Electrochemical Microsensors for the Detection of Cadmium(II) and Lead(II) Ions in Plants
Abstract
:1. Introduction
2. Results and Discussion
2.1. Automated Electrochemical Detection of Cadmium(II) and Lead(II) Ions at a Hanging Mercury Drop Electrode—Metrohm Potentiostat
2.2. Electrochemical Detection of Cadmium(II) and Lead(II) Ions—PalmSens potentiostat
2.3. Cadmium and Lead Ions Detection by Micropotentiostat
2.4. Comparison of the Potentiostats and Working Electrodes Used for Detection of Cadmium(II) and Lead(II) Ions
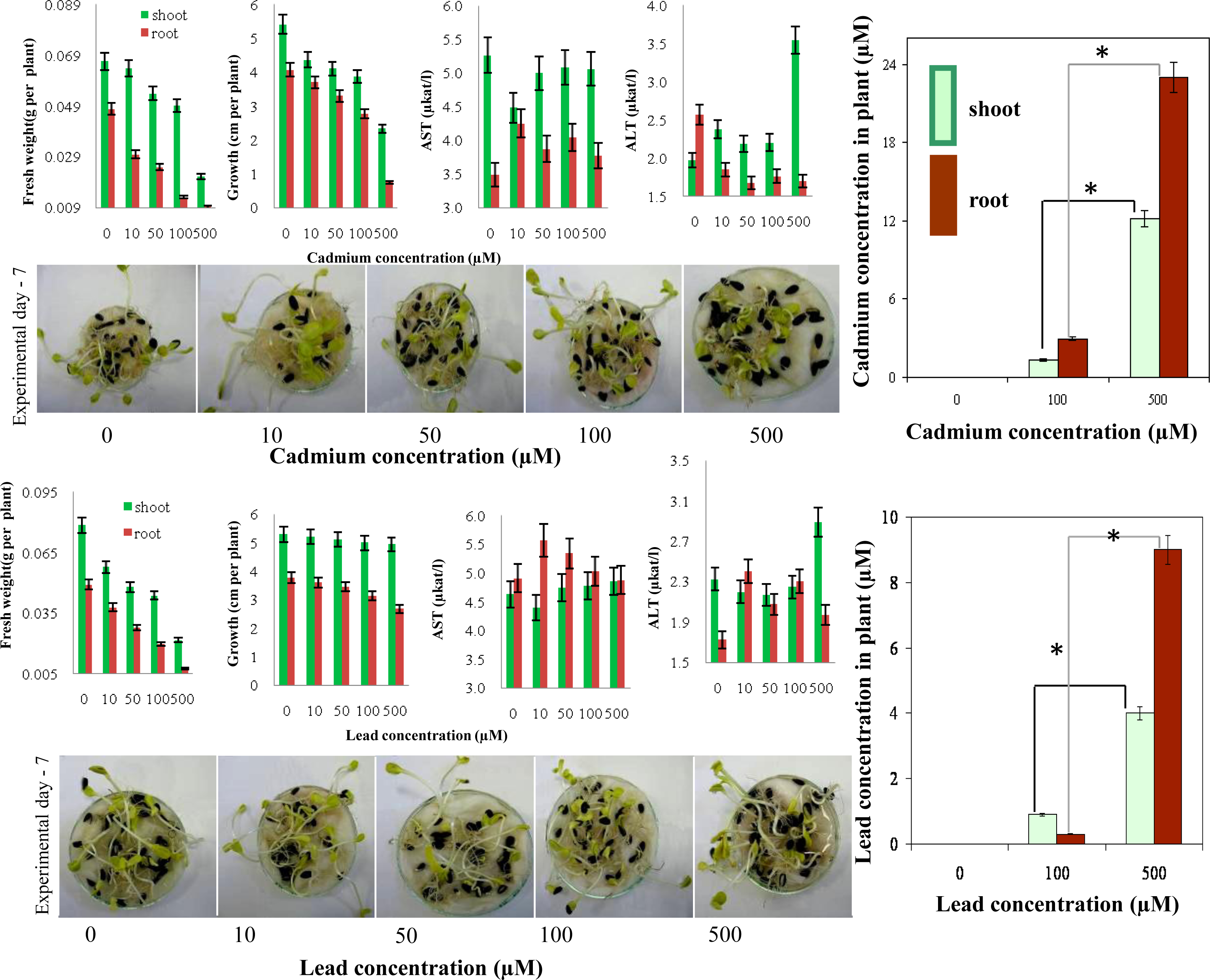
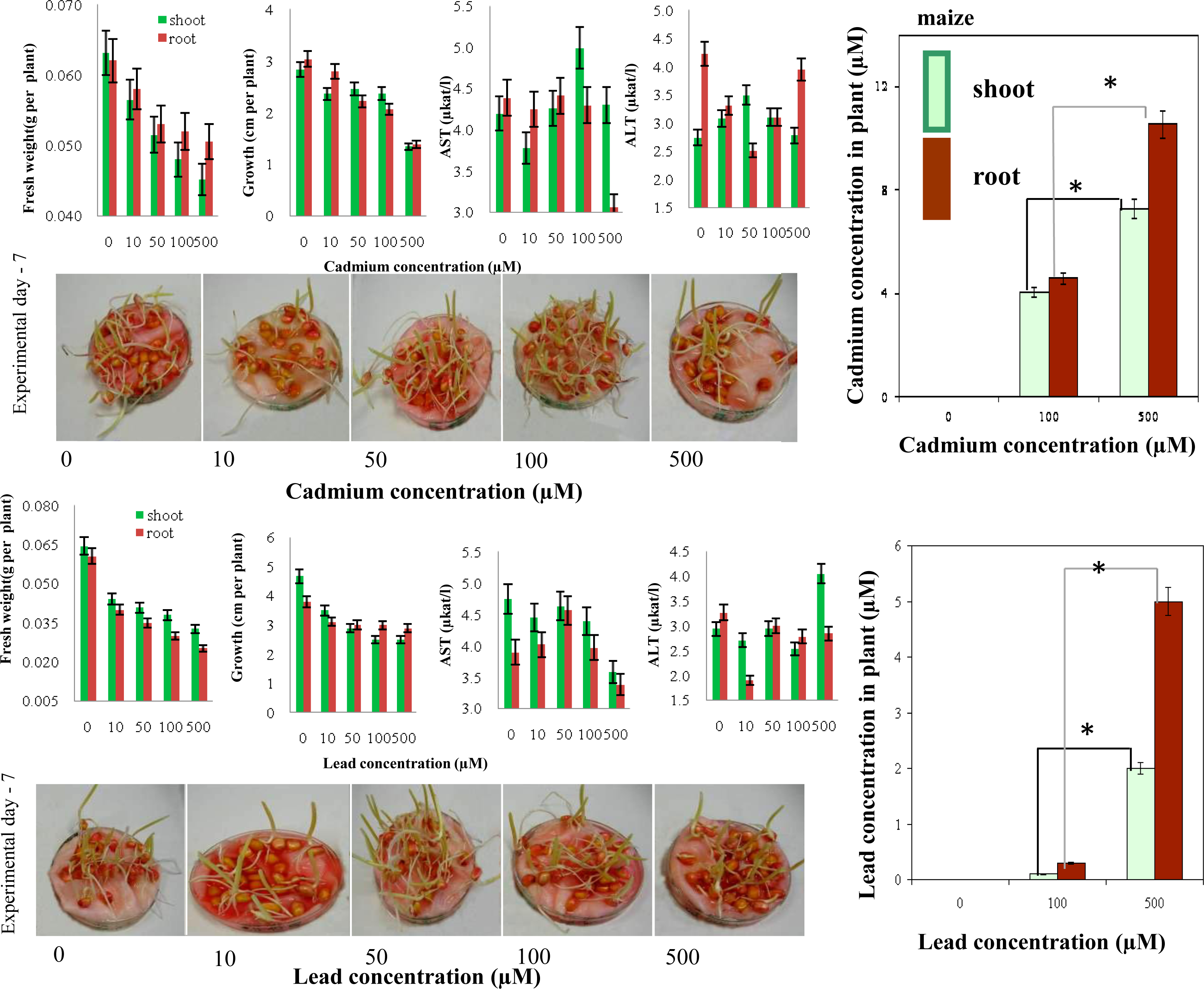
2.5. Determination of Cadmium(II) and Lead(II) Ions in Maize and Sunflower Seedlings
2.6. Cadmium(II) and Lead(II) Ions Monitoring in Wetlands
3. Experimental Section
3.1. Chemicals, Materials and pH Measurements
3.2. Plant Cultivation
3.3. Sample Preparation
3.4. Automated Spectrometric Measurements
3.4.1. Determination of ALT (AST) activity
3.5. Autolab
3.6. PalmSens
3.7. Homemade Potentiostat
3.8. Fabrication of Screen Printed Electrodes
3.9. Biotope Knizeci Forest and Rainfall Water
3.10. Descriptive Statistics
4. Conclusions
Acknowledgments
References
- Di Tioppi, S.L.; Gabbrielli, R. Response to cadmium in higher plants. Environ. Exp. Bot 1999, 41, 105–130. [Google Scholar]
- Petrek, J.; Baloun, J.; Vlasinova, H.; Havel, L.; Adam, V.; Vitecek, J.; Babula, P.; Kizek, R. Image analysis and activity of intracellular esterases as new analytical tools for determination of growth and viability of embryonic cultures of spruce (Picea sp.) treated with cadmium. Chem. Listy 2007, 101, 569–577. [Google Scholar]
- Supalkova, V.; Huska, D.; Diopan, V.; Hanustiak, P.; Zitka, O.; Stejskal, K.; Baloun, J.; Pikula, J.; Havel, L.; Zehnalek, J.; Adam, V.; Trnkova, L.; Beklova, M.; Kizek, R. Electroanalysis of plant thiols. Sensors 2007, 7, 932–959. [Google Scholar]
- Supalkova, V.; Petrek, J.; Baloun, J.; Adam, V.; Bartusek, K.; Trnkova, L.; Beklova, M.; Diopan, V.; Havel, L.; Kizek, R. Multi-instrumental investigation of affecting of early somatic embryos of spruce by cadmium(II) and lead(II) ions. Sensors 2007, 7, 743–759. [Google Scholar]
- Zehnalek, J.; Adam, V.; Kizek, R. Influence of heavy metals on production of protecting compounds in agriculture plants. Lis. Cukrov. Reparske 2004, 120, 222–224. [Google Scholar]
