A Novel Biosensor for Evaluation of Apoptotic or Necrotic Effects of Nitrogen Dioxide during Acute Pancreatitis in Rat
Abstract
:1. Introduction
2. Results and Disscusion
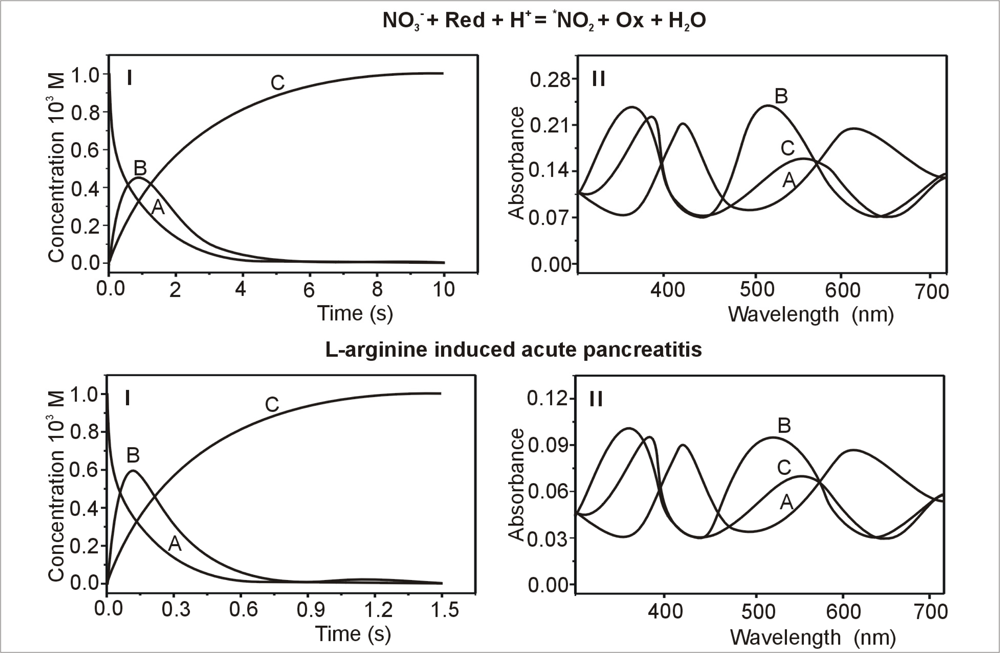
2.1. Kinetics of •NO2 Uptake Generated from Pancreatic Cytosol Fractions
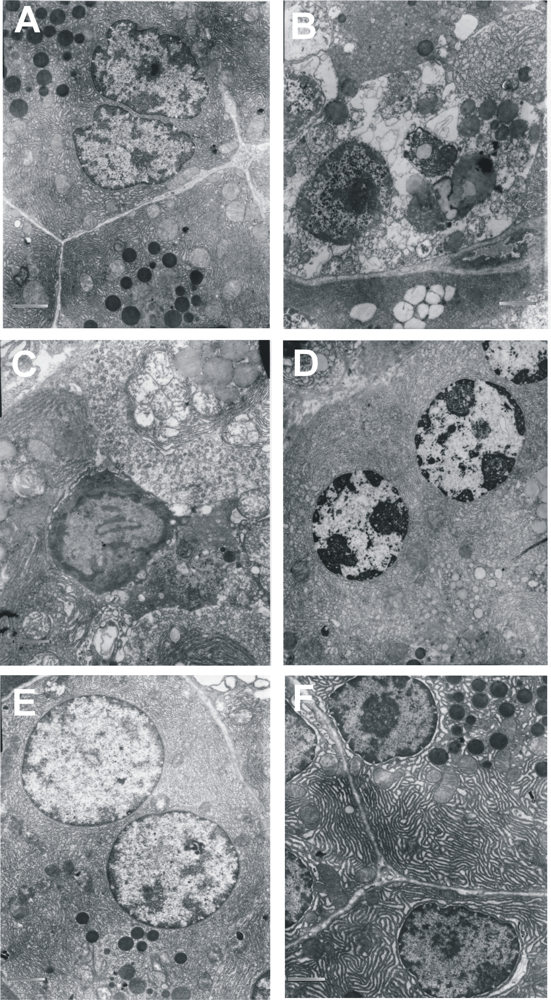
2.2. Biological Study
2.3. Discussion
3. Materials and Methods
3.1. Reagents
3.2. Animals-Experimental Groups
- control rats that received 0.9% NaCl (ip) (n = 8),
- rats injected (ip) with L-arg/HCl solution (pH = 7.4) at a dose of 1.5 g of free amino acid per 1 kg bw (n = 8),
- rats injected (ip) with L-arg/HCl solution (pH = 7.4) at a dose of 3.0 g of free amino acid per 1 kg bw (n = 8),
- rats injected (ip) with 20 μg 4-OH-TEMPO dissolved in 0.9% NaCl; 20 min. later the rats were injected (ip) with L-arg/HCl solution (pH = 7.4) at a dose of 1.5 g of free amino acid per 1 kg bw (n = 8),
- rats injected (ip) with 20μg 4-OH-TEMPO dissolved in 0.9% NaCl; 20 min. later the rats were injected (ip) with L-arg/HCl solution (pH = 7.4) at a dose of 3.0 g of free amino acid per 1 kg of bw (n = 8),
Sample preparation for •NO2 measurements
Samples for microscopic examination
3.3. Spectroscopic Measurements
3.4. Determination of Nitric Dioxide Concentration
3.5. Biochemical Assays
3.6. GSH Assessment
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
References and Notes
- Cho, D.I.; Koo, N.Y.; Chung, W.J.; Kim, T.S.; Ryu, S.Y.; Im, S.Y.; Kim, K.M. Effects of resveratrol-related hydroxystilbenes on the nitric oxide production in macrophage cells: structural requirements and mechanism of action. Life Sci 2002, 71, 2071–2082. [Google Scholar]
- Sledzinski, Z.; Wozniak, M.; Brunelli, A.; Lezoche, E.; Scutti, G.; Kossowska, E.; Jankowski, K.; Stanek, A.; Bertoli, E. Experimental pancreatitis induced by synthetic prooxidant tert-butyl hydroperoxide. Pancreas 2000, 20, 146–151. [Google Scholar]
- Norman, J.; Franz, M.; Messina, J.; Riker, A.; Fabri, P.J.; Rosemurgy, A.S.; Gower, W.R., Jr. Interleukin-1 receptor antagonist decreases severity of experimental acute pancreatitis. Surgery 1995, 117, 648–655. [Google Scholar]
- Viedma, J.A.; Perez-Mateo, M.; Dominguez, J.E.; Carballo, F. Role of interleukin-6 in acute pancreatitis. Comparison with C-reactive protein and phospholipase A. Gut 1992, 33, 1264–1267. [Google Scholar]
- Mizunuma, T.; Kawamura, S.; Kishino, Y. Effects of injecting excess arginine on rat pancreas. J. Nutr 1984, 114, 467–471. [Google Scholar]
