The Structure of Ca2+ Sensor Case16 Reveals the Mechanism of Reaction to Low Ca2+ Concentrations
Abstract
:1. Introduction
2. Experimental Section
2.1. Cloning and Protein Purification
2.2. Crystallization Conditions
2.3. Data Collection and Analysis
2.4. Structure Determination and Refinement
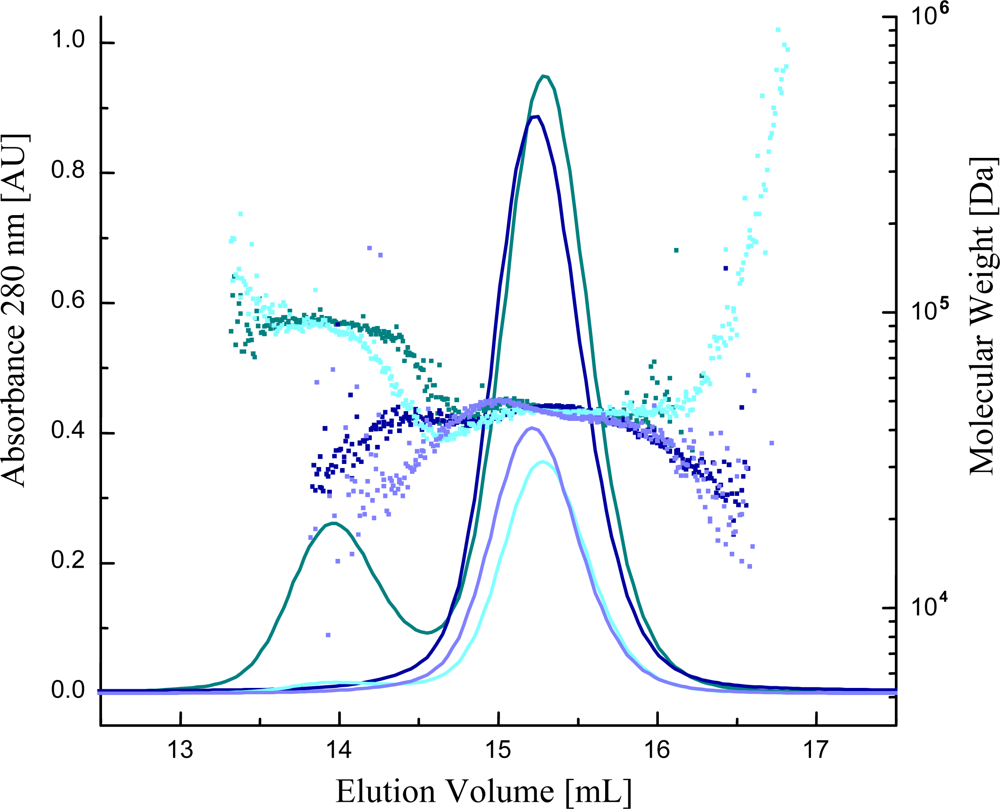