- Zehnalek, J.; Vacek, J.; Kizek, R. Application of higher plants in phytoremetiation of heavy metals. Lis. Cukrov. Reparske 2004, 120, 220–221. [Google Scholar]
- Soylak, M.; Narin, I.; Divrikli, U.; Saracoglu, S.; Elci, L.; Dogan, M. Preconcentration-separation of heavy metal ions in environmental samples by membrane filtration-atomic absorption spectrometry combination. Anal. Lett 2004, 37, 767–780. [Google Scholar]
- Sneddon, J.; Vincent, M.D. ICP-OES and ICP-MS for the determination of metals: Application to oysters. Anal. Lett 2008, 41, 1291–1303. [Google Scholar]
- Ferreira, S.L.C.; de Andrade, J.B.; Korn, M.D.A.; Pereira, M.D.; Lemos, V.A.; dos Santos, W.N.L.; Rodrigues, F.D.; Souza, A.S.; Ferreira, H.S.; da Silva, E.G.P. Review of procedures involving separation and preconcentration for the determination of cadmium using spectrometric techniques. J. Hazard. Mater 2007, 145, 358–367. [Google Scholar]
- Pyrzynska, K. Online sample pretreatment systems for determination of cadmium by the ETAAS method. Crit. Rev. Anal. Chem 2007, 37, 39–49. [Google Scholar]
- Davis, A.C.; Wu, P.; Zhang, X.F.; Hou, X.D.; Jones, B.T. Determination of cadmium in biological samples. Appl. Spectrosc. Rev 2006, 41, 35–75. [Google Scholar]
- Yantasee, W.; Lin, Y.; Hongsirikarn, K.; Fryxell, G.E.; Addleman, R.; Timchalk, C. Electrochemical sensors for the detection of lead and other toxic heavy metals: The next generation of personal exposure biomonitors. Environ. Health Perspect 2007, 115, 1683–1690. [Google Scholar]
- Korn, M.D.A.; dos Santos, D.S.S.; Welz, B.; Vale, M.G.R.; Teixeira, A.P.; Lima, D.D.; Ferreira, S.L.C. Atomic spectrometric methods for the determination of metals and metalloids in automotive fuels—A review. Talanta 2007, 73, 1–11. [Google Scholar]
- Korn, M.D.A.; de Andrade, J.B.; de Jesus, D.S.; Lemos, V.A.; Bandeira, M.; dos Santos, W.N.L.; Bezerra, M.A.; Amorim, F.A.C.; Souza, A.S.; Ferreira, S.L.C. Separation and preconcentration procedures for the determination of lead using spectrometric techniques: A review. Talanta 2006, 69, 16–24. [Google Scholar]
- Shaw, M.J.; Haddad, P.R. The determination of trace metal pollutants in enviromental matrices using ion chromatography. Environ. Int 2004, 30, 403–431. [Google Scholar]
