High-level Expression of Cecropin X in Escherichia coli
Abstract
:1. Introduction
2. Results and Discussion
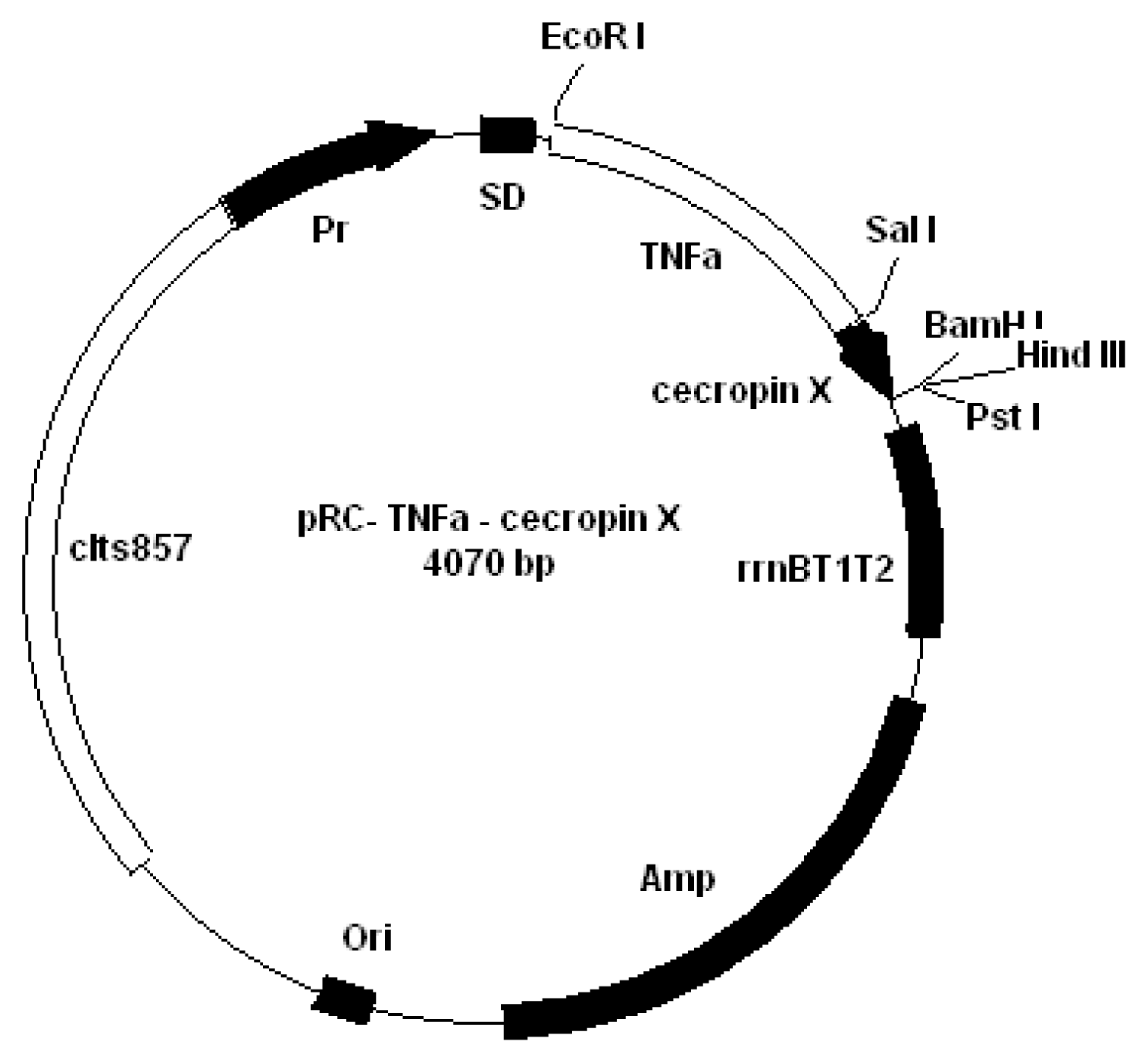
2.1 Plasmid construction
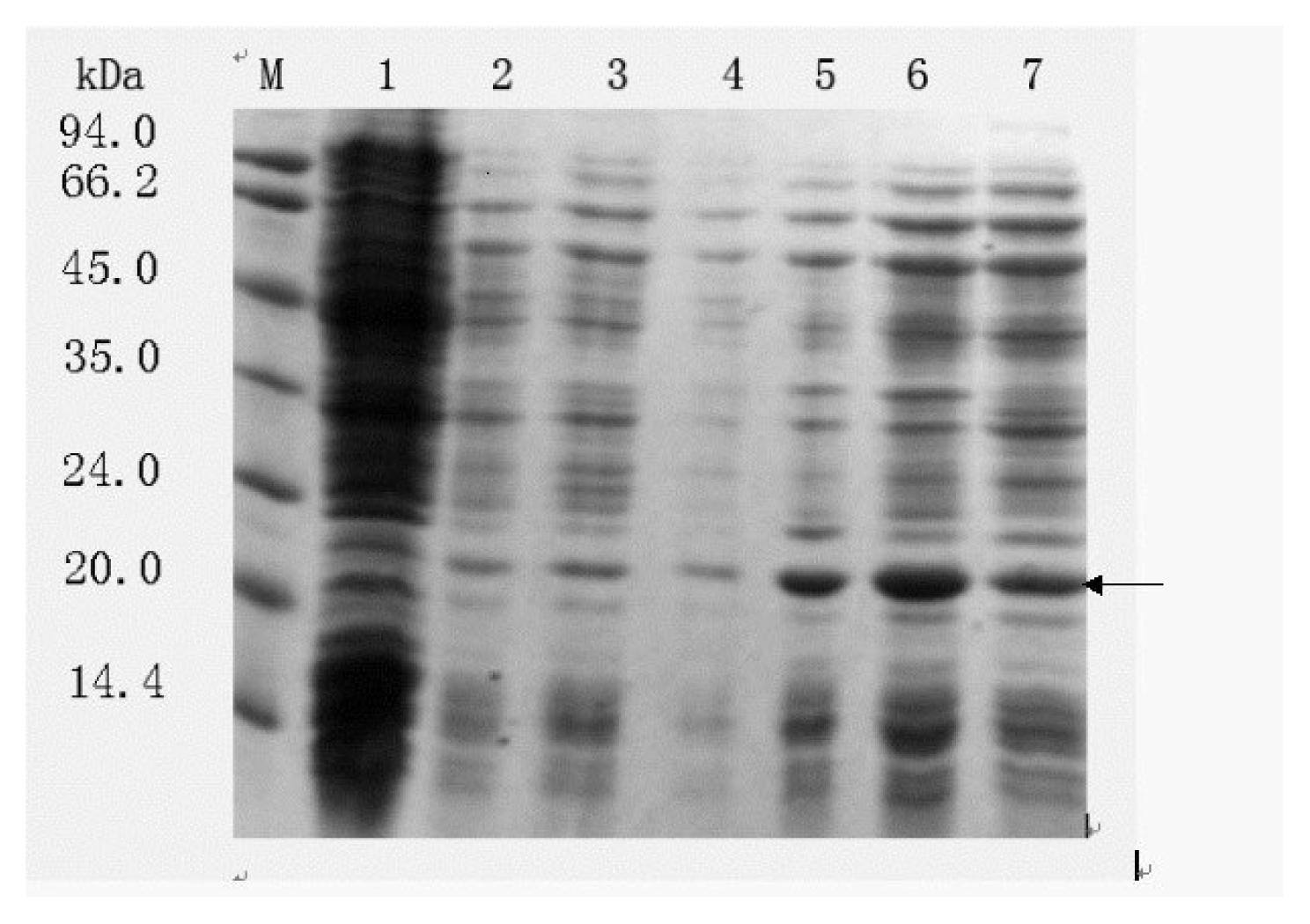
2.2. Expression of TNFα–cecropin X fusion protein in the E. coli strain TG1
2.3. Optimization of culture conditions for the overexpression of TNFα–cecropin X fusion protein in E. coli
2.3.1 Effect of host cells on fusion protein formation
2.3.2 Fermentation medium
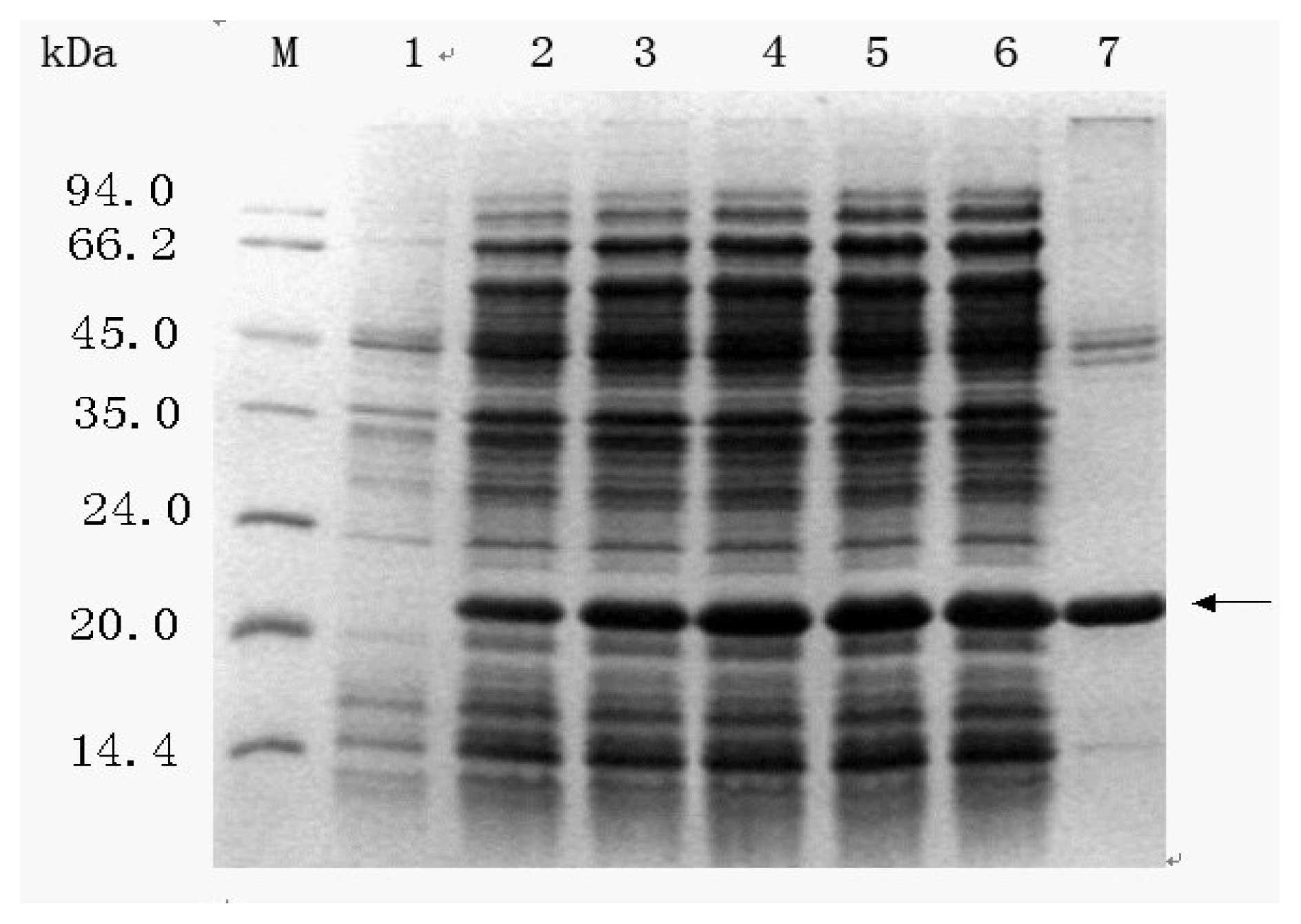
2.3.3 Induction timing
2.3.4 Post-induction time
2.3.5 Effect of aeration
2.3.6 Batch fermentation in a 30 l fermentor
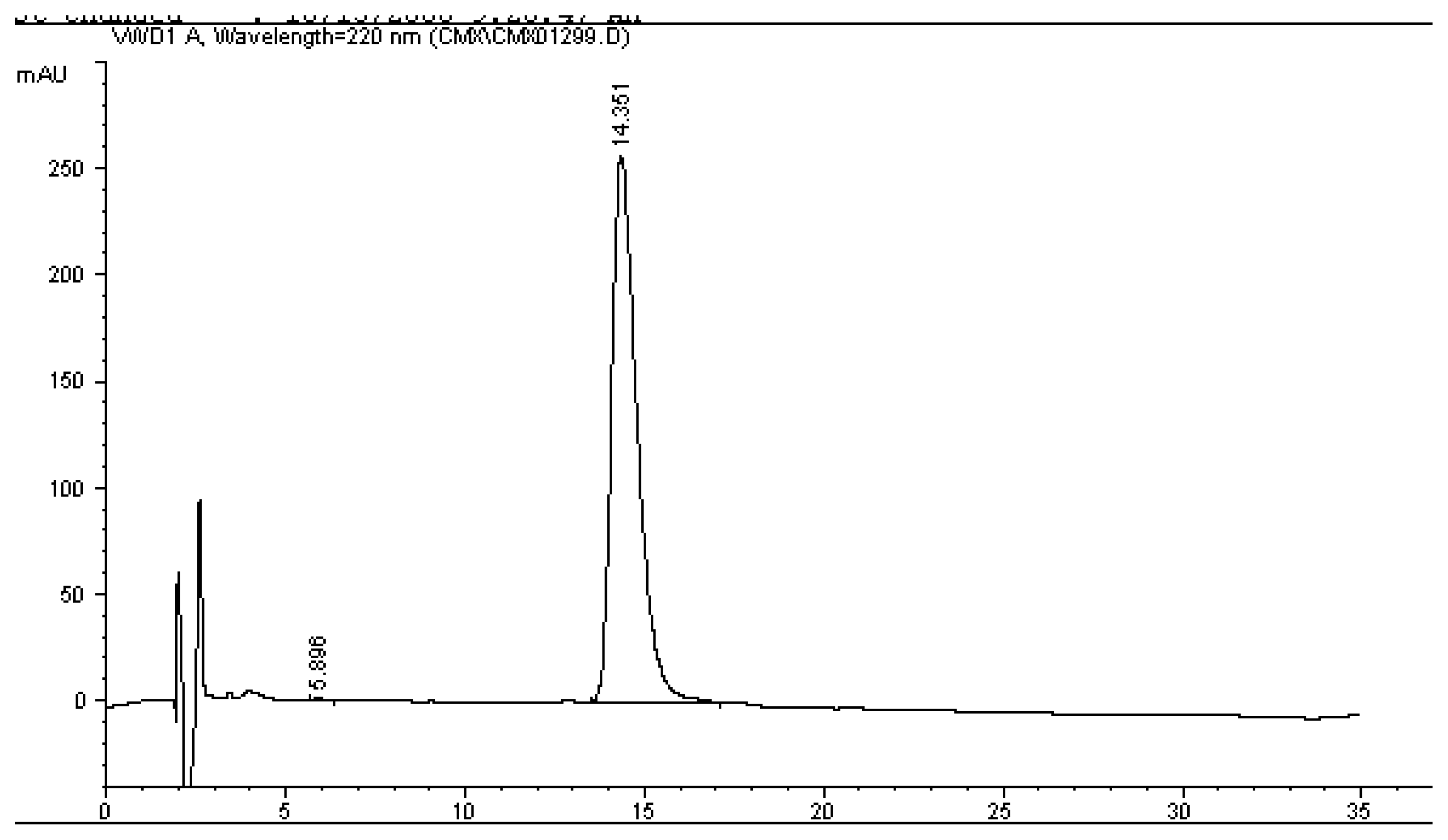
2.4 RP-HPLC analysis of cecropin X
2.5 Assay of biological activity
3. Conclusions
4. Materials and methods
4.1 Plasmid construction
4.2 Medium [31]
4.3 Strains [31]
4.4 Expression of fusion protein
4.5 Expression of fusion protein in a 30 l fermentor and purification of cecropin X
4.6 SDS-PAGE and reverse-phase HPLC analysis
4.7 Assay of biological activity of cecropin X [32]
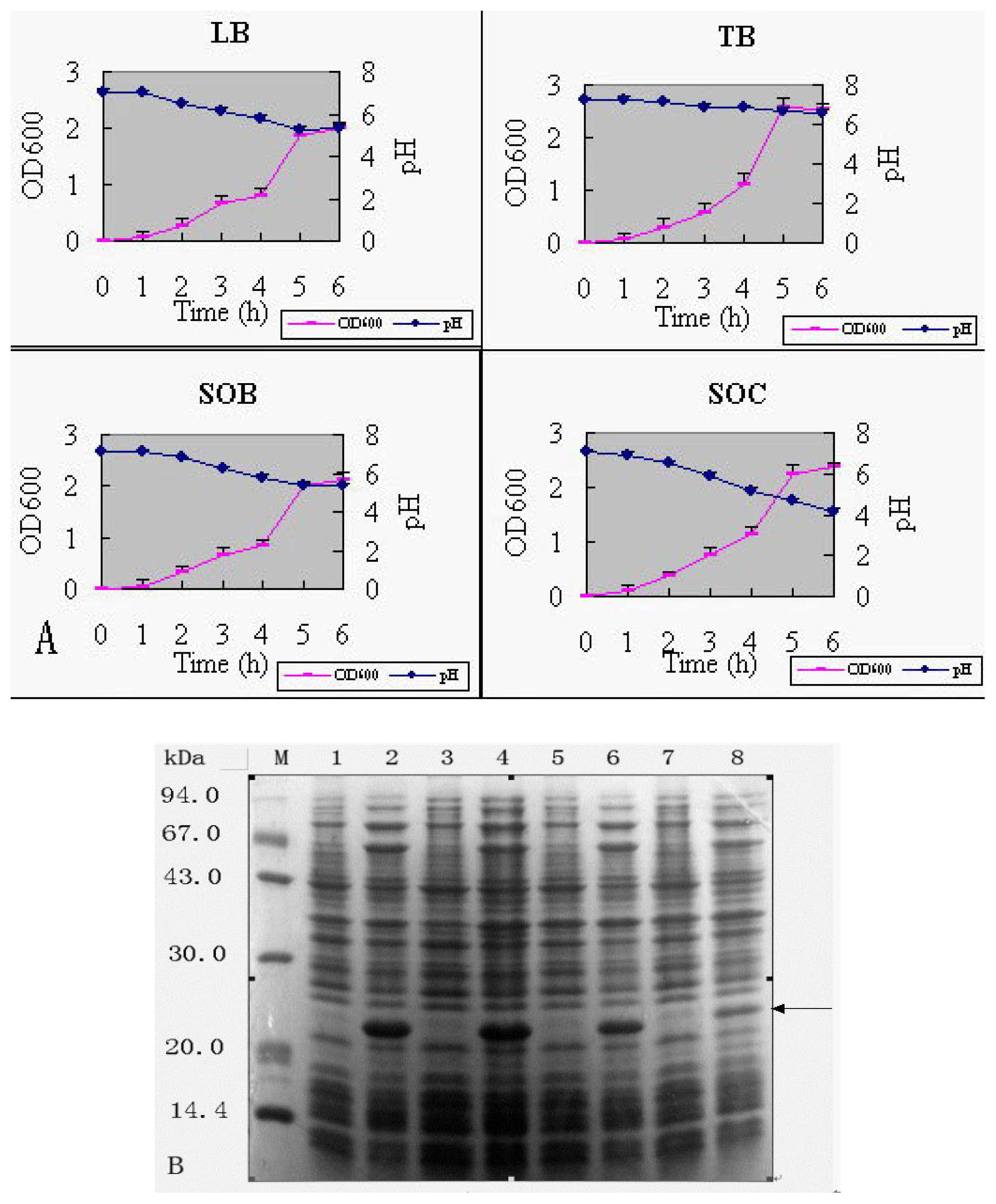
| Per liter | LB | TB | SOB | SOC |
|---|---|---|---|---|
| Tryptone | 10 g | 12 g | 20 g | 20 g |
| Yeast extract | 5 g | 24 g | 5 g | 5 g |