2.5. Characterization of the Oligomerization State of Ca2+ Sensors with Multi Angle Light Scattering (MALS)
2.6. Testing the Sensitivity of Case12 and Case16 Sensors to Free CaM or M13-peptide
3. Results and Discussion
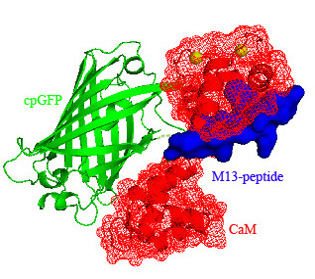
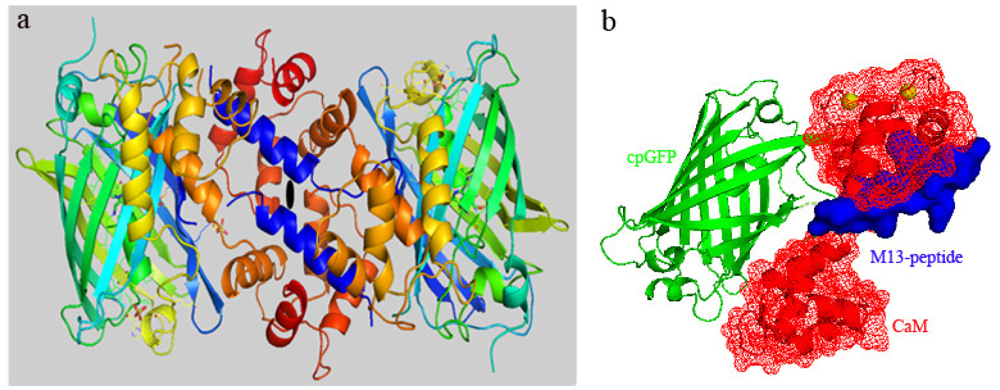
3.1. Structure of Case16 Sensor Freezed in Its “half Ca2+-bound” State
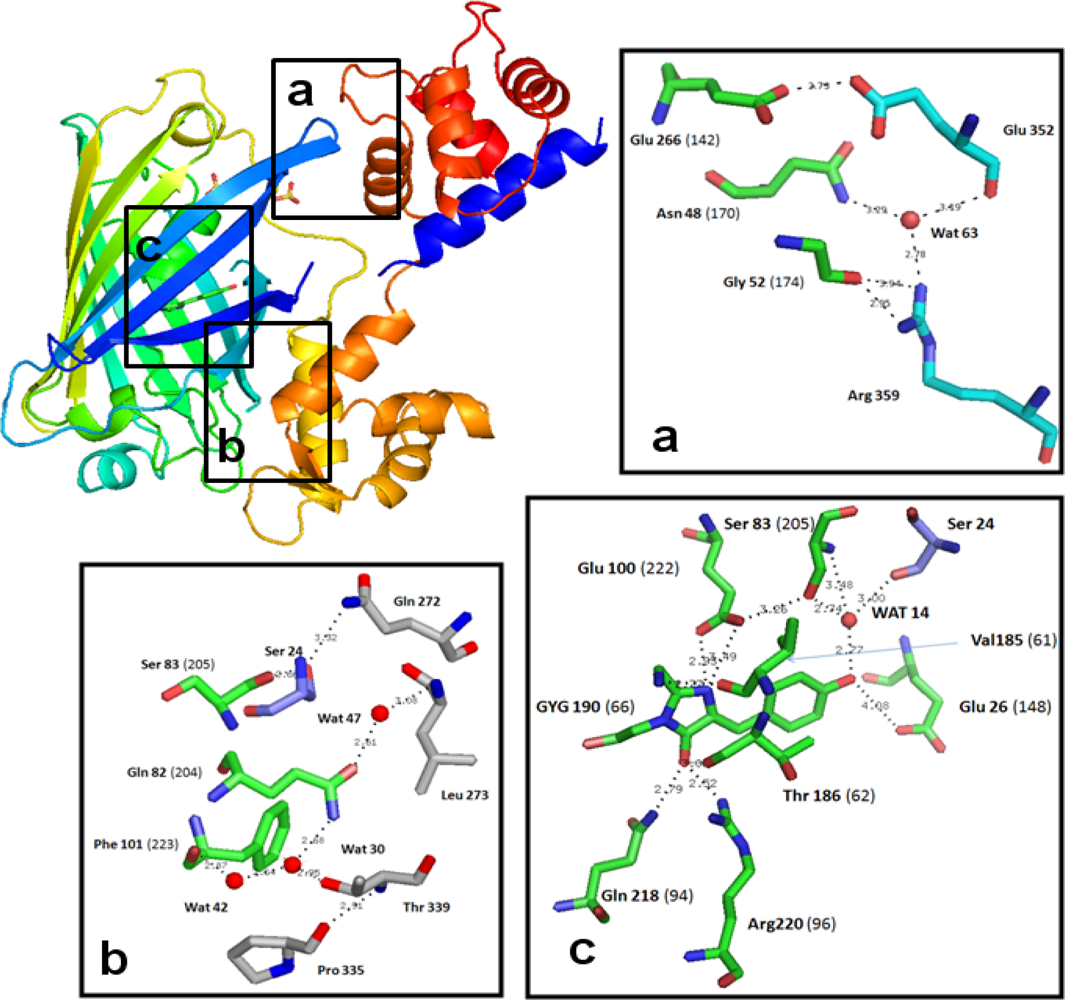