- Tu, B.; Wallin, A.; Moldeus, P.; Cotgreave, I.A. Cytotoxicity of NO2 gas to cultured human and murine cells in an inverted monolayer exposure system. Toxicology 1995, 96, 7–18. [Google Scholar]
- Dabrowska, A.; Jacewicz, D.; Chylewska, A.; Szkatula, A.; Knap, N.; Kubasik-Juraniec, J.; Wozniak, M.; Chmurzynski, L. Nitric oxide as biologically important radical and its role in molecular mechanism of pancreatic inflammation. Curr. Pharm. Anal 2008, 4, 183–196. [Google Scholar]
- Jacewicz, D.; Lapinska, A.; Dabrowska, A.; Figarski, A.; Wozniak, M.; Chmurzynski, L. Reactions of *NO2 with chromium(III) complexes with histamine and pyridoxamine ligands studied by the stopped-flow technique. Anal. Biochem 2006, 350, 256–262. [Google Scholar]
- Dabrowska, A.; Sikorski, A.; Jacewicz, D.; Chmurzynski, L. X-ray and conformational analysis of methyl 3-amino-2,3-dideoxy-alpha-D-arabino-hexopyranoside. Carbohydr. Res 2004, 339, 1195–1199. [Google Scholar]
- Dabrowska, A.; Jacewicz, D.; Sikorski, A.; Chmurzynski, L. Crystal structure of methyl 3-amino-2,3-dideoxy-beta-D-arabino-hexopyranoside. Stabilization of the crystal lattice by a double network of N-H...O, O-H...N and O-H...O interactions. Carbohydr. Res 2005, 340, 2201–2205. [Google Scholar]
- Dabrowska, A.; Jacewicz, D.; Makowska, J.; Makowski, M.; Chmurzynski, L. Ab initio study of the energetics of protonation and deprotonation of the methyl 3-amino-2,3-dideoxyhexopyranosides isomers. J. Mol. Struct. : THEOCHEM 2005, 718, 87–92. [Google Scholar]
- Dabrowska, A.; Jacewicz, D.; Lapinska, A.; Banecki, B.; Figarski, A.; Szkatula, M.; Lehman, J.; Krajewski, J.; Kubasik-Juraniec, J.; Wozniak, M.; Chmurzynski, L. Pivotal participation of nitrogen dioxide in L-arginine induced acute necrotizing pancreatitis: protective role of superoxide scavenger 4-OH-TEMPO. Biochem. Biophys. Res. Commun 2005, 326, 313–320. [Google Scholar]
- Goldstein, S.; Samuni, A.; Russo, A. Reaction of cyclic nitroxides with nitrogen dioxide: the intermediacy of the oxoammonium cations. J. Am. Chem. Soc 2003, 125, 8364–8370. [Google Scholar]
- Bonini, M.G.; Mason, R.P.; Augusto, O. The Mechanism by which 4-hydroxy-2,2,6,6-tetramethylpiperidene-1-oxyl (tempol) diverts peroxynitrite decomposition from nitrating to nitrosating species. Chem. Res. Toxicol 2002, 15, 506–511. [Google Scholar]
- Goldstein, S.; Samuni, A.; Merenyi, G. Reactions of nitric oxide, peroxynitrite, and carbonate radicals with nitroxides and their corresponding oxoammonium cations. Chem. Res. Toxicol 2004, 17, 250–257. [Google Scholar]