| Salt | NaCl 10 g | 2.31 g KH2PO4 12.54 g K2HPO4 | NaCl 0.5 g KCl 0.186 g MgCl2 0.95 g | NaCl 0.5 g KCl 0.186 g MgCl2 0.95 g |
| Carbon |  | Glycerol 4 ml |  | Glucose 3.6 g |
| Sample (h) A | OD600 | pH | Total protein (g/l) B | Fusion protein (%) C | Cecropin X (g/l)D |
|---|---|---|---|---|---|
| 0 | 8.128 | 7.15 | 9.255 | 0 | 0 |
| 1 | 12.972 | 6.92 | 12.438 | 15.08 | 0.357 |
| 2 | 16.293 | 6.87 | 17.238 | 19.92 | 0.654 |
| 3 | 18.621 | 6.77 | 19.347 | 22.32 | 0.823 |
| 4 | 20.138 | 6.68 | 21.973 | 22.43 | 0.939 |
| 5 | 21.453 | 6.70 | 22.778 | 22.51 | 0.977 |
| Purification steps | cecropin X (mg) | Yield (%) |
|---|---|---|
| Inclusion bodies 5.2 gA | 812 B | 100 |
| Supernatant liquidC | 143 D | 17.6 |
| Purified cecropin X | 106 D | 13.1 |
| Group | Dose (mg/kg day) | Given pathway | Animal number Start/End | Weight (g) Start/End | Tumor weight (g) X+SD | Inhibitory rate (%) |
|---|---|---|---|---|---|---|
| Cecropin X | 32 | i.v. | 10/10 | 20.1/25.1 | 1.21+0.14 | 54.34 |
| Cecropin X | 8 | i.v. | 10/10 | 20.7/25.9 | 1.39+0.11 | 47.55 |
| Cecropin X | 2 | i.v. | 10/10 | 20.5/25.3 | 1.69+0.13 | 36.23 |
| Cecropin X | 0.5 | i.v. | 10/10 | 20.4/25.5 | 1.93+0.11 | 27.17 |
| CTX | 30 | i.p. | 10/10 | 20.3/23.4 | 0.27+0.12 | 89.74 |