3.2. Chromophore Environment in Case16 Structure A
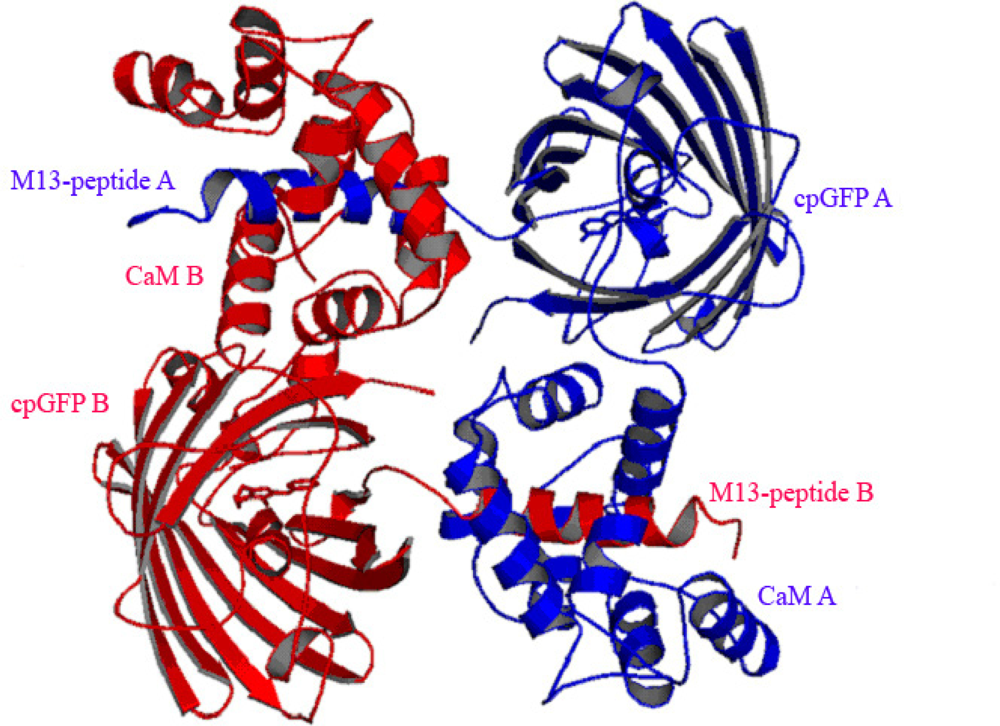
3.3. At high Concentrations Ca2+ Sensors Can form Non-Functional Homodimers in Presence of Saturating Ca2+ Concentrations
3.4. The Dependence of Case12 and Case16 Fluorescent Response on High Concentrations of Free CaM or M13-Peptide
4. Conclusions
Supplementary Data
Supplementary Figure 1.
