Efficacy of a Computerized Sensor System for Evaluation and Training of Dizzy Patients
Abstract
:1. Introduction
2. Experimental Section
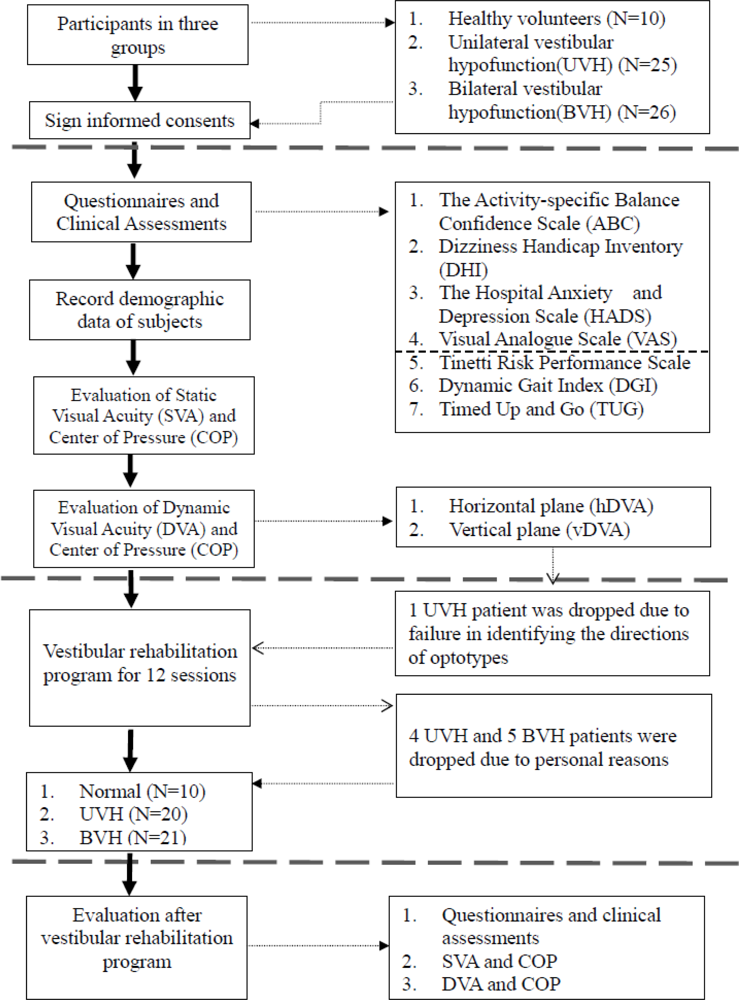
2.1. Participants
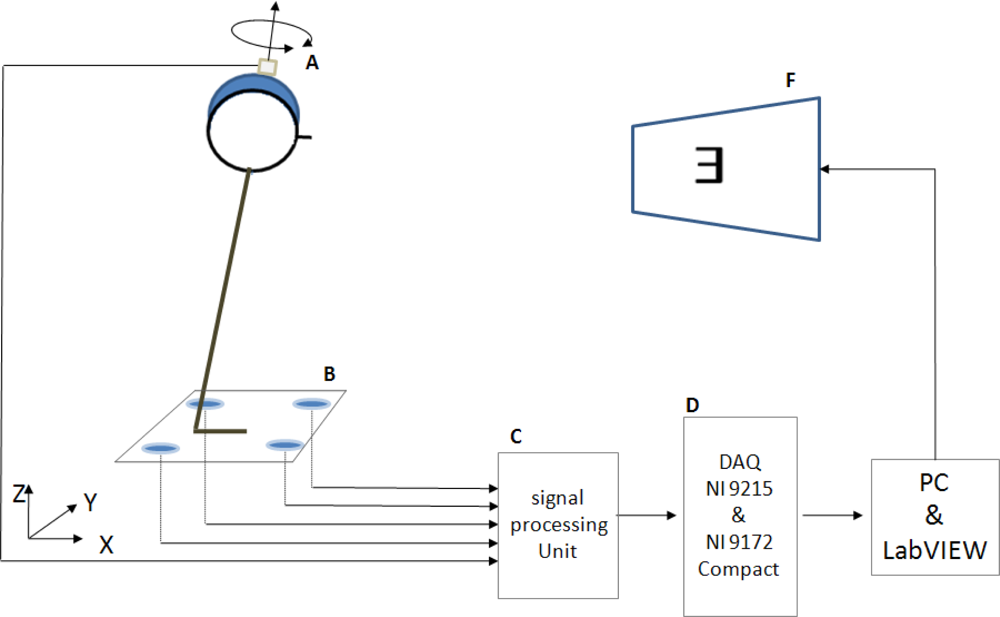
2.2. Equipment and Devices
2.3. SVA and DVA Measurements
2.4. Data Reduction
2.5. Clinical Assessment of Outcome Effects
2.5.1. Dizziness Handicap Inventory (DHI)
2.5.2. The Activities-specific Balance Confidence Scale (ABC)
2.5.3. The Hospital Anxiety and Depression Score (HADS)
2.5.4. Visual Analog Scale (VAS)
2.5.5 Tinetti Fall Risk Performance Scale (TFRPS)
2.5.6. Dynamic Gait Index (DGI)
2.5.7. Timed “Up and Go” Test (TUG)
2.6. Rehabilitation Program
2.7. Statistical Analysis
3. Results and Discussion
3.1. Subjects Characteristics
3.2. Dynamic Visual Acuity and COP Differences between Normal and Vestibular Deficits Individuals
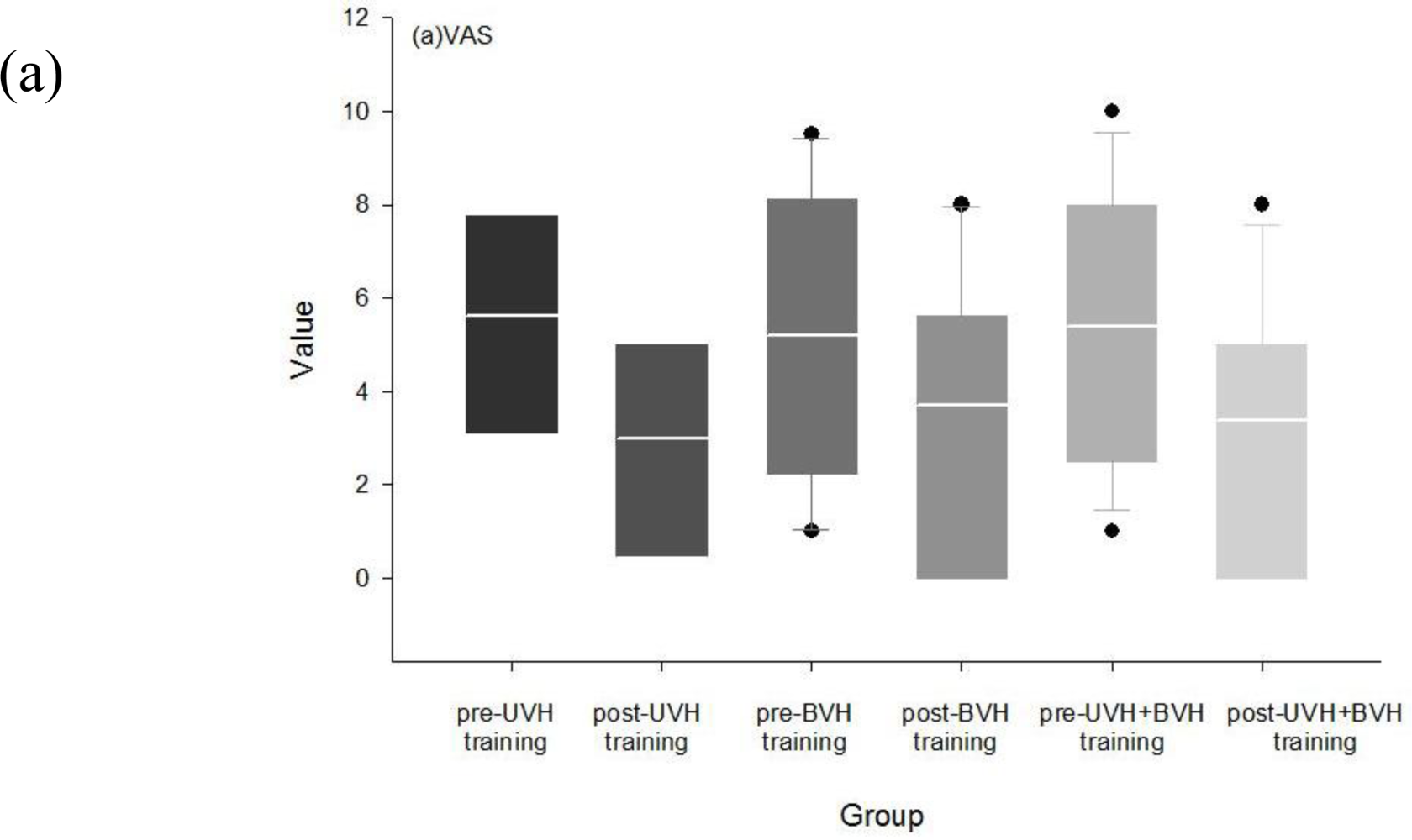
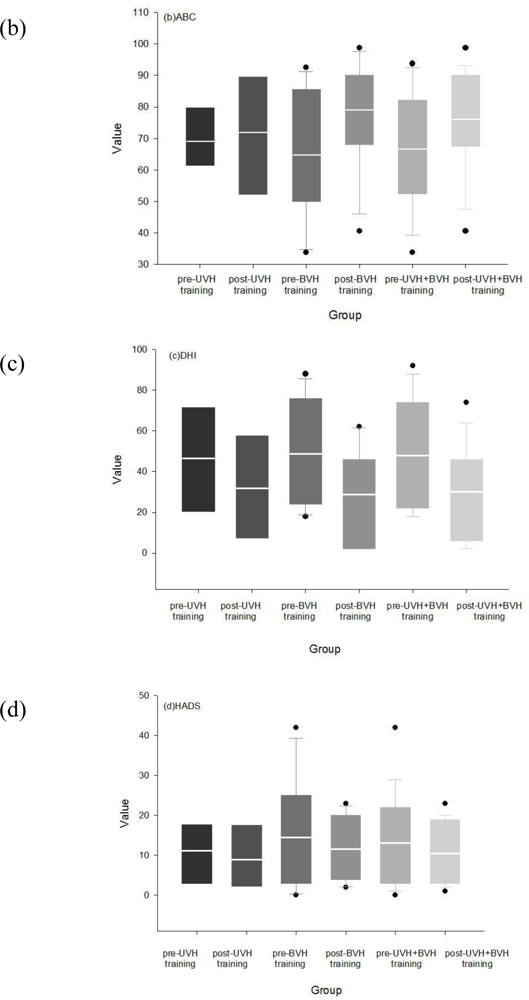
3.3. Effects of Rehabilitation in Visual Analog, DHI and ABC Scores
3.4. Effects of Rehabilitation on Dynamic Gait Index (DGI), Tinetti Scores and Timed “Up and Go” Test
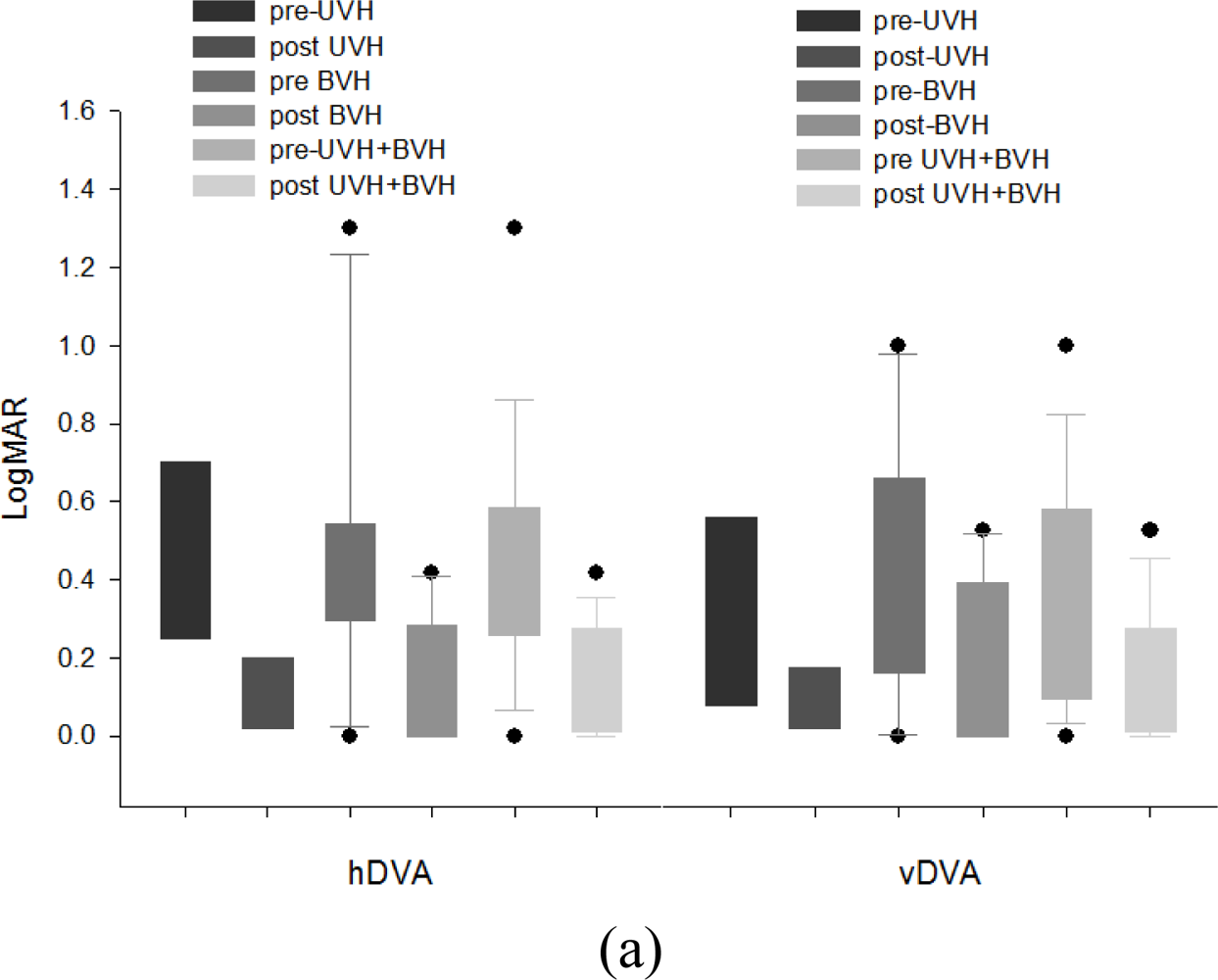
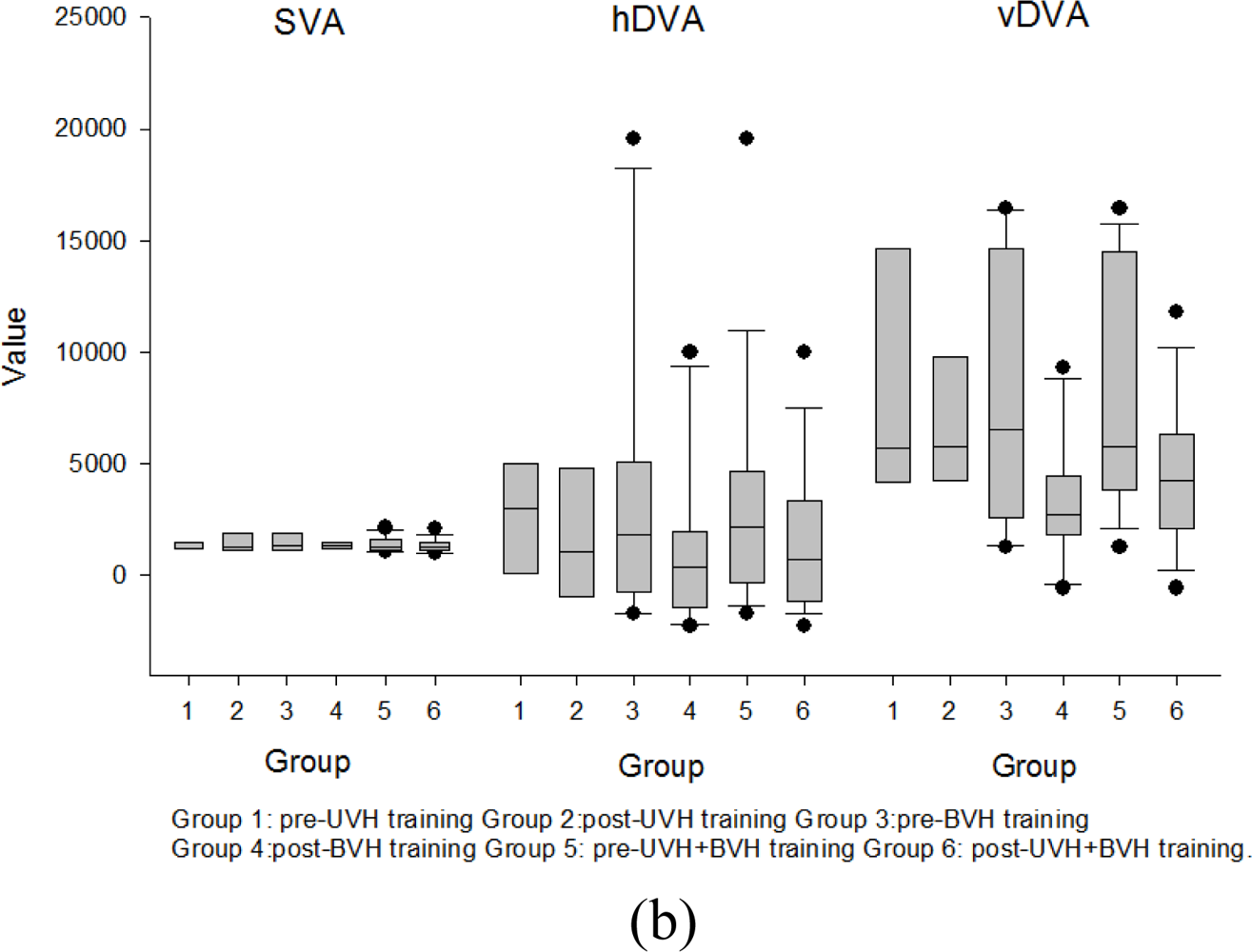
3.5. Effects of Rehabilitation Training on DVA and COP
3.6. Discussion
3.6.1. Advantages of the Computerized Sensor System
3.6.2. Differences of DVA and COP between Normal and Vestibular Deficits Groups before Rehabilitation
3.6.3. Improvements in Dynamic Visual Acuity & Balance Functions
3.6.4. Improvements in Quality of Life
3.6.5. Study Limitations
4. Conclusions
Acknowledgments
References
- Colledge, NR; Wilson, JA; Macintyre, CC; Mac Lennan, WJ. The prevalence and characteristics of dizziness in an elderly community. Age Ageing 1994, 23, 117–120. [Google Scholar]
