance of cAMP compartmentation on pulmonary microvascular endothelial cell barrier. Circ. Res 2006, 98, 675–681. [Google Scholar]
- Shelly, M.; Lim, B.K.; Cancedda, L.; Heilshorn, S.C.; Gao, H.; Poo, M.M. Local and long-range reciprocal regulation of cAMP and cGMP in axon/dendrite formation. Science 2010, 327, 547–552. [Google Scholar]
- Acin-Perez, R.; Salazar, E.; Brosel, S.; Yang, H.; Schon, E.A.; Manfredi, G. Modulation of mitochondrial protein phosphorylation by soluble adenylyl cyclase ameliorates cytochrome oxidase defects. Embo Mol. Med 2009, 1, 392–406. [Google Scholar]
- Schmid, A.; Sutto, Z.; Nlend, M.C.; Horvath, G.; Schmid, N.; Buck, J.; Levin, L.R.; Conner, G.E.; Fregien, N.; Salathe, M. Soluble adenylyl cyclase is localized to cilia and contributes to ciliary beat frequency regulation via production of cAMP. J. Gen. Physiol 2007, 130, 99–109. [Google Scholar]
- Hess, K.C.; Jones, B.H.; Marquez, B.; Chen, Y.; Ord, T.S.; Kamenetsky, M.; Miyamoto, C.; Zippin, J.H.; Kopf, G.S.; Suarez, S.S.; Levin, L.R.; Williams, C.J.; Buck, J.; Moss, S.B. The “soluble” adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev. Cell 2005, 9, 249–259. [Google Scholar]
- Xie, F.; Garcia, M.A.; Carlson, A.E.; Schuh, S.M.; Babcock, D.F.; Jaiswal, B.S.; Gossen, J.A.; Esposito, G.; van Duin, M.; Conti, M. Soluble adenylyl cyclase (sAC) is indispensable for sperm function and fertilization. Dev. Biol 2006, 296, 353–362. [Google Scholar]
- Carlson, A.E.; Hille, B.; Babcock, D.F. External Ca2+ acts upstream of adenylyl cyclase SACY in the bicarbonate signaled activation of sperm motility. Dev. Biol 2007, 312, 183–192. [Google Scholar]
- Esposito, G.; Jaiswal, B.S.; Xie, F.; Krajnc-Franken, M.A.; Robben, T.J.; Strik, A.M.; Kuil, C.; Philipsen, R.L.; Van Duin, M.; Conti, M.; Gossen, J.A. Mice deficient for soluble adenylyl cyclase are infertile because of a severe sperm-motility defect. Proc. Natl. Acad. Sci. USA 2004, 101, 2993–2998. [Google Scholar]
- Chen, J.; Iyengar, R. Suppression of Ras-induced transformation of NIH 3T3 cells by activated G alphas. Science 1994, 263, 1278–1281. [Google Scholar]
- Fan, C.M.; Porter, J.A.; Chiang, C.; Chang, D.T.; Beachy, P.A.; Tessier-Lavigne, M. Long-range sclerotome induction by sonic hedgehog: Direct role of the amino-terminal cleavage product and modulation by the cyclic AMP signaling pathway. Cell 1995, 81, 457–465. [Google Scholar]
- Jiang, J.; Struhl, G. Protein kinase A and hedgehog signaling in Drosophila limb development. Cell 1995, 80, 563–572. [Google Scholar]
- Li, W.; Ohlmeyer, J.T.; Lane, M.E.; Kalderon, D. Function of protein kinase A in hedgehog signal transduction and Drosophila imaginal disc development. Cell 1995, 80, 553–562. [Google Scholar]
- Blitzer, R.D.; Wong, T.; Nouranifar, R.; Iyengar, R.; Landau, E.M. Postsynaptic cAMP pathway gates early LTP in hippocampal CA1 region. Neuron 1995, 15, 1403–1414. [Google Scholar]
- Meyer-Franke, A.; Kaplan, M.R.; Pfrieger, F.W.; Barres, B.A. Characterization of the signaling interactions that promote the survival and growth of developing retinal ganglion cells in culture. Neuron 1995, 15, 805–819. [Google Scholar]
- Choi, H.B.; Gordon, G.R.J.; Zhou, N.; Tai, C.; Ryu, J.K.; McLarnon, J.G.; Levin, L.R.; Buck, J.; MacVIcar, B.A. Metabolic communication between astrocytes and neurons via bicarbonate responsive soluble adenylyl cyclase. Unpublished work..