Acknowledgments
References
- Miyawaki, A; Llopis, J; Heim, R; McCaffery, JM; Adams, JA; Ikura, M; Tsien, RY. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 1997, 388, 882–887. [Google Scholar]
- Baird, GS; Zacharias, DA; Tsien, RY. Circular permutation and receptor insertion within green fluorescent proteins. Proc. Natl. Acad. Sci. USA 1999, 96, 11241–11246. [Google Scholar]
- Nagai, T; Yamada, S; Tominaga, T; Ichikawa, M; Miyawaki, A. Expanded dynamic range of fluorescent indicators for Ca2+ by circularly permuted yellow fluorescent proteins. Proc. Natl. Acad. Sci. USA 2004, 101, 10554–10559. [Google Scholar]
- Nakai, J; Ohkura, M; Imoto, K. A high signal-to-noise Ca2+ probe composed of a single green fluorescent protein. Nat. Biotechnol 2001, 19, 137–141. [Google Scholar]
- Ohkura, M; Matsuzaki, M; Kasai, H; Imoto, K; Nakai, J. Genetically encoded bright Ca2+ probe applicable for dynamic Ca2+ imaging of dendritic spines. Analyt. Chem 2005, 77, 5861–5869. [Google Scholar]
- Tallini, YN; Ohkura, M; Choi, BR; Ji, G; Imoto, K; Doran, R; Lee, J; Plan, P; Wilson, J; Xin, HB; Sanbe, A; Gulick, J; Mathai, J; Robbins, J; Salama, G; Nakai, J; Kotlikoff, MI. Imaging cellular signals in the heart in vivo: Cardiac expression of the high-signal Ca2+ indicator GCaMP2. Proc. Natl. Acad. Sci. USA 2006, 103, 4753–4758. [Google Scholar]
- Souslova, EA; Belousov, VV; Lock, JG; Strömblad, S; Kasparov, S; Bolshakov, AP; Pinelis, VG; Labas, YA; Lukyanov, S; Mayr, LM; Chudakov, DM. Single fluorescent protein-based Ca2+ sensors with increased dynamic range. BMC Biotechnol 2007, e7–37. [Google Scholar]
- Tian, L; Hires, SA; Mao, T; Huber, D; Chiappe, ME; Chalasani, SH; Petreanu, L; Akerboom, J; McKinney, SA; Schreiter, ER; Bargmann, CI; Jayaraman, V; Svoboda, K; Looger, LL. Imaging neural activity in worms, flies and mice with improved GCaMP calcium indicators. Nat. Methods 2009, 6, 875–881. [Google Scholar]
- Heim, N; Griesbeck, O. Genetically encoded indicators of cellular calcium dynamics based on troponin C and green fluorescent protein. J. Biol. Chem 2004, 279, 14280–14286. [Google Scholar]
- Mank, M; Reiff, DF; Heim, N; Friedrich, MW; Borst, A; Griesbeck, O. A FRET-based calcium biosensor with fast signal kinetics and high fluorescence change. Biophys. J 2006, 90, 1790–1796. [Google Scholar]
- Mank, M; Santos, AF; Direnberger, S; Mrsic-Flogel, TD; Hofer, SB; Stein, V; Hendel, T; Reiff, DF; Levelt, C; Borst, A; Bonhoeffer, T; Hübener, M; Griesbeck, O. A genetically encoded calcium indicator for chronic in vivo two-photon imaging. Nat. Methods 2008, 5, 805–811. [Google Scholar]
- Patterson, GH; Lippincott-Schwartz, J. A photoactivatable GFP for selective photolabeling of proteins and cells. Science 2002, 13, 1873–1877. [Google Scholar]
- Chudakov, DM; Verkhusha, VV; Staroverov, DB; Souslova, EA; Lukyanov, S; Lukyanov, KA. Photoswitchable cyan fluorescent protein for protein tracking. Nat. Biotechnol 2004, 22, 1435–1439. [Google Scholar]
- Wang, Q; Shui, B; Kotlikoff, MI; Sondermann, H. Structural basis for calcium sensing by GCaMP2. Structure 2008, 16, 1817–1827. [Google Scholar]