- Espey, M.G.; Xavier, S.; Thomas, D.D.; Miranda, K.M.; Wink, D.A. Direct real-time evaluation of nitration with green fluorescent protein in solution and within human cells reveals the impact of nitrogen dioxide vs. peroxynitrite mechanisms. Proc. Natl. Acad. Sci. USA 2002, 99, 3481–3486. [Google Scholar]
- Schafer, F.Q.; Buettner, G.R. Redox environment of the cell as viewed through the redox state of the glutathione disulfide/glutathione couple. Free Radic. Biol. Med 2001, 30, 1191–1212. [Google Scholar]
- Hancock, J.T.; Desikan, R.; Neill, S.J. Cytochrome c, glutathione, and the possible role of redox potentials in apoptosis. Ann. N. Y. Acad. Sci 2003, 1010, 446–448. [Google Scholar]
- Jacewicz, D.; Banecki, B.; Dabrowska, A.; Wozniak, M.; Chmurzynski, L. Kinetics and mechanisms of the CO2 and SO2 uptake by coordinate ion, cis-[Cr(C2O4)(L-L)(OH2)2]+ {(L-L) = methyl 3-amino-2,3-dideoxy-alpha-D-arabino-hexopyranoside)} studied by stopped-flow spectrophotometry. Inorg. Chim. Acta 2004, 357, 4467–4475. [Google Scholar]
- Gornall, A.G.; Bardawill, C.J.; David, M.M. Determination of serum proteins by means of the biuret reaction. J. Biol. Chem 1949, 177, 751–766. [Google Scholar]
- Caraway, W.T. A stable starch substrate for the determination of amylase in serum and other body fluids. Am. J. Clin. Pathol 1959, 32, 97–99. [Google Scholar]
- Baker, M.A.; Cerniglia, G.J.; Zaman, A. Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal. Biochem 1990, 190, 360–365. [Google Scholar]


©2010 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/)
Share and Cite
Jacewicz, D.; Dabrowska, A.; Wyrzykowski, D.; Pranczk, J.; Wozniak, M.; Kubasik-Juraniec, J.; Knap, N.; Siedlecka, K.; Neuwelt, A.J.; Chmurzynski, L. A Novel Biosensor for Evaluation of Apoptotic or Necrotic Effects of Nitrogen Dioxide during Acute Pancreatitis in Rat. Sensors 2010, 10, 280-291. https://doi.org/10.3390/s100100280
Jacewicz D, Dabrowska A, Wyrzykowski D, Pranczk J, Wozniak M, Kubasik-Juraniec J, Knap N, Siedlecka K, Neuwelt AJ, Chmurzynski L. A Novel Biosensor for Evaluation of Apoptotic or Necrotic Effects of Nitrogen Dioxide during Acute Pancreatitis in Rat. Sensors. 2010; 10(1):280-291. https://doi.org/10.3390/s100100280
Chicago/Turabian StyleJacewicz, Dagmara, Aleksandra Dabrowska, Dariusz Wyrzykowski, Joanna Pranczk, Michal Wozniak, Jolanta Kubasik-Juraniec, Narcyz Knap, Kamila Siedlecka, Alexander J. Neuwelt, and Lech Chmurzynski. 2010. "A Novel Biosensor for Evaluation of Apoptotic or Necrotic Effects of Nitrogen Dioxide during Acute Pancreatitis in Rat" Sensors 10, no. 1: 280-291. https://doi.org/10.3390/s100100280



