A Novel PCC-Catalyzed Process Involving the Oxidative Cleavage of an α-Bromomethyl-tetrahydrofuran Bond. Synthesis of (2S,3R)-2-{(R)-Bromo[(2R,3R,5S,6R)-3,5-dibromo-6-ethyltetrahydro-2H-pyran-2-yl]methyl}-5-oxotetrahydrofuran-3-yl Benzoate
Abstract
:1. Introduction
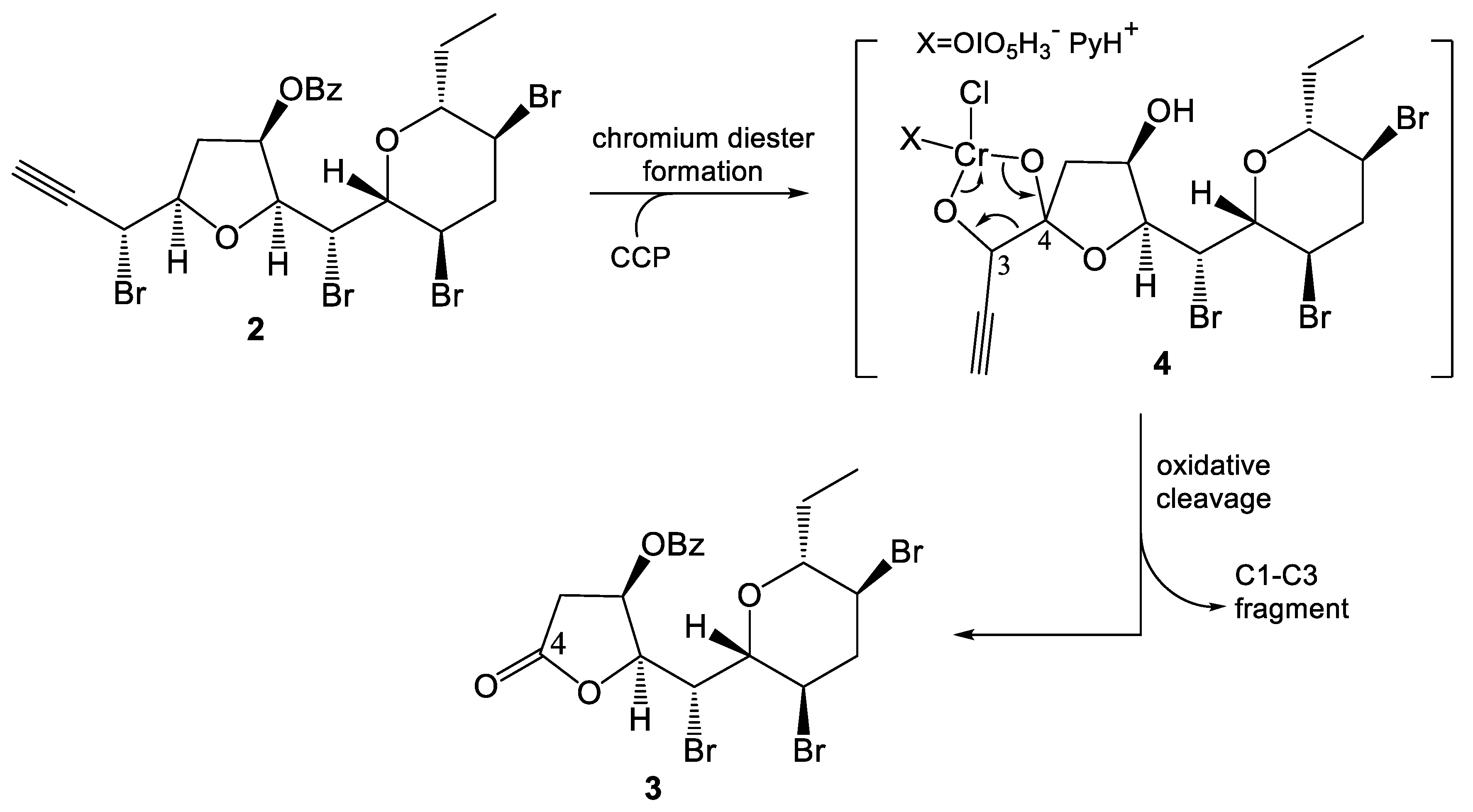
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthesis of (2S,3R,5R)-2-{(R)-Bromo[(2R,3R,5S,6R)-3,5-dibromo-6-ethyltetrahydro-2H-pyran-2-yl]methyl}-5-[(R)-1-bromoprop-2-yn-1-yl]tetrahydrofuran-3-yl Benzoate (2)
3.3. (2S,3R)-2-{(R)-Bromo[(2R,3R,5S,6R)-3,5-dibromo-6-ethyltetrahydro-2H-pyran-2-yl]methyl}-5-oxotetrahydrofuran-3-yl Benzoate (3)
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References
- Mijs, W.J.; De Jonge, C.R.H.I. (Eds.) Organic Syntheses by Oxidation with Metal Compounds; Plenum Press: New York, NY, USA, 1986. [Google Scholar]
- Baeckvall, J.-E. (Ed.) Modern Oxidation Methods, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2010. [Google Scholar]
- Piccialli, V. Oxidative cyclization of dienes and polyenes mediated by transition metal oxo-species. Synthesis 2007, 2585–2607. [Google Scholar] [CrossRef]
- Piccialli, V. Ruthenium tetroxide and perruthenate chemistry. Recent advances and related transformations mediated by other transition metal oxo-species. Molecules 2014, 19, 6534–6582. [Google Scholar] [CrossRef] [PubMed]
- Piancatelli, G.; Scettri, A.; D’Auria, M. Pyridinium chlorochromate: A versatile oxidant in organic synthesis. Synthesis 1982, 245–258. [Google Scholar] [CrossRef]
- Piccialli, V.; Zaccaria, S.; Borbone, N.; Oliviero, G.; D’Errico, S.; Hemminki, A.; Cerullo, V.; Romano, V.; Tuzi, A.; Centore, R. Discovery of a new PCC-mediated stereoselective oxidative spiroketalization process. An access to a new type of poly-THF spiroketal compound displaying anticancer activity. Org. Biomol. Chem. 2009, 7, 3036–3039. [Google Scholar] [CrossRef]
- Piccialli, V.; Zaccaria, S.; Oliviero, G.; D’Errico, S.; D’Atri, V.; Borbone, N. Insight into pyridinium chlorochromate chemistry: Catalytic oxidation of tetrahydrofuran compounds and synthesis of umbelactone. Eur. J. Org. Chem. 2012, 2012, 4293–4305. [Google Scholar] [CrossRef]