- Schubert, MC; Minor, LB. Vestibulo-ocular physiology underlying vestibular hypofunction. Phys. Ther 2004, 84, 373–385. [Google Scholar]
- Bhansali, SA; Stockwell, CW; Bojrab, DI. Oscillopsia in patients with loss of vestibular function. Otolaryngol. Head Neck Surg 1993, 109, 120–125. [Google Scholar]
- Herdman, SJ; Schubert, MC; Das, VE; Tusa, RJ. Recovery of dynamic visual acuity in unilateral vestibular hypofunction. Arch. Otolaryngol. Head Neck Surg 2003, 129, 819–824. [Google Scholar]
- Cohen, HS; Bloomberg, JJ. Modified dynamic visual acuity tests after acoustic neuroma resection. Acta Oto-Laryngologica 2007, 127, 825–828. [Google Scholar]
- Minor, LB; Lasker, DM; Backous, DD; Hullar, TE. Horizontal vestibuloocular reflex evoked by high-acceleration rotations in the squirrel monkey, I: Normal responses. J. Neurophysiol 1999, 82, 1254–1270. [Google Scholar]
- Demer, JL; Oas, JG; Baloh, RW. Visual-vestibular interaction in humans during active and passive, vertical head movement. J. Vestib. Res 1993, 3, 101–114. [Google Scholar]
- Herdman, SJ; Tusa, RJ; Blatt, PJ; Suzuki, A; Venuto, PJ; Roberts, D. Computerized dynamic visual acuity test in the assessment of vestibular deficits. Am. J. Otol 1998, 19, 790–796. [Google Scholar]
- Paloski, WH; Wood, SJ; Feiveson, AH; Black, FO; Hwang, EY; Reschke, MF. Destabilization of human balance control by static and dynamic head tilts. Gait Posture 2006, 23, 315–323. [Google Scholar]
- Clark, S; Tolhurst, RF. Postural stability comparison between athletes and non-athletes using the sensory organization test and the headshake sensory organization test. Med. Sci. Sports Exerc 2000, 32, S144–S150. [Google Scholar]
- Meli, A; Zimatore, G; Badaracco, C; De Angelis, E; Tufarelli, D. Vestibular rehabilitation and 6-month follow-up using objective and subjective measures. Acta. Oto-Laryngologica 2006, 126, 259–266. [Google Scholar]