- Ramos, L.S.; Zippin, J.H.; Kamenetsky, M.; Buck, J.; Levin, L.R. Glucose and GLP-1 stimulate cAMP production via distinct adenylyl cyclases in INS-1E insulinoma cells. J. Gen. Physiol 2008, 132, 329–338. [Google Scholar]
- Stessin, A.M.; Zippin, J.H.; Kamenetsky, M.; Hess, K.C.; Buck, J.; Levin, L.R. Soluble adenylyl cyclase mediates nerve growth factor-induced activation of Rap1. J. Biol. Chem 2006, 281, 17253–17258. [Google Scholar]
- Wu, K.Y.; Zippin, J.H.; Huron, D.R.; Kamenetsky, M.; Hengst, U.; Buck, J.; Levin, L.R.; Jaffrey, S.R. Soluble adenylyl cyclase is required for netrin-1 signaling in nerve growth cones. Nat. Neurosci 2006, 9, 1257–1264. [Google Scholar]
- Tresguerres, M.; Buck, J.; Levin, L.R. Physiological carbon dioxide, bicarbonate, and pH sensing. Pflugers Arch 2010, 460, 953–964. [Google Scholar]
- Buck, J.; Sinclair, M.L.; Schapal, L.; Cann, M.J.; Levin, L.R. Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc. Natl. Acad. Sci. USA 1999, 96, 79–84. [Google Scholar]
- Kamenetsky, M.; Middelhaufe, S.; Bank, E.M.; Levin, L.R.; Buck, J.; Steegborn, C. Molecular details of cAMP generation in Mammalian cells: A tale of two systems. J. Mol. Biol 2006, 362, 623–639. [Google Scholar]
- Geng, W.; Wang, Z.; Zhang, J.; Reed, B.Y.; Pak, C.Y.; Moe, O.W. Cloning and characterization of the human soluble adenylyl cyclase. Am. J. Physiol. Cell Physiol 2005, 288, 1305–1316. [Google Scholar]
- Jaiswal, B.S.; Conti, M. Identification and functional analysis of splice variants of the germ cell soluble adenylyl cyclase. J. Biol. Chem 2001, 276, 31698–31708. [Google Scholar]
- Farrell, J.; Ramos, L.; Tresguerres, M.; Kamenetsky, M.; Levin, L.R.; Buck, J. Somatic “soluble” adenylyl cyclase isoforms are unaffected in Sacy tm1Lex/Sacy tm1Lex “knockout” mice. Plos One 2008, 3, e3251. [Google Scholar]
- Garbers, D.L.; Tubb, D.J.; Hyne, R.V. A requirement of bicarbonate for Ca2+-induced elevations of cyclic AMP in guinea pig spermatozoa. J. Biol. Chem 1982, 257, 8980–8984. [Google Scholar]
- Garty, N.B.; Salomon, Y. Stimulation of partially purified adenylate cyclase from bull sperm by bicarbonate. FEBS Lett 1987, 218, 148–152. [Google Scholar]
- Okamura, N.; Tajima, Y.; Soejima, A.; Masuda, H.; Sugita, Y. Sodium bicarbonate in seminal plasma stimulates the motility of mammalian spermatozoa through direct activation of adenylate cyclase. J. Biol. Chem 1985, 260, 9699–9705. [Google Scholar]
- Visconti, P.E.; Muschietti, J.P.; Flawia, M.M.; Tezon, J.G. Bicarbonate dependence of cAMP accumulation induced by phorbol esters in hamster spermatozoa. Biochim. Biophy. Acta 1990, 1054, 231–236. [Google Scholar]
- Jaiswal, B.S.; Conti, M. Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc. Natl. Acad. Sci. USA 2003, 100, 10676–10681. [Google Scholar]
- Litvin, T.N.; Kamenetsky, M.; Zarifyan, A.; Buck, J.; Levin, L.R. Kinetic properties of “soluble” adenylyl cyclase. Synergism between calcium and bicarbonate. J. Biol. Chem 2003, 278, 15922–15926. [Google Scholar]
- Steegborn, C.; Litvin, T.N.; Levin, L.R.; Buck, J.; Wu, H. Bicarbonate activation of adenylyl cyclase via promotion of catalytic active site closure and metal recruitment. Nat. Struct. Mol. Biol 2005, 12, 32–37. [Google Scholar]