- Piccialli, V.; D’Errico, S.; Borbone, N.; Oliviero, G.; Centore, R.; Zaccaria, S. A general synthesis of bis-α-acyloxy-1,4- and -1,5-diketones through catalytic oxidative opening of acylated THF and THP diols. Eur. J. Org. Chem. 2013, 2013, 1781–1789. [Google Scholar] [CrossRef]
- Zaccaria, S.; Borbone, N.; Oliviero, G.; D’Errico, S.; Piccialli, V. Pyridinium chlorochromate chemistry. New insight into oxidation of tetrahydrofurans. Arkivoc 2017, iv, 273–290. [Google Scholar] [CrossRef]
- Ferraz, H.M.C.; Longo, L.S. Bicyclic β-hydroxytetrahydrofurans as precursors of medium ring keto-lactones. J. Org. Chem. 2007, 72, 2945–2950. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Liu, Y.; Tong, R. Cephalosporolide B serving as a versatile synthetic precursor: Asymmetric biomimetic total syntheses of cephalosporolides C, E, F, G, and (4-OMe-)G. Org. Lett. 2013, 15, 5850–5853. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Lee, K.-H.; Lin, Z.; Tong, R. Structural revision of cephalosporolide J and bassianolone. J. Org. Chem. 2014, 79, 1493–1497. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tong, R. Total synthesis of purported cephalosporolides H and I, penisporolide B, and their stereoisomers. J. Org. Chem. 2016, 81, 4325–4339. [Google Scholar] [CrossRef] [PubMed]
- Norris, M.D.; Perkins, M.V. Total synthesis of plakilactones C, B and des-hydroxyplakilactone B by the oxidative cleavage of gracilioether furanylidenes. J. Org. Chem. 2016, 81, 6848–6854. [Google Scholar] [CrossRef] [PubMed]
- Bifulco, G.; Caserta, T.; Gomez-Paloma, L.; Piccialli, V. RuO4-promoted syn-oxidative polycyclization of isoprenoid polyenes: A new stereoselective cascade process. Tetrahedron Lett. 2002, 43, 9265–9269. [Google Scholar] [CrossRef]
- Bifulco, G.; Caserta, T.; Gomez-Paloma, L.; Piccialli, V. Corrigendum to “RuO4-promoted syn-oxidative polycyclization of isoprenoid polyenes: A new stereoselective cascade process”: [Tetrahedron Lett. 43 (2002) 9265]. Tetrahedron Lett. 2003, 44, 3429. [Google Scholar] [CrossRef]
- Piccialli, V.; Borbone, N.; Oliviero, G. Ruthenium-catalyzed oxidative cyclization of 1,7-dienes. A novel diasteroselective synthesis of 2,7-disubstituted trans-oxepane diols. Tetrahedron Lett. 2007, 48, 5131–5135. [Google Scholar] [CrossRef]