| NS | — | i.v. | 20/20 | 20.4/25.9 | 2.65+0.23 | — |
Acknowledgements
References and Notes
- Steiner, H.; Hultmark, D.; Engstrom, A.; Bennich, H.; Boman, H.G. Sequence and specificity of two antibacterial proteins involved in insect immunity. Nature 1981, 292, 246–248. [Google Scholar]
- Boman, H.G.; Hultmark, D. Cell-free immunity in insects. Ann. Rev. Microbiol 1987, 41, 103–126. [Google Scholar]
- Zhang, S.Q.; Qu, X.M.; Qi, Z.W. Insect immunity and the applied prospect of cecropins. J. Biochem 1987, 3, 11–18. [Google Scholar]
- Zhang, S.Q.; Jia, H.W.; Zhu, Y.D. Ultrastructure observation of K562 leukemia cells treated with antibacterial peptide CM4 component. Prog. Biochem. Biophys 1997, 24, 159–163. [Google Scholar]
- Xu, J.; Zhang, S.Q. The mechanism of antibacterial of antibacterial peptide CMIV component was observed by confocal laser scanning microscope. Prog. Nat. Sci 2001, 10, 1105–1109. [Google Scholar]
- Dou, F.; Xie, W.; Dong, X.Y.; Xu, X.X. The terminal structure plays an important role in the biological activity of cecropin CMIV. Sci. China (Ser. C) 1999, 42, 494–500. [Google Scholar]
- Nakajima, Y.K.; Qu, X.M.; Natori, S.J. Interaction between liposomes and Sarcotoxin IA, a potent antibacterial protein of Sarcophaga Peregrina (Flesh Fly). J. Bio. Chem 1987, 262, 1665–1669. [Google Scholar]