- Tesmer, J.J.; Sunahara, R.K.; Gilman, A.G.; Sprang, S.R. Crystal structure of the catalytic domains of adenylyl cyclase in a complex with Gsalpha.GTP gamma S. Science 1997, 278, 1907–1916. [Google Scholar]
- Linder, J.U.; Schultz, J.E. The class III adenylyl cyclases: Multi-purpose signalling modules. Cell. Signal 2003, 15, 1081–1089. [Google Scholar]
- Cann, M.J.; Hammer, A.; Zhou, J.; Kanacher, T. A defined subset of adenylyl cyclases is regulated by bicarbonate ion. J. Biol. Chem 2003, 278, 35033–35038. [Google Scholar]
- Kobayashi, M.; Buck, J.; Levin, L.R. Conservation of functional domain structure in bicarbonate-regulated “soluble” adenylyl cyclases in bacteria and eukaryotes. Dev. Genes Evol 2004, 214, 503–509. [Google Scholar]
- Tresguerres, M.; Levin, L.R.; Buck, J.; Grosell, M. Modulation of NaCl absorption by [HCO3–] in the marine teleost intestine is mediated by soluble adenylyl cyclase. Amer. J. Physiol.-Regul. Integr. C 2010, 299, R62–R71. [Google Scholar]
- Tresguerres, M.; Parks, S.K.; Salazar, E.; Levin, L.R.; Goss, G.G.; Buck, J. Bicarbonate-sensing soluble adenylyl cyclase is an essential sensor for acid/base homeostasis. Proc. Natl. Acad. Sci. USA 2010, 107, 442–447. [Google Scholar]
- Klengel, T.; Liang, W.J.; Chaloupka, J.; Ruoff, C.; Schroppel, K.; Naglik, J.R.; Eckert, S.E.; Mogensen, E.G.; Haynes, K.; Tuite, M.F.; Levin, L.R.; Buck, J.; Muhlschlegel, F.A. Fungal adenylyl cyclase integrates CO(2) sensing with cAMP signaling and virulence. Curr. Biol 2005, 15, 2021–2026. [Google Scholar]
- Mogensen, E.G.; Janbon, G.; Chaloupka, J.; Steegborn, C.; Fu, M.S.; Moyrand, F.; Klengel, T.; Pearson, D.S.; Geeves, M.A.; Buck, J.; Levin, L.R.; Muhlschlegel, F.A. Cryptococcus neoformans senses CO2 through the carbonic anhydrase Can2 and the adenylyl cyclase Cac1. Eukaryot. Cell 2006, 5, 103–111. [Google Scholar]
- Hall, R.A.; De Sordi, L.; Maccallum, D.M.; Topal, H.; Eaton, R.; Bloor, J.W.; Robinson, G.K.; Levin, L.R.; Buck, J.; Wang, Y.; Gow, N.A.; Steegborn, C.; Muhlschlegel, F.A. CO(2) acts as a signalling molecule in populations of the fungal pathogen Candida albicans. Plos Pathog 2010, 6, e1001193. [Google Scholar]
- Chao, Y.C.; Cheng, C.J.; Hsieh, H.T.; Lin, C.C.; Chen, C.C.; Yang, R.B. Guanylate cyclase-G, expressed in the Grueneberg ganglion olfactory subsystem, is activated by bicarbonate. Biochem. J 2010, 432, 267–273. [Google Scholar]
- Duda, T.; Sharma, R.K. Distinct ONE-GC transduction modes and motifs of the odorants: Uroguanylin and CO(2). Biochem. Biophys. Res. Commun 2010, 391, 1379–1384. [Google Scholar]
- Guo, D.; Zhang, J.J.; Huang, X.Y. Stimulation of guanylyl cyclase-D by bicarbonate. Biochemistry 2009, 48, 4417–4422. [Google Scholar]
- Sun, L.; Wang, H.; Hu, J.; Han, J.; Matsunami, H.; Luo, M. Guanylyl cyclase-D in the olfactory CO2 neurons is activated by bicarbonate. Proc. Natl. Acad. Sci. USA 2009, 106, 2041–2046. [Google Scholar]
- Tresguerres, M.; Levin, L.R.; Buck, J. Intracellular cAMP signaling by soluble adenylyl cyclase. Kidney Int. in press..
