Synthesis of 10-Methoxydiamantan-3-One
Abstract
:1. Introduction
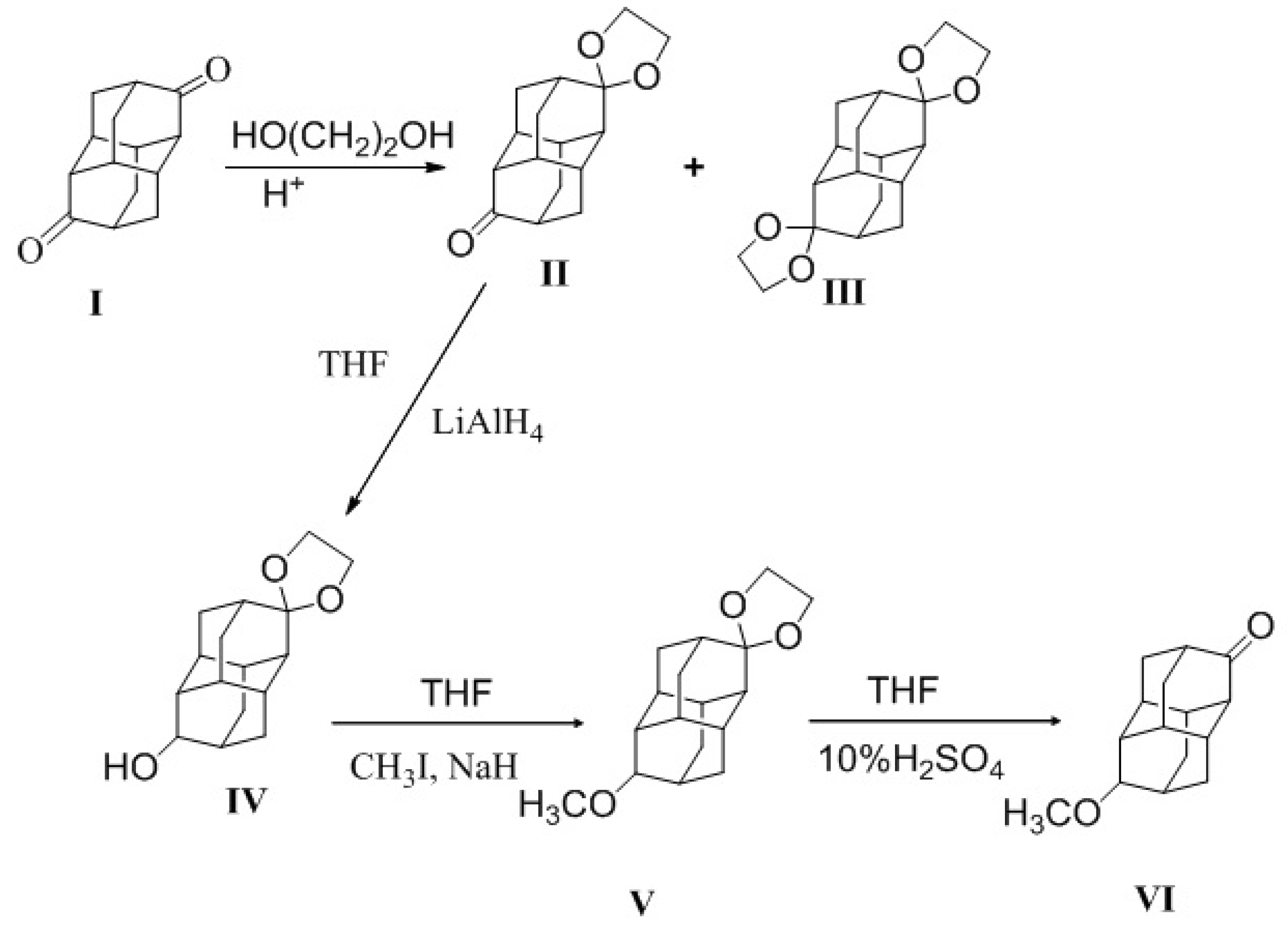
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. 10-Ethylene Ketal Diamantan-3-One (II)
3.3. 3,10 Diethylene Ketal Diamantan (III)
3.4. Ethylene Ketal of 10-Hydroxydiamantan-3-One (IV)
3.5. Ethylene Ketal of 10-Methoxydiamantan-3-One (V)
3.6. 10-Methoxydiamantan-3-One (VI)
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhuk, T.S.; Koso, T.; Pashenko, A.E.; Trung Hoc, N.; Rodionov, V.N.; Serafin, M.; Schreiner, P.R.; Fokin, A.A. Toward an Understanding of Diamond sp2-Defects with Unsaturated Diamondoid Oligomer Models. J. Am. Chem. Soc. 2015, 137, 6577–6586. [Google Scholar] [CrossRef] [PubMed]
- Randel, J.C.; Niestemski, F.C.; Botello-Mendez, A.R.; Mar, W.; Ndabashimiye, G.; Melinte, S.; Dahl, J.E.P.; Carlson, R.M.K.; Butova, E.D.; Fokin, A.A.; et al. Unconventional molecule-resolved current rectification in diamondoid–fullerene hybrids. Nat. Commun. 2014, 5, 4877. [Google Scholar] [CrossRef] [PubMed]
- Shiwata, H.; Acremann, Y.; Scholl, A.; Rotenberg, E.; Hellwig, O.; Dobisz, E.; Doran, A.; Tkachenko, B.A.; Fokin, A.A.; Schreiner, P.R.; et al. Diamondoid coating enables disruptive approach for chemical and magnetic imaging with 10 nm spatial resolution. Appl. Phys. Lett. 2012, 101, 163101. [Google Scholar] [CrossRef]
- Clay, W.A.; Maldonado, J.R.; Pianetta, P.; Dahl, J.E.P.; Carlson, R.M.K.; Schreiner, P.R.; Fokin, A.A.; Tkachenko, B.A.; Melosh, N.A.; Shen, Z.X. Photocathode device using diamondoid and cesium bromide films. Appl. Phys. Lett. 2012, 101, 241605. [Google Scholar] [CrossRef]