- Xie, W.; Qiu, Q.F.; Chen, J.N.; Ma, Z.; Xu, X.X. Chemical synthesis and cloning of cecropin CMIV gene from Chinese silkworm. J. Nanjing Univ. (Nat. Sci.) 1996, 32, 474–478. [Google Scholar]
- Xie, W.; Qiu, Q.F.; Dong, X.Y.; Hua, Z.C.; Xu, X.X. Fusion expression of mutated cecropin CM IV in E. coli. Sci. China (Ser. C) 1997, 40, 225–231. [Google Scholar]
- Xie, W.; Dou, F.; Qiu, Q.F.; Zhang, J.; Zhang, X.; Xu, X.X. Production and activities against clinical pathogenic bacteria of recombinant CMIV mutant. Prog. Microbiol. Immunol 1997, 25, 46–49. [Google Scholar]
- Wang, L.; Wu, H.H.; Dou, F.; Xie, W.; Xu, X.X. High-level expression of cecropin CMIV in E. coli from a fusion construct containing the human tumor necrosis factor. Biochem. Mol. Biol. Int 1997, 41, 1051–1056. [Google Scholar]
- Seegers Jos, F.M.L.; Franke, C.M.; Kieweit, R.; Venema, G.; Bron, S. Use of continuous culture for the selection of plasmids with improved segregational stability. Plasmid 1995, 33, 71–77. [Google Scholar]
- Nayak, D.P.; Vyas, V.V. Improved stability and expression of a recombinant shuttle plasmid in Escherichia coli during fedbatch cultivation. World. J. Microbiol. Biotechnol 1999, 15, 65–71. [Google Scholar]