- Yanagimachi, R. Mammalian fertilization. In The Physiology of Reproduction; Knobil, E., Neill, J.D., Eds.; Raven Press, Ltd.: New York, NY, USA, 1994; pp. 189–317. [Google Scholar]
- Boatman, D.F.; Robbins, R.S. Bicarbonate: Carbon-dioxide regulation of sperm capacitation, hyperactivated motility, and acrosome reactions. Biol. Reprod 1991, 44, 806–813. [Google Scholar]
- Lee, M.A.; Storey, B.T. Bicarbonate is essential for ferilization of mouse eggs: Mouse sperm require it to undergo the acrosome reaction. Biol. Reprod 1986, 34, 349–356. [Google Scholar]
- Neill, J.M.; Olds-Clarke, P. A computer-assisted assay for mouse sperm hyperactivation demonstrates that bicarbonate but not bovine serum albumin is required. Gamete Res 1987, 18, 121–140. [Google Scholar]
- Visconti, P.E.; Galantino-Homer, H.; Moore, G.D.; Bailey, J.L.; Ning, X.; Fornes, M.; Kopf, G.S. The molecular basis of sperm capacitation. J. Androl 1998, 19, 242–248. [Google Scholar]
- Tash, J.S.; Means, A.R. Cyclic adenosine 3′,5′ monophosphate, calcium and protein phosphorylation in flagellar motility. Biol. Reprod 1983, 28, 75–104. [Google Scholar]
- Visconti, P.E.; Moore, G.D.; Bailey, J.L.; Leclerc, P.; Connors, S.A.; Pan, D.; Olds-Clarke, P.; Kopf, G.S. Capacitation of mouse spermatozoa. II. Protein tyrosine phosphorylation and capacitation are regulated by a cAMP-dependent pathway. Development 1995, 121, 1139–1150. [Google Scholar]
- Fraser, L.R.; Monks, N.J. Cyclic nucleotides and mammalian sperm capacitation. J. Reprod. Fertil. Suppl 1990, 42, 9–21. [Google Scholar]
- Sinclair, M.L.; Wang, X.Y.; Mattia, M.; Conti, M.; Buck, J.; Wolgemuth, D.J.; Levin, L.R. Specific expression of soluble adenylyl cyclase in male germ cells. Mol. Reprod. Dev 2000, 56, 6–11. [Google Scholar]
- Chaloupka, J.A.; Bullock, S.A.; Iourgenko, V.; Levin, L.R.; Buck, J. Autoinhibitory regulation of soluble adenylyl cyclase. Mol. Reprod. Dev 2006, 73, 361–368. [Google Scholar]
- Wang, D.; Hu, J.; Bobulescu, I.A.; Quill, T.A.; McLeroy, P.; Moe, O.W.; Garbers, D.L. A sperm-specific Na+/H+ exchanger (sNHE) is critical for expression and in vivo bicarbonate regulation of the soluble adenylyl cyclase (sAC). Proc. Natl. Acad. Sci. USA 2007, 104, 9325–9330. [Google Scholar]
- Xie, F.; Conti, M. Expression of the soluble adenylyl cyclase during rat spermatogenesis: Evidence for cytoplasmic sites of cAMP production in germ cells. Dev. Biol 2004, 265, 196–206. [Google Scholar]
- Schuh, S.M.; Carlson, A.E.; McKnight, G.S.; Conti, M.; Hille, B.; Babcock, D.F. Signaling pathways for modulation of mouse sperm motility by adenosine and catecholamine agonists. Biol. Reprod 2006, 74, 492–500. [Google Scholar]
- Levine, N.; Marsh, D.J. Micropuncture studies of the electrochemical aspects of fluid and electrolyte transport in individual seminiferous tubules, the epididymis, and the vas deferens in rats. J. Physiol 1971, 213, 557–570. [Google Scholar]
- Pastor-Soler, N.; Beaulieu, V.; Litvin, T.N.; Da Silva, N.; Chen, Y.; Brown, D.; Buck, J.; Levin, L.R.; Breton, S. Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J. Biol. Chem 2003, 278, 49523–49529. [Google Scholar]
- Tresguerres, M.; Parks, S.K.; Wood, C.M.; Goss, G.G. V-H+-ATPase translocation during blood alkalosis in dogfish gills: Interaction with carbonic anhydrase and involvement in the postfeeding alkaline tide. Am. J. Physiol 2007, 292, 2012–2019. [Google Scholar]