- Lin, T.J.; Chung, M.F. Using monoclonal antibody to determine lead ions with a localized surface plasmon resonance fiber-optic biosensor. Sensors 2008, 8, 582–593. [Google Scholar]
- Lewen, N.; Mathew, S.; Schenkenberger, M.; Raglione, T. A rapid ICP-MS screen for heavy metals in pharmaceutical compounds. J. Pharm. Biomed. Anal 2004, 35, 739–752. [Google Scholar]
- Szlyk, E.; Szydlowska-Czerniak, A. Determination of cadmium, lead, and copper in margarines and butters by galvanostatic stripping chronopotentiometry. J. Agric. Food Chem 2004, 52, 4064–4071. [Google Scholar]
- Mikkelsen, O.; Schroder, K.H. Amalgam electrodes for electroanalysis. Electroanalysis 2003, 15, 679–687. [Google Scholar]
- May, L.M.; Russell, D.A. Novel determination of cadmium ions using an enzyme self-assembled monolayer with surface plasmon resonance. Anal. Chim. Acta 2003, 500, 119–125. [Google Scholar]
- Pei, J.H.; Tercier-Waeber, M.L.; Buffle, J. Simultaneous determination and speciation of zinc, cadmium, lead, and copper in natural water with minimum handling and artifacts, by voltammetry on a gel-integrated microelectrode array. Anal. Chem 2000, 72, 161–171. [Google Scholar]
- Fernandez-Bobes, C.; Fernandez-Abedul, M.T.; Costa-Garcia, A. Anodic stripping of heavy metals using a hanging mercury drop electrode in a flow system. Electroanalysis 1998, 10, 701–706. [Google Scholar]
- Adam, V.; Hanustiak, P.; Krizkova, S.; Beklova, M.; Zehnalek, J.; Trnkova, L.; Horna, A.; Sures, B.; Kizek, R. Palladium biosensor. Electroanalysis 2007, 19, 1909–1914. [Google Scholar]
- Adam, V.; Petrlova, J.; Potesil, D.; Zehnalek, J.; Sures, B.; Trnkova, L.; Jelen, F.; Kizek, R. Study of metallothionein modified electrode surface behavior in the presence of heavy metal ions-biosensor. Electroanalysis 2005, 17, 1649–1657. [Google Scholar]
- Krizkova, S.; Adam, V.; Petrlova, J.; Zitka, O.; Stejskal, K.; Zehnalek, J.; Sures, B.; Trnkova, L.; Beklova, M.; Kizek, R. A suggestion of electrochemical biosensor for study of platinum(II)-DNA interactions. Electroanalysis 2007, 19, 331–338. [Google Scholar]
- Petrlova, J.; Potesil, D.; Zehnalek, J.; Sures, B.; Adam, V.; Trnkova, L.; Kizek, R. Cisplatin electrochemical biosensor. Electrochim. Acta 2006, 51, 5169–5173. [Google Scholar]