- Akerboom, J; Rivera, JD; Guilbe, MM; Malavé, EC; Hernandez, HH; Tian, L; Hires, SA; Marvin, JS; Looger, LL; Schreiter, ER. Crystal structures of the GCaMP calcium sensor reveal the mechanism of fluorescence signal change and aid rational design. J. Biol. Chem 2009, 284, 6455–6464. [Google Scholar]
- Palmer, AE; Giacomello, M; Kortemme, T; Hires, SA; Lev-Ram, V; Baker, D; Tsien, RY. Ca2+ indicators based on computationally redesigned calmodulin-peptide pairs. Chem. Biol 2006, 13, 521–530. [Google Scholar]
- Zeng, G. Sticky-end PCR: new method for subcloning. Biotechniques 1998, 25, 206–208. [Google Scholar]
- Freuler, F; Stettler, T; Meyerhofer, M; Leder, L; Mayr, LM. Development of a novel Gateway-based vector system for efficient, multiparallel protein expression in Escherichia coli. Protein Expr. Purif 2008, 59, 232–241. [Google Scholar]
- Kabsch, W. Automatic indexing of rotation diffraction patterns. J. Appl. Crystallogr 1988, 21, 67–71. [Google Scholar]
- Kroemer, M; Dreyer, MK; Wendt, KU. APRV—A program for automated data processing, refinement and visualization. Acta Crystallogr. D. Biol. Crystallogr 2004, 60, 1679–1682. [Google Scholar]
- Vagin, AA; Teplyakov, A. MOLREP: An automated program for molecular replacement. J. Appl. Crystallogr 1997, 30, 1022–1025. [Google Scholar]
- Emsley, P; Cowtan, K. COOT: model-building tools for molecular graphics. Acta Crystallogr. D. Biol. Crystallogr 2004, 60, 2126–2132. [Google Scholar]
- Murshudov, GN; Vagin, AA; Dodson, EJ. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D. Biol. Crystallogr 1997, 53, 240–255. [Google Scholar]
- Maune, JF; Klee, CB; Beckingham, K. Ca2+ binding and conformational change in two series of point mutations to the individual Ca(2+)-binding sites of calmodulin. J. Biol. Chem 1992, 267, 5286–5295. [Google Scholar]
- Linse, S; Helmersson, A; Forsen, S. Calcium binding to calmodulin and its globular domains. J. Biol. Chem 1991, 266, 8050–8059. [Google Scholar]
- Meador, WE; Means, AR; Quiocho, FA. Target enzyme recognition by calmodulin: 2.4 Å structure of a calmodulin-complex. Science 1992, 257, 1251–1255. [Google Scholar]
- Hasan, MT; Rainer, WF; Euler, T; Larkum, ME; Giese, G; Both, M; Duebel, J; Waters, J; Bujard, H; Griesbeck, O; Tsien, RY; Nagai, T; Miyawaki, A; Denk, W. Functional fluorescent Ca2+ indicator proteins in transgenic mice under TET control. PLoS Biol 2004, 2, e163. [Google Scholar]
- Wallace, DJ; Alten-Borghof, SM; Astori, S; Yang, Y; Bausen, M; Kugler, S; Palmer, AE; Tsien, RY; Sprengel, R; Kerr, JND; Denk, W; Hasan, MT. Single-spike detection in vitro and in vivo with a genetic Ca2+ sensor. Nat. Methods 2008, 5, 797–804. [Google Scholar]
- Guo, F; Liu, B; Lane, S; Souslova, EA; Chudakov, DM; Paton, JFR; Kasparov, S. Astroglia is a possible cellular substrate of angiotensin-II effects in the rostral ventro-lateral medulla. Cardiovasc Res 2010, in press. [Google Scholar]
- Palmer, AE; Giacomello, M; Kortemme, T; Hires, SA; Lev-Ram, V; Baker, D; Tsien, RY. Ca2+ indicators based on computationally redesigned calmodulin-peptide pairs. Chem. Biol 2006, 13, 521–530. [Google Scholar]

| Case16 structure A | Case12 structure B | |