- Whitney, SL; Hudak, MT; Marchetti, GF. The dynamic gait index relates to self-reported fall history in individuals with vestibular dysfunction. J. Vestib. Res 2000, 10, 99–105. [Google Scholar]
- Honrubia, V; Bell, TS; Harris, MR; Baloh, RW; Fisher, LM. Quantitative evaluation of dizziness characteristic and impact on quality of life. Am. J. Otol 1996, 17, 595–602. [Google Scholar]
- Enloe, LJ; Shields, RK. Evaluation of health-related quality of life in individuals with vestibular disease using disease specific and general outcomes measures. Phys. Ther 1997, 77, 890–903. [Google Scholar]
- Perez, N; Garmendia, I; Garcia-Granero, M; Martin, E; Garcia-Tapia, R. Factor analysis correlation between dizziness handicap inventory and dizziness characteristics and impact on quality of life scales. Acta Otolaryngol. Suppl 2001, 545, 145–154. [Google Scholar]
- Jacobson, PG; Newman, WC. The Development of the Dizziness Handicap Inventory. Arch. Otolaryngol. Head Neck Surg 1990, 116, 424–427. [Google Scholar]
- Whitney, SL; Hudack, MT; Marchetti, GF. The activitiesspecific balance confidence scale and the dizziness handicap inventory: A comparison. J. Vestib. Res 1999, 9, 253–259. [Google Scholar]
- Tinetti, ME. Performance-oriented assessment of mobility problems in elderly patients. J. Am. Geriatr. Soc 1986, 34, 119–126. [Google Scholar]
- Whitney, SL; Marchetti, GF; Schade, A; Wrisley, DM. The sensitivity and specificity of the Timed “Up & Go” and the dynamic gait index for self-reported falls in persons with vestibular disorders. J. Vestib. Res 2004, 14, 397–409. [Google Scholar]
- Cohen, HS. Specialized knowledge and skills in adult vestibular rehabilitation for occupational therapy practice. Am. J. Occup. Ther 2001, 55, 661–665. [Google Scholar]