- Wu, K.B.; Hu, S.S.; Fei, J.J.; Bai, W. Mercury-free simultaneous determination of cadmium and lead at a glassy carbon electrode modified with multi-wall carbon nanotubes. Anal. Chim. Acta 2003, 489, 215–221. [Google Scholar]
- Yantasee, W.; Lin, Y.H.; Fryxell, G.E.; Busche, B.J. Simultaneous detection of cadmium, copper, and lead using a carbon paste electrode modified with carbamoylphosphonic acid self-assembled monolayer on mesoporous silica (SAMMS). Anal. Chim. Acta 2004, 502, 207–212. [Google Scholar]
- Hu, C.G.; Wu, K.B.; Dai, X.; Hu, S.S. Simultaneous determination of lead(II) and cadmium(II) at a diacetyldioxime modified carbon paste electrode by differential pulse stripping voltammetry. Talanta 2003, 60, 17–24. [Google Scholar]
- Palchetti, I.; Cagnini, A.; Mascini, M.; Turner, A.P.F. Characterisation of screen-printed electrodes for detection of heavy metals. Mikrochim. Acta 1999, 131, 65–73. [Google Scholar]
- Guell, R.; Aragay, G.; Fontas, C.; Antico, E.; Merkoci, A. Sensitive and stable monitoring of lead and cadmium in seawater using screen-printed electrode and electrochemical stripping analysis. Anal. Chim. Acta 2008, 627, 219–224. [Google Scholar]
- Roa, G.; Ramirez-Silva, M.T.; Romero-Romo, M.A.; Galicia, L. Determination of lead and cadmium using a polycyclodextrin-modified carbon paste electrode with anodic stripping voltammetry. Anal. Bioanal. Chem 2003, 377, 763–769. [Google Scholar]
- Cooper, J.; Bolbot, J.A.; Saini, S.; Setford, S.J. Electrochemical method for the rapid on site screening of cadmium and lead in soil and water samples. Water Air Soil Pollut 2007, 179, 183–195. [Google Scholar]
- Palchetti, H.; Laschi, S.; Mascini, M. Miniaturised stripping-based carbon modified sensor for in field analysis of heavy metals. Anal. Chim. Acta 2005, 530, 61–67. [Google Scholar]
- Locatelli, C. Peak area: The instrumental datum to improve the determination at ultratrace level concentration of mercury(II) at gold electrode. Electroanalysis 2008, 20, 1330–1338. [Google Scholar]
- Locatelli, C. Voltammetric analysis of trace levels of platinum group metals principles and applications. Electroanalysis 2007, 19, 2167–2175. [Google Scholar]
- Locatelli, C. Voltammetric peak area as instrumental datum. A possibility to improve the determination at ultratrace level concentration of platinum group metals (PGMs) and lead— Application to particulate matter. Electroanalysis 2007, 19, 445–452. [Google Scholar]