|---|---|---|
| Data collectiona | ||
| X-ray source | Swiss Light Source (PX2) | Rigaku FRE |
| Detector type | CCDCHESS-3072-SLS-PX2 225 mm | MAR345-2300-PSU-345 mm |
| Wavelength (Å) | 0.99990 | 1.54179 |
| Space group | P21212 | P21 |
| Cell dimensions | ||
| a, b. c (Å) | 92.5, 106.9, 43.3 | 46.4, 101.6, 82.2 |
| α, β, γ (deg) | 90.0, 90.0, 90.0 | 90.0, 91.5, 90.0 |
| Resolution | 92.50–2.35 (2.54–2.35) | 100.00–2.60 (2.71–2.60) |
| Rsym (%) | 8.8 (57.7) | 10.1 (51.7) |
| I/sig(I) (%) | 14.4 (3.3) | 12.0 (2.8) |
| Completeness (%) | 98.7 (90.1) | 99.8 (99.8) |
| Completeness (highest shell I/sig(I) > 3)(%) | 38.7 | 31.3 |
| Redundancy (%) | 6.9 (6.9) | 3.7 (3.7) |
| Number of observed reflections | 128,528 | 87,933 |
| Number of unique Reflections | 18,555 | 23,464 |
| Wilson Bfactor (Å2) | 49.6 | 41.9 |
| Refinementb | ||
| Resolution range (Å) | 70.01–2.35 (2.411–2.35) | 50.83–2.60 (2.667–2.60) |
| Completeness (%) | 99.94 (100.0) | 99.83 (99.77) |
| Number of reflections | 17.624 (1,278) | 22.288 (1,626) |
| R value | 0.1995 | 0.245 |
| R free | 0.2730 | 0.316 |
| R free test set size (%) | 5.0 | 5.0 |
| R value | 0.217 | 0.340 |
| R free | 0.348 | 0.453 |
| Number of atoms | 3169 | 6434 |
| Mean B (Å2) | 51.129 | 22.432 |
| PDB ID | 3077 | 3078 |
| Estimated coordinate error based on | ||
| R value | 0.380 | 0.939 |
| R free | 0.278 | 0.450 |
| ML | 0.212 | 0.376 |
| B ML (Å2) | 16.931 | 24.100 |
| Bond lengths (Å) | 0.022 | 0.015 |
| Bond angles (deg) | 1.978° | 1.726° |
| Sensor protein concentration | Ca2+ concentration | Case12 | Case16 |
|---|---|---|---|
| 4 mg/mL | 1 mM | 3% dimer | 2% dimer |
| 4 mg/mL | 0 mM (5 mM EGTA) | 0% dimer | 0% dimer |
| 16 mg/mL | 1 mM | 22% dimer | 26% dimer |
| 16 mg/mL | 0 mM (5 mM EGTA) | 0% dimer | 0% dimer |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Leder, L.; Stark, W.; Freuler, F.; Marsh, M.; Meyerhofer, M.; Stettler, T.; Mayr, L.M.; Britanova, O.V.; Strukova, L.A.; Chudakov, D.M.; et al. The Structure of Ca2+ Sensor Case16 Reveals the Mechanism of Reaction to Low Ca2+ Concentrations. Sensors 2010, 10, 8143-8160. https://doi.org/10.3390/s100908143
Leder L, Stark W, Freuler F, Marsh M, Meyerhofer M, Stettler T, Mayr LM, Britanova OV, Strukova LA, Chudakov DM, et al. The Structure of Ca2+ Sensor Case16 Reveals the Mechanism of Reaction to Low Ca2+ Concentrations. Sensors. 2010; 10(9):8143-8160. https://doi.org/10.3390/s100908143
Chicago/Turabian StyleLeder, Lukas, Wilhelm Stark, Felix Freuler, May Marsh, Marco Meyerhofer, Thomas Stettler, Lorenz M. Mayr, Olga V. Britanova, Lydia A. Strukova, Dmitriy M. Chudakov, and et al. 2010. "The Structure of Ca2+ Sensor Case16 Reveals the Mechanism of Reaction to Low Ca2+ Concentrations" Sensors 10, no. 9: 8143-8160. https://doi.org/10.3390/s100908143
APA StyleLeder, L., Stark, W., Freuler, F., Marsh, M., Meyerhofer, M., Stettler, T., Mayr, L. M., Britanova, O. V., Strukova, L. A., Chudakov, D. M., & Souslova, E. A. (2010). The Structure of Ca2+ Sensor Case16 Reveals the Mechanism of Reaction to Low Ca2+ Concentrations. Sensors, 10(9), 8143-8160. https://doi.org/10.3390/s100908143