- Tresguerres, M.; Katoh, F.; Fenton, H.; Jasinska, E.; Goss, G.G. Regulation of branchial V-H(+)-ATPase, Na(+)/K(+)-ATPase and NHE2 in response to acid and base infusions in the Pacific spiny dogfish (Squalus acanthias). J. Exp. Biol 2005, 208, 345–354. [Google Scholar]
- Tresguerres, M.; Parks, S.K.; Katoh, F.; Goss, G.G. Microtubule-dependent relocation of branchial V-H+-ATPase to the basolateral membrane in the Pacific spiny dogfish (Squalus acanthias): A role in base secretion. J. Exp. Biol 2006, 209, 599–609. [Google Scholar]
- Paunescu, T.G.; Da Silva, N.; Russo, L.M.; McKee, M.; Lu, H.A.; Breton, S.; Brown, D. Association of soluble adenylyl cyclase with the V-ATPase in renal epithelial cells. Am. J. Physiol. Renal Physiol 2008, 294, 130–138. [Google Scholar]
- Paunescu, T.G.; Ljubojevic, M.; Russo, L.M.; Winter, C.; McLaughlin, M.M.; Wagner, C.A.; Breton, S.; Brown, D. cAMP stimulates apical V-ATPase accumulation, microvillar elongation, and proton extrusion in kidney collecting duct A-intercalated cells. Am. J. Physiol. Renal Physiol 2010, 298, 643–654. [Google Scholar]
- Brown, D.; Paunescu, T.G.; Breton, S.; Marshansky, V. Regulation of the V-ATPase in kidney epithelial cells: Dual role in acid-base homeostasis and vesicle trafficking. J. Exp. Biol 2009, 212, 1762–1772. [Google Scholar]
- Gudis, D.A.; Cohen, N.A. Cilia dysfunction. Otolaryngol. Clin. North Am 2010, 43, 461–472. [Google Scholar]
- Kumar, S.; Kostin, S.; Flacke, J.P.; Reusch, H.P.; Ladilov, Y. Soluble adenylyl cyclase controls mitochondria-dependent apoptosis in coronary endothelial cells. J. Biol. Chem 2009, 284, 14760–14768. [Google Scholar]
- Breton, S.; Brown, D. New insights into the regulation of V-ATPase-dependent proton secretion. Am. J. Physiol. Renal Physiol 2007, 292, 1–10. [Google Scholar]
- Geng, W.; Hill, K.; Zerwekh, J.E.; Kohler, T.; Muller, R.; Moe, O.W. Inhibition of osteoclast formation and function by bicarbonate: Role of soluble adenylyl cyclase. J. Cell. Physiol 2009, 220, 332–340. [Google Scholar]
- Reed, B.Y.; Gitomer, W.L.; Heller, H.J.; Hsu, M.C.; Lemke, M.; Padalino, P.; Pak, C.Y. Identification and characterization of a gene with base substitutions associated with the absorptive hypercalciuria phenotype and low spinal bone density. J. Clin. Endocrinol. Metab 2002, 87, 1476–1485. [Google Scholar]
- Ichikawa, S.; Koller, D.L.; Curry, L.R.; Lai, D.; Xuei, X.; Edenberg, H.J.; Hui, S.L.; Peacock, M.; Foroud, T.; Econs, M.J. Association of adenylate cyclase 10 (ADCY10) polymorphisms and bone mineral density in healthy adults. Calcif. Tissue Int 2009, 84, 97–102. [Google Scholar]
- Kishida, K.; Sasabe, T.; Iizuka, S.; Manabe, R.; Otori, T. Sodium and chloride transport across the isolated rabbit ciliary body. Curr. Eye Res 1982, 2, 149–152. [Google Scholar]
- Maren, T.H. Bicarbonate formation in cerebrospinal fluid: Role in sodium transport and pH regulation. Am. J. Physiol 1972, 222, 885–899. [Google Scholar]
- Maren, T.H. The kinetics of HCO3− synthesis related to fluid secretion, pH control, and CO2 elimination. Annu. Rev. Physiol 1988, 50, 695–717. [Google Scholar]
- Mittag, T.W.; Guo, W.B.; Kobayashi, K. Bicarbonate-activated adenylyl cyclase in fluid-transporting tissues. Am. J. Physiol 1993, 264, 1060–1064. [Google Scholar]