- Locatelli, C. Possible interference in the sequential voltammetric determination at trace and ultratrace concentration level of platinum group metals (PGMs) and lead—Application to environmental matrices. Electrochim. Acta 2006, 52, 614–622. [Google Scholar]
- Locatelli, C. Simultaneous square wave, stripping voltammetric determination of platinum group metals (PGMs) and lead at trace and ultratrace concentration level—Application to surface water. Anal. Chim. Acta 2006, 557, 70–77. [Google Scholar]
- Locatelli, C.; Melucci, D.; Torsi, G. Determination of platinum-group metals and lead in vegetable environmental bio-monitors by voltammetric and spectroscopic techniques: critical comparison. Anal. Bioanal. Chem 2005, 382, 1567–1573. [Google Scholar]
- Locatelli, C. Overlapping voltammetric peaks—An analytical procedure for simultaneous determination of trace metals. Application to food and environmental matrices. Anal. Bioanal. Chem 2005, 381, 1073–1081. [Google Scholar]
- Paneli, M.G.; Voulgaropoulos, A. Applications of Adsorptive Stripping Voltammetry in the Determination of Trace and Ultratrace Metals. Electroanalysis 1993, 5, 355–373. [Google Scholar]
- Wang, J. Analytical Electrochemistry; VCH Publishers: New York, NY, USA, 1994. [Google Scholar]
- White, N.M.; Turner, J.D. Thick-film sensors: Past, present and future. Meas. Sci. Technol 1997, 8, 1–20. [Google Scholar]
- Fujcik, L.; Prokop, R.; Prasek, J.; Hubalek, J.; Vrba, R. New CMOS potentiostat as ASIC for several electrochemical microsensors construction. Microelectron. Int 2009, 27, 3–10. [Google Scholar]
- Adam, V.; Zitka, O.; Dolezal, P.; Zeman, L.; Horna, A.; Hubalek, J.; Sileny, J.; Krizkova, S.; Trnkova, L.; Kizek, R. Lactoferrin isolation using monolithic column coupled with spectrometric or micro-amperometric detector. Sensors 2008, 8, 464–487. [Google Scholar]
- Krystofova, O.; Shestivska, V.; Galiova, M.; Novotny, K.; Kaiser, J.; Zehnalek, J.; Babula, P.; Opatrilova, R.; Adam, V.; Kizek, R. Sunflower plants as bioindicators of environmental pollution with lead(II) ions. Sensors 2009, 9, 5040–5058. [Google Scholar]
- Vacek, J.; Petrek, J.; Kizek, R.; Havel, L.; Klejdus, B.; Trnkova, L.; Jelen, F. Electrochemical determination of lead and glutathione in a plant cell culture. Bioelectrochemistry 2004, 63, 347–351. [Google Scholar]
