Hydrolysis and Partial Recycling of a Chloroaluminate Ionic Liquid
Abstract
:1. Introduction
2. Experimental Section
2.1 Materials
- (1)
- 1:1 Nitric acid was prepared with a 1:1 volume ratio of nitric acid (65 % – 68 %, m/m) to deionized water and 1 mol/l nitric acid was prepared with nitric acid (65 % – 68 %, m/m, 63 ml) diluted to 1l in a volumetric flask.
- (2)
- Bromophenol blue indicator was prepared by dissolving bromophenol blue (0.1 g) in ethanol (20 %) to 100 ml in a volumetric flask.
- (3)
- Phenylazoformic acid 2-phenylhydrazide indicator was prepared with phenylazoformic acid 2-phenylhydrazide (0.5 g) dissolved by ethanol (95 %) and made up to 100 ml in a volumetric flask.
- (4)
- Phenolphthalein indicator was prepared with phenolphthalein (0.5 g) dissolved by ethanol (95 %) and made up to 100 ml in a volumetric flask.
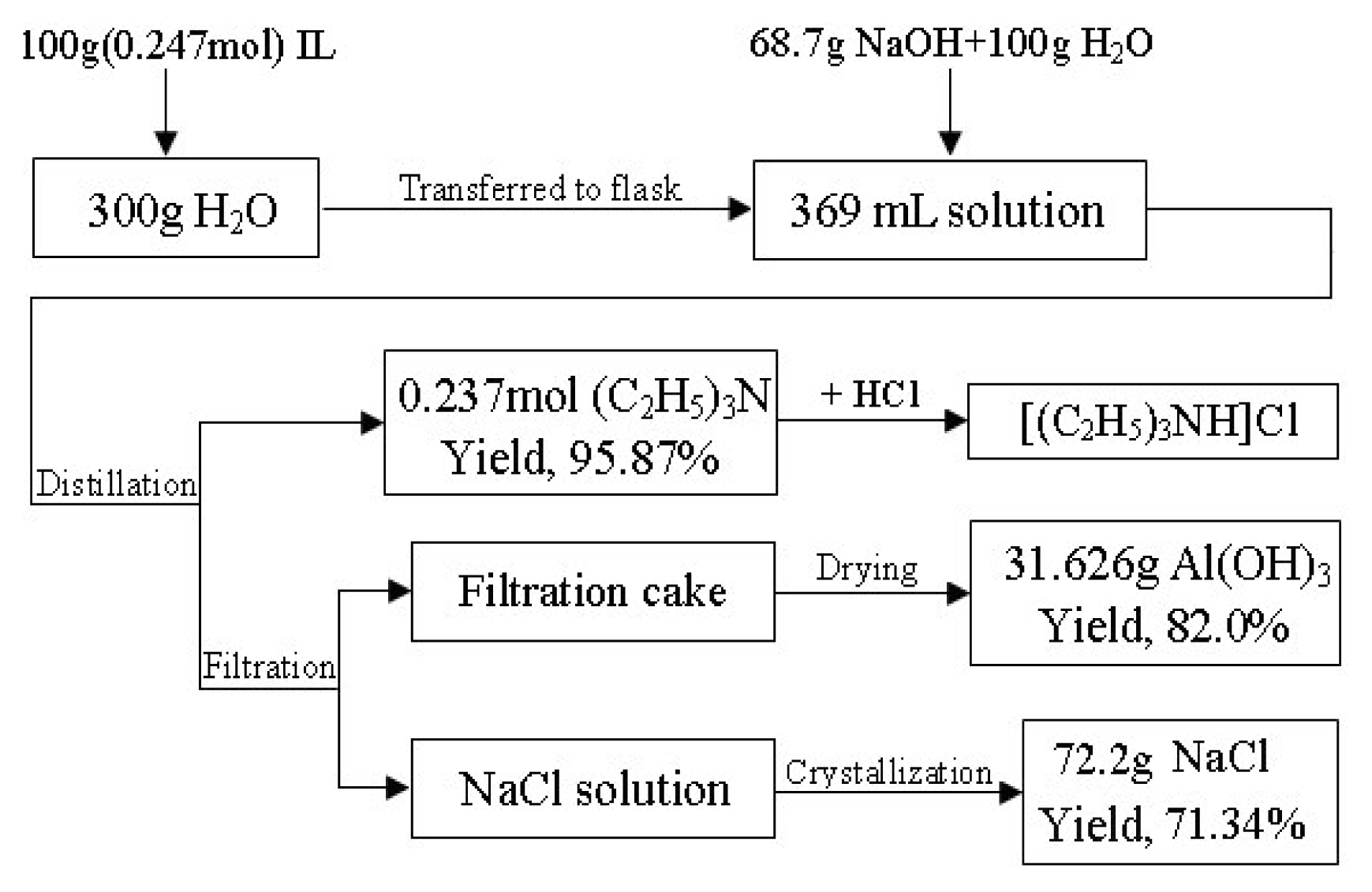