- Cohen, HS; Kimball, KT. Increased independence and decreased vertigo after vestibular rehabilitation. Otolaryngol. Head Neck Surg 2003, 128, 60–70. [Google Scholar]
- Cohen, HS; Kimball, KT. Changes in a repetitive head movement task after vestibular rehabilitation. Clin. Rehabil 2004, 18, 125–131. [Google Scholar]
- Cohen, HS; Kimball, KT. Treatment variations on the Epley maneuver for benign paroxysmal positional vertigo. Am. J. Otolaryngol 2004, 25, 33–37. [Google Scholar]
- Cohen, HS. Disability and rehabilitation in the dizzy patient. Curr. Opin. Neurol 2006, 19, 49–54. [Google Scholar]
- Piirtola, M; Era, P. Force platform measurements as predictors of falls among older people—A review. Gerontology 2006, 52, 1–16. [Google Scholar]
- Satu Pajala, PE; Koskenvuo, M; Kaprio, J; Tormakangas, T; Rantane, T. Force platform balance measures as predictors of indoor and outdoor falls in Community-dwelling women aged 63–76 years. J. Gerontol. Med. Sci 2008, 63A, 171–178. [Google Scholar]
- Era, P; Schroll, M; Ytting, H; Gause-Nilsson, I; Heikkinen, E; Steen, B. Postural balance and its sensory-motor correlates in 75-year-old men and women: A cross-national comparative study. J. Gerontol. Med. Sci 1996, 51A, M53–M63. [Google Scholar]
- Maki, BE; Holliday, PJ; Topper, AK. A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. J. Gerontol. Med. Sci 1994, 49, M72–M84. [Google Scholar]
- Thapa, PB; Gideon, P; Brockman, KG; Fought, RL; Ray, WA. Clinical and biomechanical measures of balance as fall predictors in ambulatory nursing home residents. J. Gerontol. Med. Sci 1996, 51A, M239–M246. [Google Scholar]
- Powell, LE; Myers, AM. The Activities-specific Balance Confidence (ABC) scale. J Gerontol. Med. Sci 1995, 50A, M28–M34. [Google Scholar]
- Zigmond, AS; Snaith, RP. The Hospital anxiety and depression scale. Acta. Psychiatr. Scand 1983, 67, 361–370. [Google Scholar]