- Burham, N.; Abdel-Azeem, S.M.; El-Shahat, M.F. Separation and determination of trace amounts of zinc, lead, cadmium and mercury in tap and Qaroun lake water using polyurethane foam functionalized with 4-hydroxytoluene and 4-hydroxyacetophenone. Anal. Chim. Acta 2006, 579, 193–201. [Google Scholar]
- Petrlova, J.; Krizkova, S.; Zitka, O.; Hubalek, J.; Prusa, R.; Adam, V.; Wang, J.; Beklova, M.; Sures, B.; Kizek, R. Utilizing a chronopotentiometric sensor technique for metallothionein determination in fish tissues and their host parasites. Sens. Actuator B-Chem 2007, 127, 112–119. [Google Scholar]
- Hubalek, J.; Hradecky, J.; Adam, V.; Krystofova, O.; Huska, D.; Masarik, M.; Trnkova, L.; Horna, A.; Klosova, K.; Adamek, M.; Zehnalek, J.; Kizek, R. Spectrometric and voltammetric analysis of urease-Nickel nanoelectrode as an electrochemical sensor. Sensors 2007, 7, 1238–1255. [Google Scholar]
- Supalkova, V.; Petrek, J.; Havel, L.; Krizkova, S.; Petrlova, J.; Adam, V.; Potesil, D.; Babula, P.; Beklova, M.; Horna, A.; Kizek, R. Electrochemical sensors for detection of acetylsalicylic acid. Sensors 2006, 6, 1483–1497. [Google Scholar]
- Huska, D.; Zitka, O.; Adam, V.; Beklova, M.; Krizkova, S.; Zeman, L.; Horna, A.; Havel, L.; Zehnalek, J.; Kizek, R. A sensor for investigating the interaction between biologically important heavy metals and glutathione. Czech J. Anim. Sci 2007, 52, 37–43. [Google Scholar]
- Vitecek, J.; Petrlova, J.; Petrek, J.; Adam, V.; Potesil, D.; Havel, L.; Mikelova, R.; Trnkova, L.; Kizek, R. Electrochemical study of S-nitrosoglutathione and nitric oxide by carbon fibre NO sensor and cyclic voltammetry—possible way of monitoring of nitric oxide. Electrochim. Acta 2006, 51, 5087–5094. [Google Scholar]
- Verma, N.; Singh, M. Biosensors for heavy metals. Biometals 2005, 18, 121–129. [Google Scholar]
- Rudnitskaya, A.; Legin, A.; Seleznev, B.; Kirsanov, D.; Vlasov, Y. Detection of ultra-low activities of heavy metal ions by an array of potentiometric chemical sensors. Microchim. Acta 2008, 163, 71–80. [Google Scholar]
- Hwang, G.H.; Han, W.K.; Park, J.S.; Kang, S.G. Determination of trace metals by anodic stripping voltammetry using a bismuth-modified carbon nanotube electrode. Talanta 2008, 76, 301–308. [Google Scholar]