2.2 Preparation of Et3NHCl-2AlCl3 ionic liquid
2.3 Hydrolysis of the ionic liquid
2.3.1 Hydrolysis
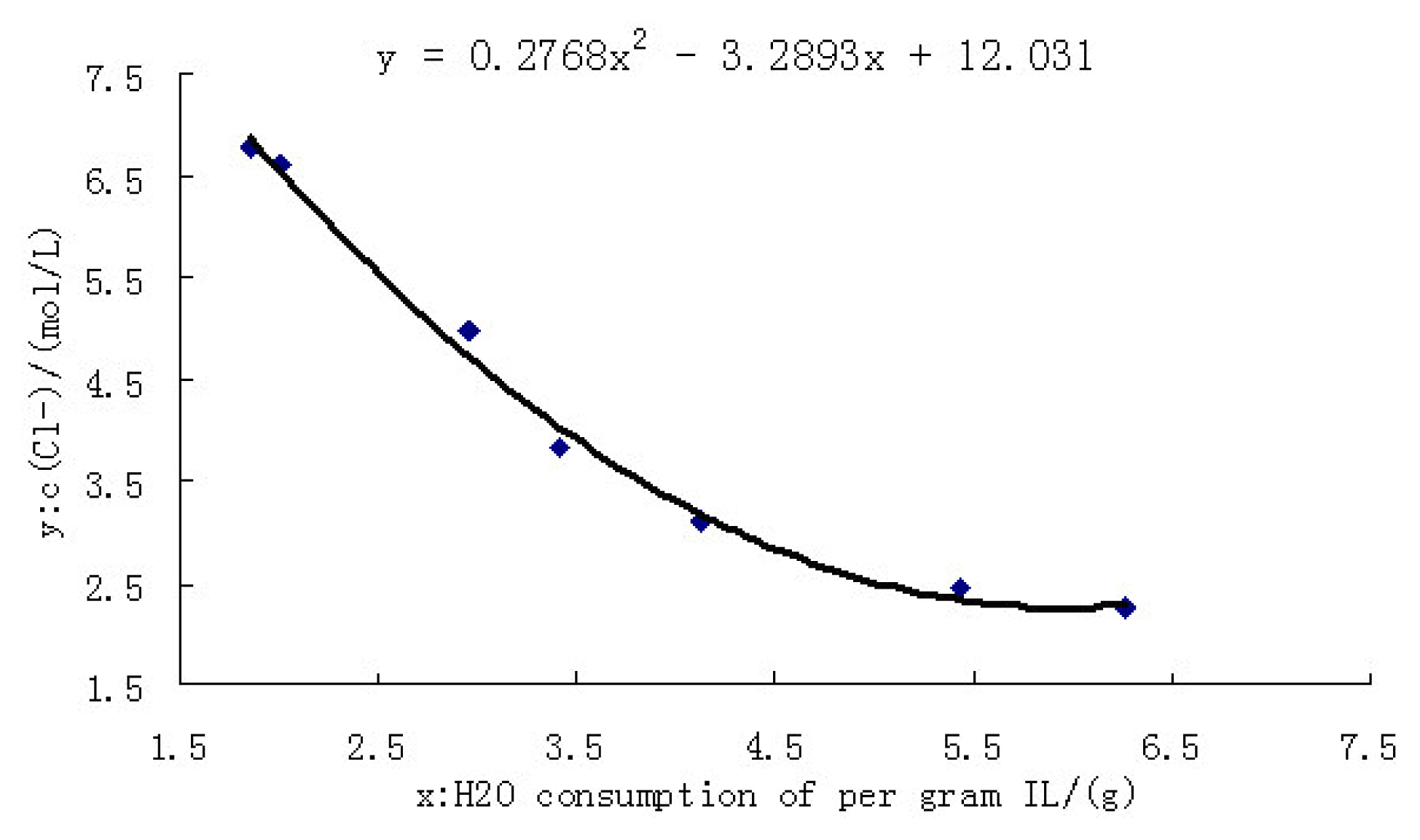
2.3.2 Calibration curve of chlorine ion concentration
2.4 Process for recycling triethylamine
2.5 Titration of the concentration of triethylamine solution
2.6 Preparation of triethylhydrogenammonium chloride from the recovered triethylamine
3. Conclusions
- (1)
- The influence of temperature of hydrolysis of the Et3NHCl-2AlCl3 chloroaluminate ionic liquid on the chlorine concentration of the obtained aqueous solution was studied. The concentration of chlorine ion was higher in the solutions where hydrolysis of the ionic liquid was carried out at a higher temperature. The aqueous solution with a higher temperature could absorb HCl released by the hydrolysis more easily and was beneficial to the safety of industrial processing.