- Andersons, D.; Engstrom, A.; Josephson, S.; Hansson, L.; Steiner, H. Biologically active and amidated cecropin produced in a baclovirus expression system from a fusion construct containing the antibody-binding part of protein A. Biochem. J 1991, 280, 219–224. [Google Scholar]
- Hara, S.; Yamakawa, M. Production in Escherichia coli of moricin, a novel type antibacterial peptide from the silkworm, Bombyx mori. Biochem. Biophys. Res. Commun 1996, 220, 664–669. [Google Scholar]
- Harrison, S.J.; McManus, A.M.; Marcus, J.P.; Goulter, K.C.; Green, J.L.; Nielsen, K.J.; Craik, D.J.; Maclean, D.J.; Manners, J.M. Purification and characterization of a plant antimicrobial peptide expressed in Escherichia coli. Protein. Expr. Purif 1999, 15, 171–177. [Google Scholar]
- Lee, J.H.; Kim, J.H.; Hwang, S.W.; Lee, W.J.; Yoon, H.K.; Lee, H.S.; Hong, S.S. High-level expression of antimicrobial peptide mediated by a fusion partner reinforcing formation of inclusion bodies. Biochem. Biophys. Res. Commun 2000, 277, 575–580. [Google Scholar]
- Hwang, S.W.; Lee, J.H.; Park, H.B.; Pyo, S.H.; So, J.E.; Lee, H.S.; Hong, S.S.; Kim, J.H. A simple method for the purification of an antimicrobial peptide in recombinant Escherichia coli. Mol. Biotechnol 2001, 18, 193–198. [Google Scholar]