- Zhu, L.D.; Tian, C.Y.; Yang, R.L.; Zhai, J.L. Anodic stripping voltammetric determination of lead in tap water at an ordered mesoporous carbon/Nafion composite film electrode. Electroanalysis 2008, 20, 527–533. [Google Scholar]
- Adami, M.; Marco, S.; Nicolini, C. A potentiometric stripping analyzer for multianalyte screening. Electroanalysis 2007, 19, 1288–1294. [Google Scholar]
- Li, G.; Wan, C.D.; Ji, Z.M.; Wu, K.B. An electrochemical sensor for Cd2+ based on the inducing adsorption ability of I. Sens. Actuator B-Chem 2007, 124, 1–5. [Google Scholar]
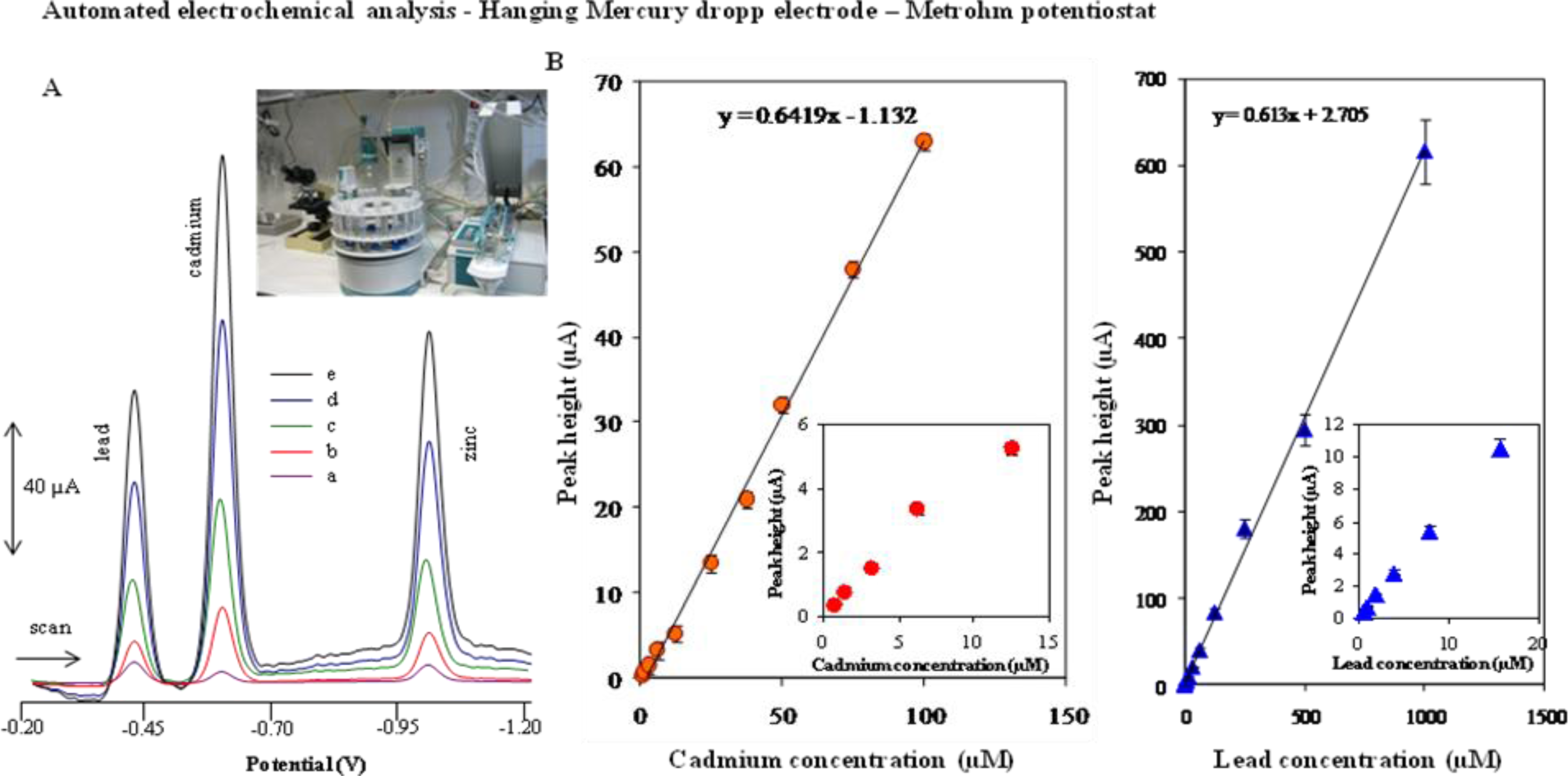

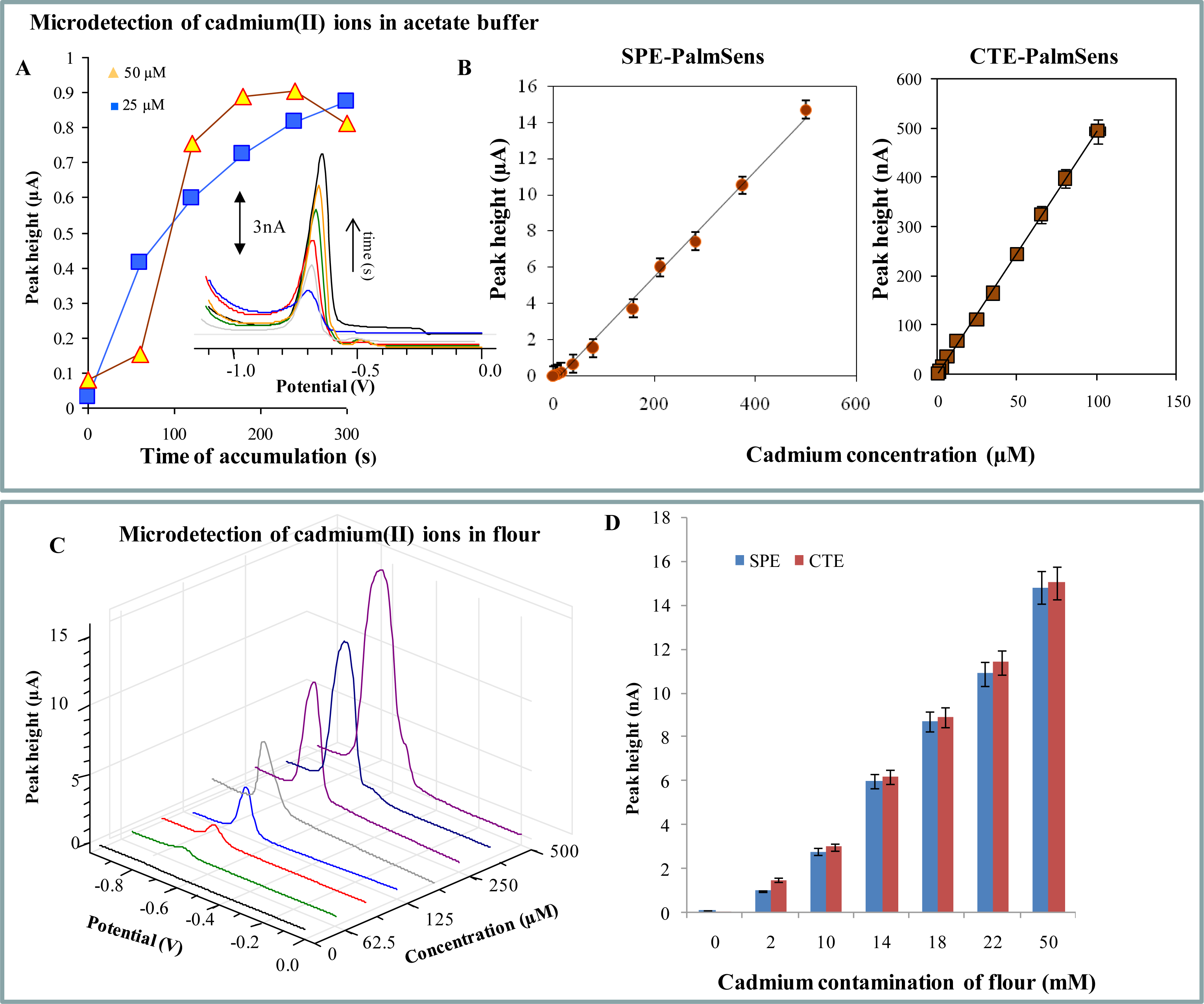
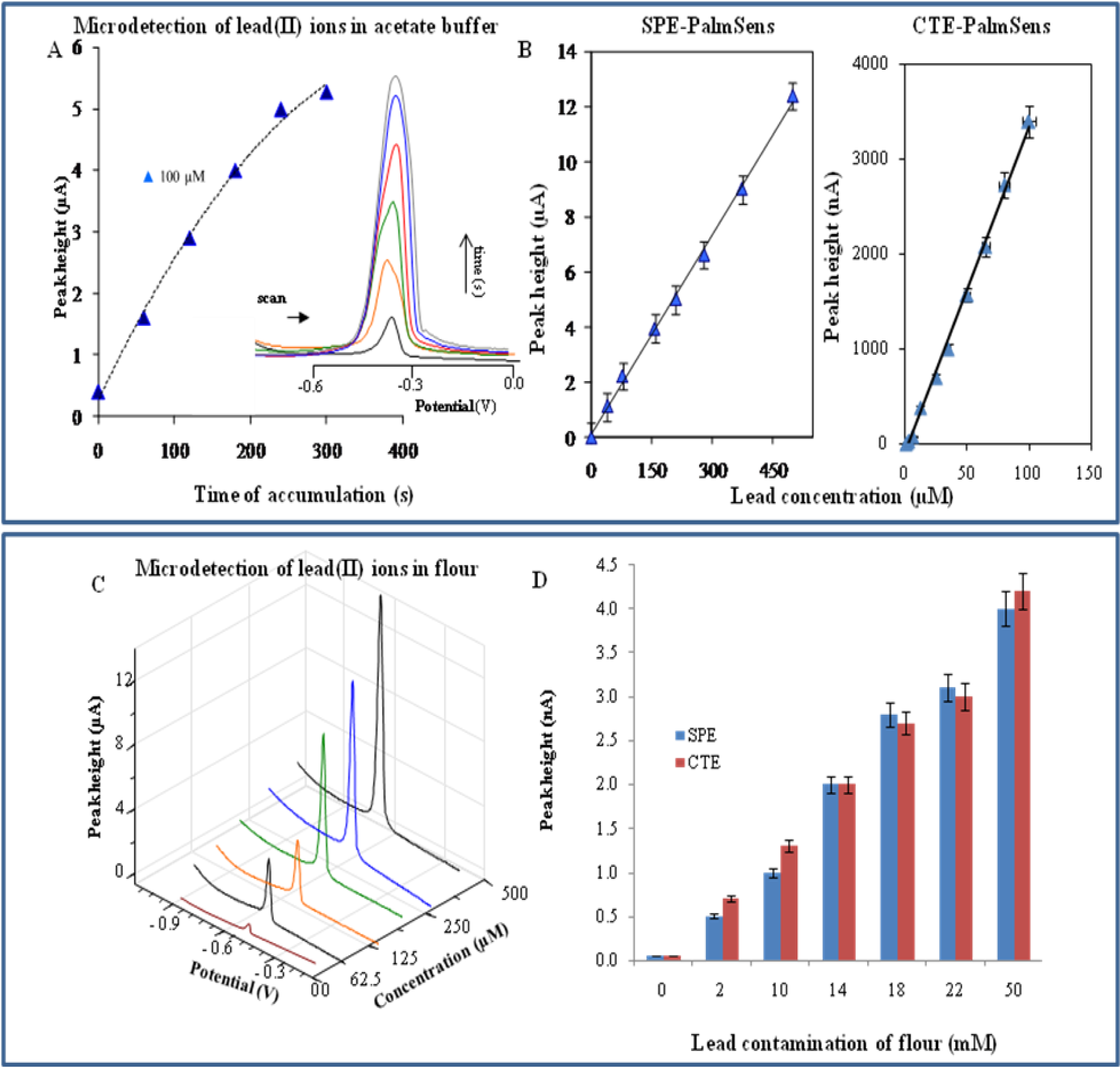
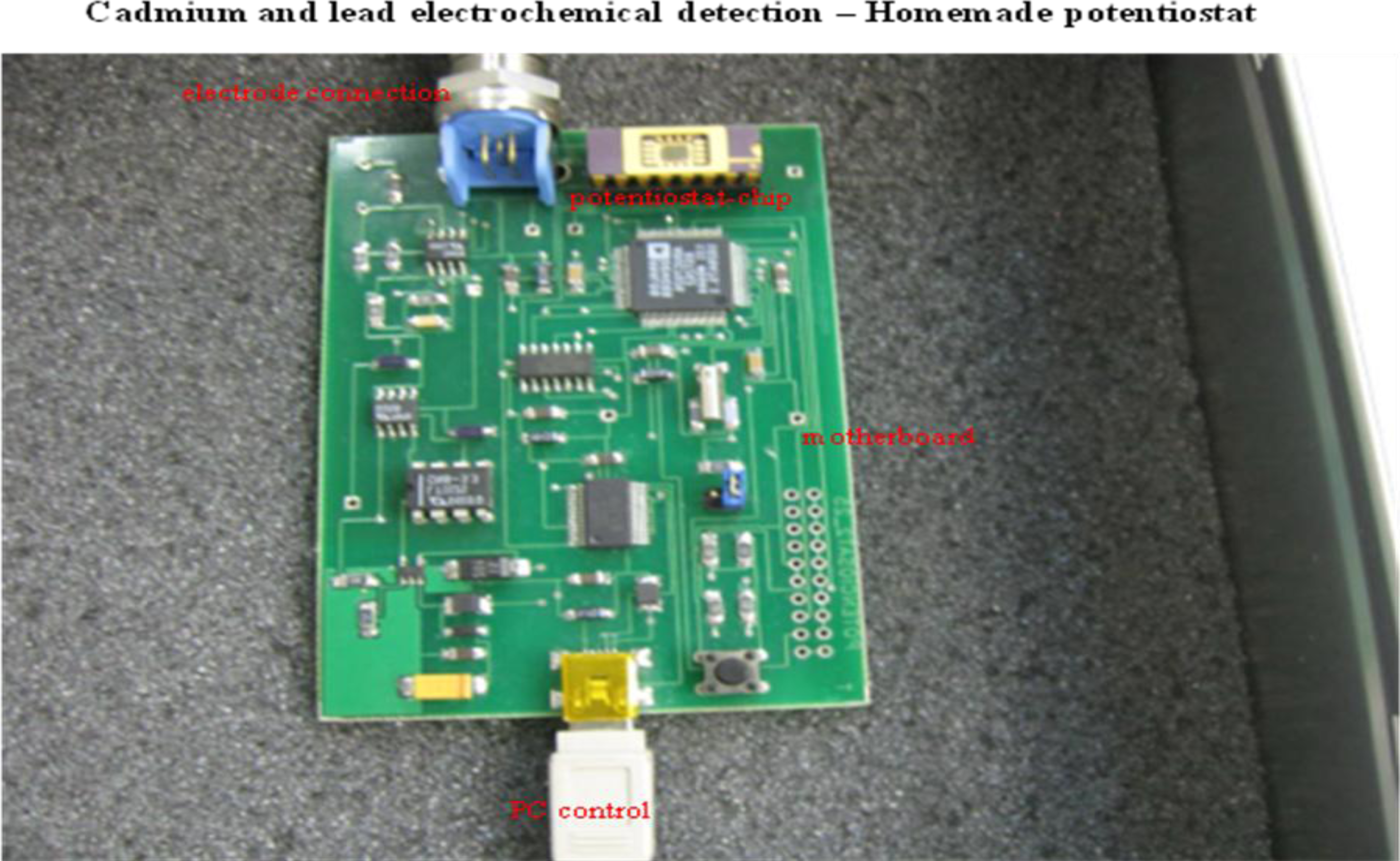
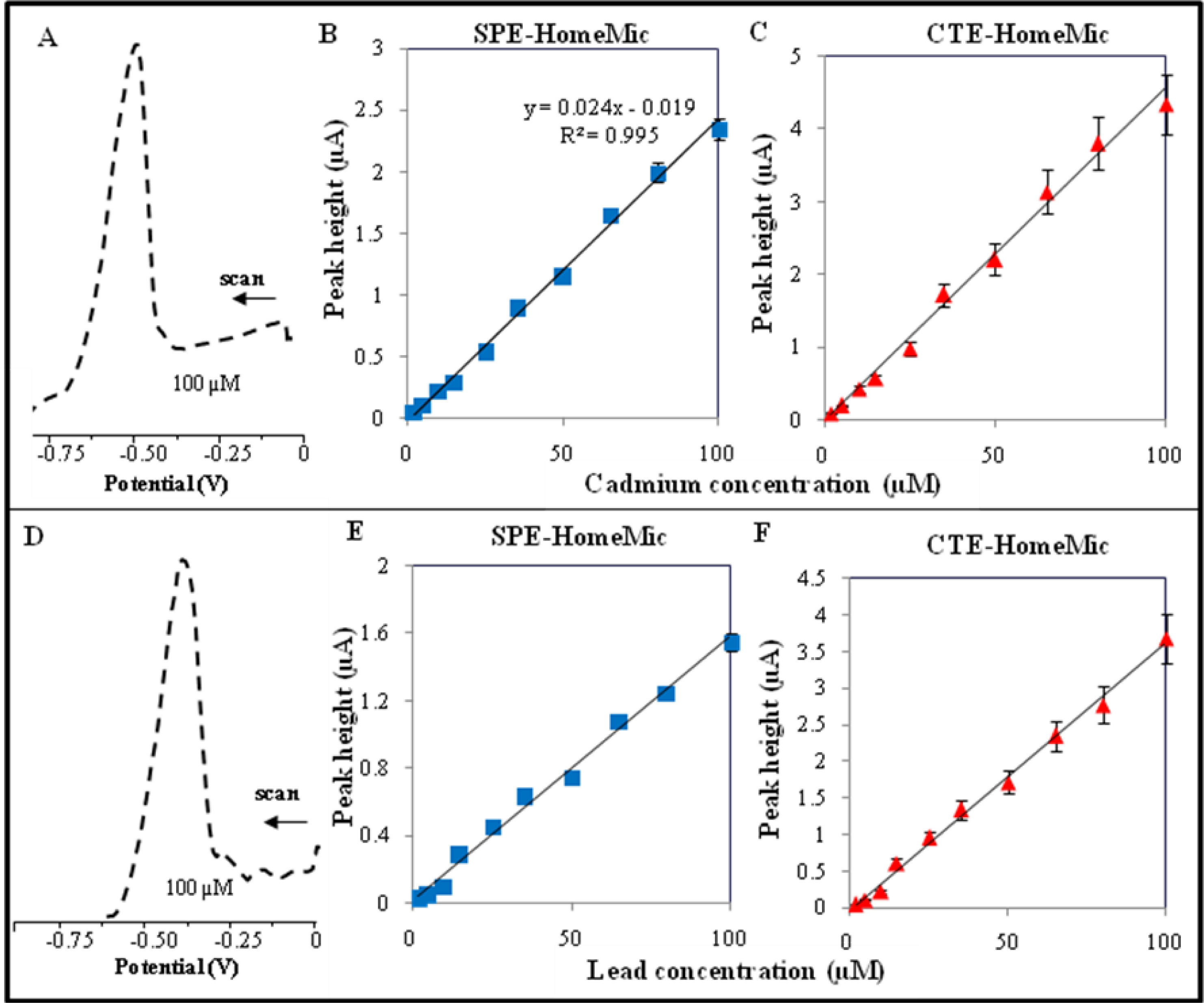

| Element | Potential (V) | Calibration curve equations (nA)— HMDE | R2 | Linear dynamic range (μM) | LOD (nM) | LOC (μM) | Relative standard deviation (%) |
|---|---|---|---|---|---|---|---|
| Cd a,b | −0.595 | I = 0.6419c − 1.132 | 0.9970 | 0.2–150 | 100 | 0.25 | 1.5 |
| Pb a,b | −0.405 | I = 0.6138c + 2.7051 | 0.9976 | 2.5–1,000 | 500 | 1.0 | 1.9 |
| Element | Potential (V) | Calibration curve equations (nA) — SPE-PalmSens | R2 | Linear dynamic range (μM) | LOD (nM) | LOC (μM) | Relative standard deviation (%) |
|---|---|---|---|---|---|---|---|
| Cd a,b | −0.725 | I = 4.955c − 0.713 | 0.9980 | 0.5–100 | 100 | 0.3 | 2.5 |
| Pb a,b | −0.455 | I = 34.37c − 93.81 | 0.9969 | 6–100 | 500 | 1.1 | 2.0 |
| Element | Potential (V) | Calibration curve equations (μA) — CTE-PalmSens | R2 | Linear dynamic range (μM) | LOD (nM) | LOC (μM) | Relative standard deviation (%) |
|---|---|---|---|---|---|---|---|
| Cd a,b | −0.640 | I = 0.0292c − 0.3462 | 0.9952 | 1–500 | 50 | 0.1 | 3.8 |
| Pb a,b | −0.455 | I = 0.0242c + 0.0656 | 0.9981 | 5–500 | 500 | 1.0 | 4.3 |
| Element | Potential (V) | Calibration curve equations (nA) — SPE-HomeMic | R2 | Linear dynamic range (μM) | LOD (nM) | LOC (μM) | Relative standard deviation (%) |
|---|---|---|---|---|---|---|---|
| Cd a,b | −0.663 | I = 24.41c − 18.13 | 0.9953 | 2–100 | 500 | 1.0 | 5.8 |
| Pb a,b | −0.453 | I = 15.68c + 12.11 | 0.9902 | 5–100 | 800 | 1.2 | 6.9 |
| Element | Potential (V) | Calibration curve equations (nA) — CTE-HomeMic | R2 | Linear dynamic range (μM) | LOD (nM) | LOC (μM) | Relative standard deviation (%) |