- (2)
- In the recovery of triethylamine, when the aluminum hydroxide precipitate was filtered before distillation, the yield of triethylamine was only 12 %–18 %, due to the fact that some of the triethylamine was volatilized and some of it remained in the filtration cake. The sharp smell of triethylamine emitted by the cake proved this point. When the mixture was directly heated to distill triethylamine before filtration, the yield was as high as 95.87 %. The sequence of distillation and filtration in the process was the key factor to achieve a high yield of triethylamine recovery.
- (3)
- The aqueous NaCl solution obtained from the above the hydrolysis process could not be reused due to the crystallization of NaCl that would happen after a small amount of ionic liquid was added, so the NaCl solution was directly heated to recover the NaCl crystals. In all, triethylamine was recycled well; aluminium hydroxide and sodium chloride were obtained as by-products. This is in accord with the spirit of Green Chemistry.
| T/°C | Cl−/(mol/l) | |
|---|---|---|
| Hydrolysis of | 25–45 | 6.225 |
| 100 g IL in 200 g H2O | 80–110 | 6.618 |
| Hydrolysis of | 25–45 | 4.903 |
| 100 g IL in 300 g H2O | 80–110 | 4.979 |
| H2O consumption of per gram IL/(g) | C(Cl−)/(mol/L) |
|---|---|
| 1.8600 | 6.7753 |
| 2.0000 | 6.6181 |
| 2.9571 | 4.9789 |
| 3.4078 | 3.8200 |
| 4.1210 | 3.1126 |
| 5.4322 | 2.4366 |
| 6.2673 | 2.2480 |
References and Notes
- Anastas, P.T.; Kirchhoff, M.M. Origins, Current Status, and Future Challenges of Green Chemistry. Acc. Chem. Res 2002, 35, 686–694. [Google Scholar]
- Seddon, K. R. Ionic Liquids for Clean Technology. J. Chem. Tech. Biotech 1997, 68, 351–356. [Google Scholar]
- Wasserscheid, P.; Welton, T. Ionic Liquids in Synthesis; Wiley-VCH: Weinheim, 2003. [Google Scholar]
- Welton, T. Room-Temperature Ionic Liquids: Solvents for Synthesis and Catalysis. Chem. Rev 1999, 99, 2071–2083. [Google Scholar]
- Earle, M.J.; Seddon, K.R. Ionic liquids. Green Solvents for the Future. Pure Appl. Chem 2000, 72, 1391–1398. [Google Scholar]
- Seddon, K.R. Biocatalysis in Ionic Liquids. Green Chem 2002, 4, 147–151. [Google Scholar]
- Dupont, J.; Consorti, C.S.; Spencer, J. Room Temperature Molten Salts: Neoteric “Green” Solvents for Chemical Reactions and Processes. J. Braz. Chem. Soc 2000, 11, 337–344. [Google Scholar]
- Kazarian, S.G.; Briscoe, B.J.; Welton, T. Combining Ionic Liquids and Supercritical Fluids in situ ATR-IR Study of CO2 Dissolved in Two Ionic Liquids at High Pressures. Chem. Commun 2000, 20, 2047. [Google Scholar]
- Blanchard, L.A.; Hancu, D.; Beckman, E.J.; Brennecke, J.F. Green Processing Using Ionic Liquids and CO2. Nature 1999, 399, 28. [Google Scholar]
- Nanjundiah, C.; McDevitt, S.F.; Koch, V.R. Differential Capacitance Measurements in Solvent-Free Ionic Liquids at Hg and C Interfaces. J. Electrochem. Soc 1997, 144, 3392. [Google Scholar]