- Leung, CM; Ho, S; Kan, CS; Hung, CH; Chen, CN. Evaluation of the Chinese version of the Hospital Anxiety and Depression Scale—A cross-cultural perspective. Int. J. Psychosom 1993, 40, 29–34. [Google Scholar]
- Szturm, T; Ireland, DJ; Lessing-Turner, M. Comparison of different exercise programs in the rehabilitation of patients with chronic peripheral vestibular dysfunction. J. Vestib. Res 1994, 4, 461–479. [Google Scholar]
- Barin, K. Background and Technique of Caloric Testing. In Balance Function Assessment and Management; Jacobson, GP, Shepard, NT, Eds.; Plural Publishing: San Diego, CA, USA, 2008; p. 200. [Google Scholar]
- Schubert, MC; Herdman, SJ; Tusa, RJ. Vertical dynamic visual acuity in normal subjects and patients with vestibular hypofunction. Otology Neurotology 2002, 23, 372–377. [Google Scholar]
- Badaracco, C; Labini, FS; Meli, A; Tufarelli, D. Oscillopsia in labyrinthine defective patients: Comparison of objective and subjective measures. Am. J. Otolaryngol 2009, in press. [Google Scholar]
- Herdman, SJ; Hall, CD; Schubert, MC; Das, VE; Tusa, RJ. Recovery of dynamic visual acuity in bilateral vestibular hypofunction. Arch. Otolaryngol. Head Neck Surg 2007, 133, 383–389. [Google Scholar]
- Whitney, SL; Wrisley, DM; Marchetti, GF; Furman, JM. The effect of age on vestibular rehabilitation outcomes. Laryngoscope 2002, 112, 1785–1790. [Google Scholar]
- Badke, MB; Shea, TA; Miedaner, JA; Grove, CR. Outcomes after rehabilitation for adults with balance dysfunction. Arch. Phys. Med. Rehabil 2004, 85, 227–233. [Google Scholar]
- Brown, M; Sinacore, DR; Host, HH. The relationship of strength to function in the older adult. J. Gerontol. A. Biol. Sci. Med. Sci 1995, 50A, 55–59. [Google Scholar]