- Skosyrev, V.S.; Kulesskiy, E.A.; Yakhnin, A.V.; Temirov, Y.V.; Vinokurov, L.M. Expression of the recombinant antibacterial peptide sarcotoxin IA in Escherichia coli cells. Protein. Expr. Purif 2003, 28, 350–356. [Google Scholar]
- Xu, X.X.; Jin, F.L.; Yu, X.Q.; Ji, S.X.; Wang, J.; Cheng, H.X.; Wang, C.; Zhang, W.Q. Expression and purification of a recombinant antibacterial peptide, cecropin, from Escherichia coli. Protein. Expr. Purif 2007, 53, 293–301. [Google Scholar]
- Chen, J.J.; Sun, M.; Chen, C.Q.; Lu, F.; Wang, D.B. Construction and application of a high level expression vector containing PR promoter. Prog. Biochem. Biophys 1996, 26, 495–499. [Google Scholar]
- Palomares, A.J.; Vazquez, M.E.; Rodriguez-Llorente, I.D.; Dary, M.; Caviedes, M.A. Plasmid transfer detection in soil using the inducible Lambda PR system fused to eukaryotic luciferase genes. Microbial. Ecology 2001, 41, 352–359. [Google Scholar]
- Lan, P.; Tseng, C.; Lin, M.; Chang, C. Expression and purification of human placenta lactogen in Escherichia coli. Protein. Expr. Purif 2006, 46, 285–293. [Google Scholar]
- Jeong, K.; Choi, J.; Yoo, W.; Keum, K.; Yoo, N.; Lee, S.; Sung, M. Constitutive production of human leptin by fed-batch culture of recombinant rpoS- Escherichia coli. Protein. Expr. Purif 2004, 36, 150–156. [Google Scholar]