|---|---|---|---|---|---|---|---|
| Cd a,b | −0.685 | I = 2.20c − 1.99 | 0.9900 | 2–100 | 650 | 1.2 | 8.8 |
| Pb a,b | −0.457 | I = 1.53c + 1.39 | 0.9922 | 5–100 | 880 | 1.8 | 7.3 |
| Metal (samples) | Added (μM) | SPE-PalSens (μM) | SPE-HomeMic (μM) | CTE-PalmSens (μM) | CTE-HomeMic (μM) | HMDE (μM) |
|---|---|---|---|---|---|---|
| Cd a | 50 | 49.5 ± 5.5 | 52.5 ± 10.5 | 50.8 ± 7.5 | 53.5 ± 11.8 | 50.5 ± 3.5 |
| Pb a | 50 | 50.4 ± 6.6 | 54.0 ± 12.0 | 50.5 ± 4.5 | 55.5 ± 10.9 | 50.3 ± 4.5 |
| Cd a | 10 | 9.5 ± 6.5 | 10.5 ± 8.8 | 10.3 ± 7.5 | 10.0 ± 6.8 | 10.5 ± 3.3 |
| Pb a | 10 | 9.9 ± 5.9 | 11.0 ± 9.3 | 10.8 ± 8.5 | 10.5 ± 10.8 | 10.3 ± 4.5 |
| Cd, Pb a | 5 | 4.7 ± 0.8/4.8 ± 0.6 | 4.9 ± 0.5/4.8 ±0.4 | 5.3. ± 0.4/4.8 ± 0.4 | 5.2. ± 0.3/4.9 ± 0.3 | 5.0. ± 0.2/4.9 ± 0.3 |
| Cd, Pb in rainwater b | 5 | 4.8 ± 0.7/4.9 ± 0.8 | 5.1 ± 0.6/4.9 ± 0.6 | 5.5. ± 0.7/5.3 ± 0.5 | 5.3. ± 0.3/5.0 ± 0.2 | 5.0. ± 0.1/4.9 ± 0.3 |
| Cd, Pb in flour c | 5 | 4.4 ± 0.8/4.5 ± 0.8 | 4.3 ± 0.7/4.6 ± 0.7 | 4.5. ± 0.7/4.6 ± 0.5 | 4.7. ± 0.6/4.6 ± 0.6 | 4.7. ± 0.5/4.6 ± 0.7 |
| Cd, Pb in plant d | 5 | 4.1 ± 0.9/4.7 ± 0.8 | 4.6 ± 0.9/4.8 ± 0.9 | 4.5. ± 0.7/4.6 ± 0.7 | 4.5. ± 0.9/4.6 ± 0.8 | 4.6. ± 0.6/4.7 ± 0.7 |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Krystofova, O.; Trnkova, L.; Adam, V.; Zehnalek, J.; Hubalek, J.; Babula, P.; Kizek, R. Electrochemical Microsensors for the Detection of Cadmium(II) and Lead(II) Ions in Plants. Sensors 2010, 10, 5308-5328. https://doi.org/10.3390/s100605308
Krystofova O, Trnkova L, Adam V, Zehnalek J, Hubalek J, Babula P, Kizek R. Electrochemical Microsensors for the Detection of Cadmium(II) and Lead(II) Ions in Plants. Sensors. 2010; 10(6):5308-5328. https://doi.org/10.3390/s100605308
Chicago/Turabian StyleKrystofova, Olga, Libuse Trnkova, Vojtech Adam, Josef Zehnalek, Jaromir Hubalek, Petr Babula, and Rene Kizek. 2010. "Electrochemical Microsensors for the Detection of Cadmium(II) and Lead(II) Ions in Plants" Sensors 10, no. 6: 5308-5328. https://doi.org/10.3390/s100605308
APA StyleKrystofova, O., Trnkova, L., Adam, V., Zehnalek, J., Hubalek, J., Babula, P., & Kizek, R. (2010). Electrochemical Microsensors for the Detection of Cadmium(II) and Lead(II) Ions in Plants. Sensors, 10(6), 5308-5328. https://doi.org/10.3390/s100605308