- Buchner, DM; Beresford, SA; Larson, EB; LaCroix, AZ; Wagner, EH. Effects of physical activity on health status in older adults. II. Intervention studies. Annu. Rev. Public Health 1992, 13, 469–488. [Google Scholar]
- Collins, JJ; De Luca, CJ; Burrows, A; Lipsitz, LA. Agerelated changes in open-loop and closed-loop postural control mechanisms. Exp. Brain Res 1995, 104, 480–492. [Google Scholar]
- Kamran, B. Interpretation and Usefulness of Caloric Testing. In Balance Function Assessment and Management; Gary, PJ, Neil, TS, Eds.; Plural Publishing Inc: San Diego, CA, USA, 2008. [Google Scholar]
- Kim, SC; Nam, KC; Lee, WS; Kim, DW. A new method for accurate and fast measurement of 3D eye movements. Med. Eng. Phys 2006, 28, 82–89. [Google Scholar]
- Zapala, DA; Sholt, KF; Lundy, LB. A comparison of water and air caloric responses and their ability to distinguish between patients with normal and impaired ears. Ear Hearing 2008, 29, 585–600. [Google Scholar]
| Week | Standing positions | Vestibular exercises |
|---|---|---|
| Week 1 | Quiet stance | 2 Hz horizontal head movement, naming the items on computer screen 2M in front |
| 2 Hz vertical head movement, naming the items on computer screen 2M in front | ||
| Tandem stance | 2 Hz horizontal head movement, naming the items on computer screen 2M in front | |
| 2 Hz vertical head movement, naming the items on computer screen 2M in front | ||
| Stepping | 2 Hz horizontal head movement, naming the items on computer screen 2M in front | |
| 2 Hz vertical head movement, naming the items on computer screen 2M in front | ||
| Week 2 | Stepping | 2 Hz horizontal head movement, naming the items on computer screen 2M in front |
| 2 Hz vertical head movement, naming the items on computer screen 2M in front | ||
| Quiet stance | 2 Hz horizontal head movement, naming revised items on computer screen 2M in front | |