- Gordon, C.M.; McLean, A.J. Photoelectron Transfer From Excited-state Ruthenium(II) Tris(bipyridyl) to Methylviologen in an Ionic Liquid. Chem. Commun. 2000, 15, 1395. [Google Scholar]
- Boulaire, V.L.; Gree, R. Wittig Reactions in the Ionic Solvent [bmim][BF4]. Chem. Commun 2000, 21, 2195. [Google Scholar]
- Lee, C.W. Diels-Alder Reactions in Chloroaluminate Ionic Liquids: Acceleration and Selectivity Enhancement. Tetrahedron Lett 1999, 40, 2461–2464. [Google Scholar]
- Adams, C.J.; Earle, M.J.; Hamill, J.; Lok, C.M.; Roberts, G.; Seddon, K.R. World Patent WO 9807680, 1998.
- Mendelson, W.L.; Spainhour, C.B.; Jones, S.S.; Lamb, B.L.; Wert, K.L. Intramolecular Friedel- Crafts Alkylations. II. An Efficient Synthesis of Biologically Active 1,2,3,4-Tetrahydro-isoquinolines. Tetrahedron Lett 1980, 21, 1393–1396. [Google Scholar]
- Boon, J.A.; Levisky, J.A.; Pflug, J.L.; Wilkes, J.S. Friedel-Crafts Reactions in Ambient-Temperature Molten Salts. J. Org. Chem 1986, 51, 480–483. [Google Scholar]
- Holbrey, J.D.; Seddon, K.R. Ionic Liquids. Clean Products and Processes 1999, 1, 223–236. [Google Scholar]
- Chen, D.C.; Wang, L.S. Solubilities of Petroleum Ether, Heptane, Phosphorus Trichloride, and Benzene with the [Triethylamine Hydrochloride]-x[AlCl3] Ionic Liquid. J. Chem. Eng. Data 2005, 50, 616–618. [Google Scholar]
- Wang, L.S.; Kang, H.B.; Wang, Z.W. Green Synthesis of Dichlorophenylphosphine Sulfide Using Chloroaluminate Ionic Liquids as a Catalyst. Phosphorus Sulfur Silicon 2007, 182, 227–236. [Google Scholar]
- Greco, C.C.; Fawzy, S.; Lieh-Jiun, S. US Patent 5824832, 1998.
- Mei, J.; Li, S.P.; Wang, X.B. A Handbook of Methods for Analyzing General Chemical Products; Chemical Industrial Press: Beijing, 1999. [Google Scholar]
- Wang, Z.W.; Wang, L.S. Friedel-Crafts Phosphylation of Benzene Catalyzed by [trEHAm]Cl-xAlCl3 Ionic Liquids. Appl. Catal. A: Gen 2004, 262, 101–104. [Google Scholar]
© 2007 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Fang, M.-H.; Wang, L.-S. Hydrolysis and Partial Recycling of a Chloroaluminate Ionic Liquid. Int. J. Mol. Sci. 2007, 8, 470-477. https://doi.org/10.3390/i8060470
Fang M-H, Wang L-S. Hydrolysis and Partial Recycling of a Chloroaluminate Ionic Liquid. International Journal of Molecular Sciences. 2007; 8(6):470-477. https://doi.org/10.3390/i8060470
Chicago/Turabian StyleFang, Ming-Hong, and Li-Sheng Wang. 2007. "Hydrolysis and Partial Recycling of a Chloroaluminate Ionic Liquid" International Journal of Molecular Sciences 8, no. 6: 470-477. https://doi.org/10.3390/i8060470
APA StyleFang, M.-H., & Wang, L.-S. (2007). Hydrolysis and Partial Recycling of a Chloroaluminate Ionic Liquid. International Journal of Molecular Sciences, 8(6), 470-477. https://doi.org/10.3390/i8060470