- Zhang, X.; Sun, T.; Liu, X.; Gu, D.; Tang, Z. Production of granulocyte-macrophage colony stimulating factor (GM-CSF) by high cell density fermentation of secretory recombination Escherichia coli. Process. Biochem 1999, 34, 55–58. [Google Scholar]
- Peng, L.; Xu, Z.; Fang, X.; Wang, F.; Cen, P. High-level expression of soluble human β-defensin- 2 in Escherichia coli. Process. Biochem 2004, 39, 2199–2205. [Google Scholar]
- Datta, A.; Kitson, R.P.; Xue, Y.M.; Al-Atrash, G.; Mazar, A.P.; Jones, T.R.; Goldfarb, R.H. Combined chemoanti-angiogenic cancer therapy against Lewis lung carcinoma (3LL) pulmonary metastases. In vivo 2002, 16, 451–457. [Google Scholar]
- Silvestro, L.; Gupta, K.; Weiser, J.N.; Axelsen, P.H. The concentration-dependent membrane activity of Cecropin A. Biochem 1997, 36, 11452–11460. [Google Scholar]
- Kaspar, A.A.; Okada, S.; Kumar, J.; Poulain, F.R.; Drouvalakis, K.A.; Kelekar, A.; Hanson, D.A.; Kluck, R.M.; Hitoshi, Y.; Johnson, D.E.; Froelich, C.J.; Thompson, C.B.; Newmeyer, D.D.; Anel, A.; Clayberger, C.; Krensky, A.M. A distinct pathway of cell-mediated apoptosis initiated by granulysin. J. Immunol 2001, 167, 350–356. [Google Scholar]