| 2 Hz vertical head movement, naming revised items on computer screen 2M in front | ||
| Tandem stance | 2 Hz horizontal head movement, naming revised items on computer screen 2M in front | |
| 2 Hz vertical head movement, naming revised items on computer screen 2M in front | ||
| Week 3 | Quiet stance on foam | 2 Hz horizontal head movement, naming revised items on computer screen 2M in front |
| 2 Hz vertical head movement, naming revised items on computer screen 2M in front | ||
| Tandem stance | 2 Hz horizontal head movement, naming revised items on computer screen 2M in front | |
| 2 Hz vertical head movement, naming revised items on computer screen 2M in front | ||
| Stepping | 2 Hz horizontal head movement, naming revised items on computer screen 2M in front | |
| 2 Hz vertical head movement, naming revised items on computer screen 2M in front | ||
| Week 4 | Quiet stance | 2 Hz horizontal head movement, naming flashed items on computer screen 2M in front |
| 2 Hz vertical head movement, naming flashed items on computer screen 2M in front | ||
| Quiet stance on foam | 2 Hz horizontal head movement, naming flashed items on computer screen 2M in front | |
| 2 Hz vertical head movement, naming flashed items on computer screen 2M in front | ||
| Stepping | 2 Hz horizontal head movement, naming flashed items on computer screen 2M in front | |
| 2 Hz vertical head movement, naming flashed items on computer screen 2M in front | ||
| hDVA LogMAR# | vDVA LogMAR# | COP in SVA (mm) | %diff | COP in hDVA (mm) | %diff | COP in vDVA (mm) | %diff | |
|---|---|---|---|---|---|---|---|---|
| Normal (N = 10) | 0.08 ± 0.02A | 0.09 ± 0.02 A | 1,230.27 ± 122.54 | -- | 4,851.52 ± 1,185.78 | -- | 7,383.43 ± 946.02 | -- |