- D’Errico, S.; Oliviero, G.; Borbone, N.; Amato, J.; D’Alonzo, D.; Piccialli, V.; Mayol, L.; Piccialli, G. A facile synthesis of 5′-fluoro-5′-deoxyacadesine (5′-F-AICAR): A novel non-phosphorylable AICAR analogue. Molecules 2012, 17, 13036–13044. [Google Scholar] [CrossRef] [PubMed]
- Giordano, F.; Mayol, L.; Notaro, G.; Piccialli, V.; Sica, D. Structure and absolute configuration of two new polybrominated C15 acetogenins from the sponge Mycale rotalis. Chem. Commun. 1990, 1559–1561. [Google Scholar] [CrossRef]
- McLafferty, F.W. Mass Spectrometric Analysis... Aliphatic Ethers. Anal. Chem. 1957, 29, 1782–1789. [Google Scholar] [CrossRef]
- Hunsen, M. Pyridinium chlorochromate catalyzed oxidation of alcohols to aldehydes and ketones with periodic acid. Tetrahedron Lett. 2005, 46, 1651–1653. [Google Scholar] [CrossRef]
- Caserta, T.; Piccialli, V.; Gomez-Paloma, L.; Bifulco, G. RuO4-catalyzed oxidative polycyclization of squalene. Determination of the configuration of the penta-tetrahydrofuranyl diol product. Tetrahedron 2005, 61, 927–939. [Google Scholar] [CrossRef]

© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piccialli, V. A Novel PCC-Catalyzed Process Involving the Oxidative Cleavage of an α-Bromomethyl-tetrahydrofuran Bond. Synthesis of (2S,3R)-2-{(R)-Bromo[(2R,3R,5S,6R)-3,5-dibromo-6-ethyltetrahydro-2H-pyran-2-yl]methyl}-5-oxotetrahydrofuran-3-yl Benzoate. Molbank 2017, 2017, M969. https://doi.org/10.3390/M969
Piccialli V. A Novel PCC-Catalyzed Process Involving the Oxidative Cleavage of an α-Bromomethyl-tetrahydrofuran Bond. Synthesis of (2S,3R)-2-{(R)-Bromo[(2R,3R,5S,6R)-3,5-dibromo-6-ethyltetrahydro-2H-pyran-2-yl]methyl}-5-oxotetrahydrofuran-3-yl Benzoate. Molbank. 2017; 2017(4):M969. https://doi.org/10.3390/M969
Chicago/Turabian StylePiccialli, Vincenzo. 2017. "A Novel PCC-Catalyzed Process Involving the Oxidative Cleavage of an α-Bromomethyl-tetrahydrofuran Bond. Synthesis of (2S,3R)-2-{(R)-Bromo[(2R,3R,5S,6R)-3,5-dibromo-6-ethyltetrahydro-2H-pyran-2-yl]methyl}-5-oxotetrahydrofuran-3-yl Benzoate" Molbank 2017, no. 4: M969. https://doi.org/10.3390/M969