- Summers, B.A.; Overholt, J.L.; Prabhakar, N.R. CO(2) and pH independently modulate L-type Ca(2+) current in rabbit carotid body glomus cells. J. Neurophysiol 2002, 88, 604–612. [Google Scholar]
- Pelligrino, D.A.; Wang, Q. Cyclic nucleotide crosstalk and the regulation of cerebral vasodilation. Prog. Neurobiol 1998, 56, 1–18. [Google Scholar]
- Bretscher, A.J.; Busch, K.E.; de Bono, M. A carbon dioxide avoidance behavior is integrated with responses to ambient oxygen and food in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2008, 105, 8044–8049. [Google Scholar]
- Hallem, E.A.; Sternberg, P.W. Acute carbon dioxide avoidance in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2008, 105, 8038–8043. [Google Scholar]
- Hallem, E.A.; Spencer, W.C.; McWhirter, R.D.; Zeller, G.; Henz, S.R.; Ratsch, G.; Miller, D.M., III; Horvitz, H.R.; Sternberg, P.W.; Ringstad, N. Receptor-type guanylate cyclase is required for carbon dioxide sensation by Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2011, 108, 254–259. [Google Scholar]
- Gray, J.M.; Karow, D.S.; Lu, H.; Chang, A.J.; Chang, J.S.; Ellis, R.E.; Marletta, M.A.; Bargmann, C.I. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature 2004, 430, 317–322. [Google Scholar]
- Jones, W.D.; Cayirlioglu, P.; Kadow, I.G.; Vosshall, L.B. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature 2007, 445, 86–90. [Google Scholar]
- Kaupp, U.B. Olfactory signalling in vertebrates and insects: Differences and commonalities. Nat. Rev. Neurosci 2010, 11, 188–200. [Google Scholar]
- Hu, J.; Zhong, C.; Ding, C.; Chi, Q.; Walz, A.; Mombaerts, P.; Matsunami, H.; Luo, M. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science 2007, 317, 953–957. [Google Scholar]
- Scott, K. Out of thin air: Sensory detection of oxygen and carbon dioxide. Neuron 2011, 69, 194–202. [Google Scholar]
| sAC | tmACs | |
|---|---|---|
| Evolutionary relatedness | (Cyano)bacteria | ‘First’ Appearance: Dictyostelium |
| Isoform variability | One gene with multiple splice variants and an alternative start site | Nine distinct genes |
| Tissue distribution | Ubiquitous | Ubiquitous |
| Subcellular localization | Cytoplasm, nucleus, mitochondria, centrioles, mitotic spindle, mid-body | Plasma membrane |
| Physiological Modulators | Bicarbonate, calcium, & ATP | G proteins & other 2nd messengers |
| Functions | HCO3− sensing in sperm pH sensing in acid/base sensing epithelia CO2 sensing in airway cilia and mitochondria | Intercellular signaling (i.e., hormones, neurotransmitters, odorants) |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Buck, J.; Levin, L.R. Physiological Sensing of Carbon Dioxide/Bicarbonate/pH via Cyclic Nucleotide Signaling. Sensors 2011, 11, 2112-2128. https://doi.org/10.3390/s110202112
Buck J, Levin LR. Physiological Sensing of Carbon Dioxide/Bicarbonate/pH via Cyclic Nucleotide Signaling. Sensors. 2011; 11(2):2112-2128. https://doi.org/10.3390/s110202112
Chicago/Turabian StyleBuck, Jochen, and Lonny R. Levin. 2011. "Physiological Sensing of Carbon Dioxide/Bicarbonate/pH via Cyclic Nucleotide Signaling" Sensors 11, no. 2: 2112-2128. https://doi.org/10.3390/s110202112
APA StyleBuck, J., & Levin, L. R. (2011). Physiological Sensing of Carbon Dioxide/Bicarbonate/pH via Cyclic Nucleotide Signaling. Sensors, 11(2), 2112-2128. https://doi.org/10.3390/s110202112