| UVH (N = 20) | 0.37 ± 0.08 B | 0.22 ± 0.07 A | 1,417.10 ± 128.61 | 15.19 | 5,877.85 ± 681.03 | 21.15 | 9,566.03 ± 1321.12 | 29.56 |
| BVH (N = 21) | 0.48 ± 0.09 B | 0.47 ± 0.10 B | 1,421.61 ± 114.53 | 15.55 | 6,885.46 ± 943.02 | 41.92 | 10,999.40 ± 1,055.77 | 48.97 |
| UVH (N = 20) | BVH (N = 21) | Vestibular-hypofunction (UVH + BVH) (N = 41) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| before | after | p | before | after | p | before | after | p | |
| DGI | 14.25 ± 0.88 | 19.5 ± 0.5 | 0.000* | 13.73 ± 1.19 | 19.45 ± 0.82 | 0.000* | 13.95 ± 0.77 | 19.47 ± 0.51 | <0.001* |
| Tinetti | |||||||||
| balance | 8 ± 0.65 | 12.25 ± 0.53 | 0.001* | 8.82 ± 0.63 | 13.27 ± 0.62 | 0.000* | 8.47 ± 0.45 | 12.84 ± 0.43 | <0.001* |
| gait | 8.88 ± 0.61 | 11 ± 0.19 | 0.004* | 9.45 ± 0.47 | 11.09 ± 0.25 | 0.003* | 9.21 ± 0.37 | 11.05 ± 0.16 | <0.001* |
| total | 16.88 ± 1.08 | 23.25 ± 0.45 | 0.001* | 18.27 ± 1.03 | 24.36 ± 0.75 | 0.000* | 17.68 ± 0.75 | 23.89 ± 0.48 | <0.001* |
| TUG | 11.58 ± 0.73 | 10.19 ± 0.72 | 0.051 | 10.85 ± 1.05 | 8.77 ± 0.68 | 0.01 | 11.15 ± 0.67 | 9.37 ± 0.51 | <0.001* |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kao, C.-L.; Hsieh, W.-L.; Wang, S.-J.; Chen, S.-J.; Wei, S.-H.; Chan, R.-C. Efficacy of a Computerized Sensor System for Evaluation and Training of Dizzy Patients. Sensors 2010, 10, 7602-7620. https://doi.org/10.3390/s100807602
Kao C-L, Hsieh W-L, Wang S-J, Chen S-J, Wei S-H, Chan R-C. Efficacy of a Computerized Sensor System for Evaluation and Training of Dizzy Patients. Sensors. 2010; 10(8):7602-7620. https://doi.org/10.3390/s100807602
Chicago/Turabian StyleKao, Chung-Lan, Wan-Ling Hsieh, Shuu-Jiun Wang, Shih-Jen Chen, Shun-Hwa Wei, and Rai-Chi Chan. 2010. "Efficacy of a Computerized Sensor System for Evaluation and Training of Dizzy Patients" Sensors 10, no. 8: 7602-7620. https://doi.org/10.3390/s100807602