- Roth, S.; Leuenberger, D.; Osterwalder, J.; Dahl, J.E.Р.; Carlson, R.M.K.; Tkachenko, B.A.; Fokin, A.A.; Schreiner, P.R.; Hengsberger, M. Negative-electron-affinity diamondoid monolayers as high-brilliance source for ultrashort electron pulses. Chem. Phys. Lett. 2010, 495, 102–108. [Google Scholar] [CrossRef]
- Fokin, A.A.; Zhuk, T.S.; Pashenko, A.E.; Dral, P.O.; Gunchenko, P.A.; Dahl, J.E.P.; Carlson, R.M.K.; Koso, T.V.; Serafin, M.; Schreiner, P.R. Oxygen-doped nanodiamonds: Synthesis and functionalizations. Org. Lett. 2009, 11, 3068–3071. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.L.; Fabbri, J.D.; Willey, T.M.; Lee, J.R.I.; Dahl, J.E.Р.; Carlson, R.M.K.; Schreiner, P.R.; Fokin, A.A.; Tkachenko, B.A.; Fokina, N.A.; et al. Monochromatic electron photoemission from diamondoid monolayers. Science 2007, 316, 1460–1462. [Google Scholar] [CrossRef] [PubMed]
- McMurry, J.E.; Felming, M.P. New method for the reductive coupling of carbonyls to olefins. Synthesis of. beta.-carotene. J. Am. Chem. Soc. 1974, 96, 4708–4709. [Google Scholar] [CrossRef]
- Hoc, N.T.; Kusko, A.O.; Fokin, A.A.; Rodionov, V.N. Functional derivatives of diamantanone. Russ. J. Org. Chem. 2016, 52, 1209–1211. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trung Hoc, N.; Fokin, A.A.; Rodionov, V.N. Synthesis of 10-Methoxydiamantan-3-One. Molbank 2018, 2018, M990. https://doi.org/10.3390/M990
Trung Hoc N, Fokin AA, Rodionov VN. Synthesis of 10-Methoxydiamantan-3-One. Molbank. 2018; 2018(2):M990. https://doi.org/10.3390/M990
Chicago/Turabian StyleTrung Hoc, Ngo, A. A. Fokin, and V. N. Rodionov. 2018. "Synthesis of 10-Methoxydiamantan-3-One" Molbank 2018, no. 2: M990. https://doi.org/10.3390/M990