- Chen, J.; Xu, X.; Underhill, C.B.; Yang, S.M.; Wang, L.P.; Chen, Y.X.; Hong, S.G.; Creswell, K.; Zhang, L.R. Tachyplesin activates the classic complement pathway to kill tumor cells. Cancer. Res 2005, 65, 4614–4622. [Google Scholar]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbour Laboratory Press: New York, 1989; pp. 16–69. [Google Scholar]
- Ge, B.S.; Tang, Z.H.; Zhao, F.Q.; Ren, Y.H.; Yang, Y.; Qin, S. Scale-up of fermentation and purification of recombinant allophycocyanin over-expressed in Escherichia coli. Process Biochem 2005, 40, 3190–3195. [Google Scholar]
© 2007 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Shen, Y.; Lao, X.G.; Chen, Y.; Zhang, H.Z.; Xu, X.X. High-level Expression of Cecropin X in Escherichia coli. Int. J. Mol. Sci. 2007, 8, 478-491. https://doi.org/10.3390/i8060479
Shen Y, Lao XG, Chen Y, Zhang HZ, Xu XX. High-level Expression of Cecropin X in Escherichia coli. International Journal of Molecular Sciences. 2007; 8(6):478-491. https://doi.org/10.3390/i8060479
Chicago/Turabian StyleShen, Yi, Xue Gang Lao, Yuan Chen, Hong Zu Zhang, and Xian Xiu Xu. 2007. "High-level Expression of Cecropin X in Escherichia coli" International Journal of Molecular Sciences 8, no. 6: 478-491. https://doi.org/10.3390/i8060479




