Investigation of Phenolic Components of Hungarian Wines
Abstract
:1. Introduction
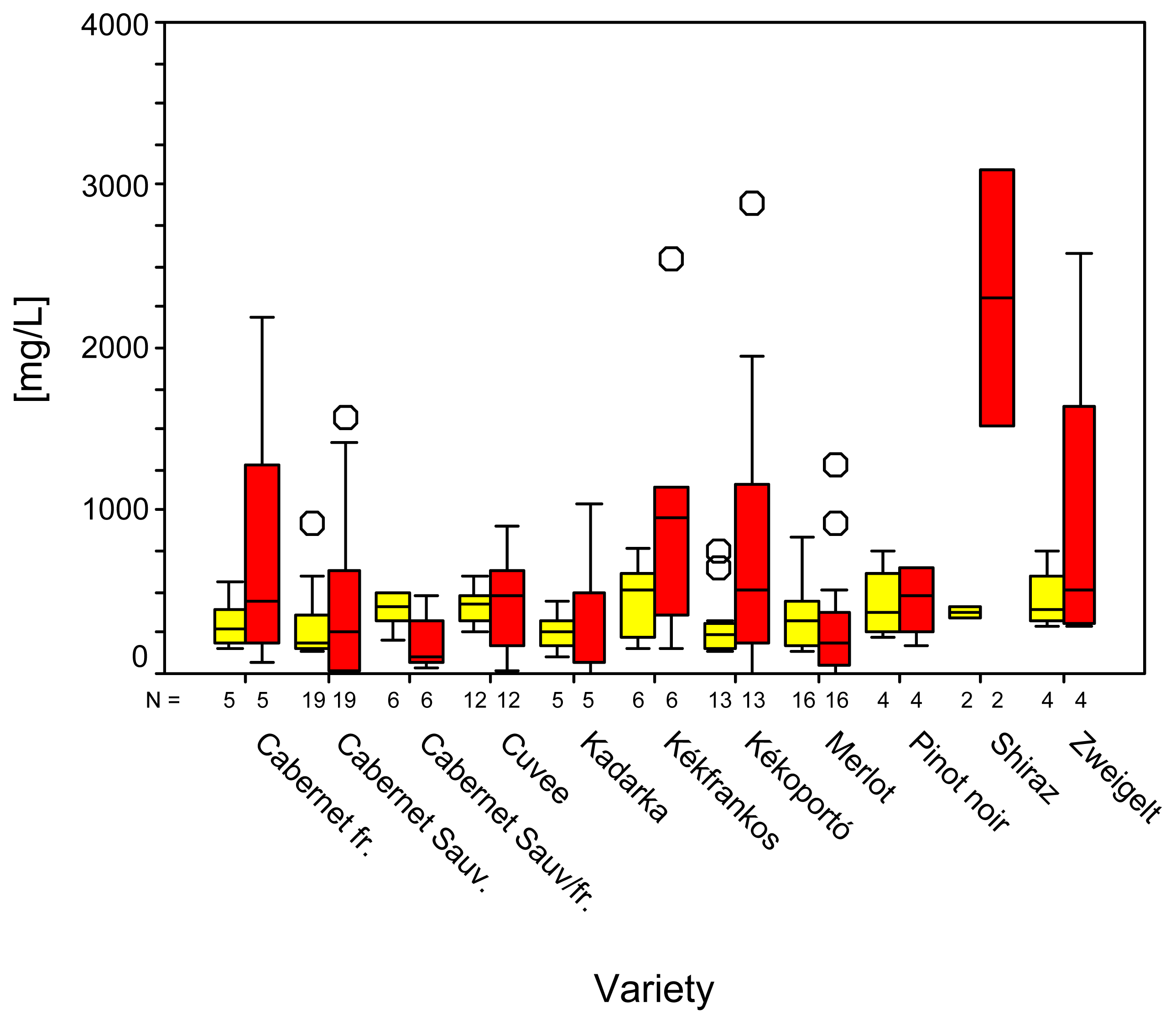
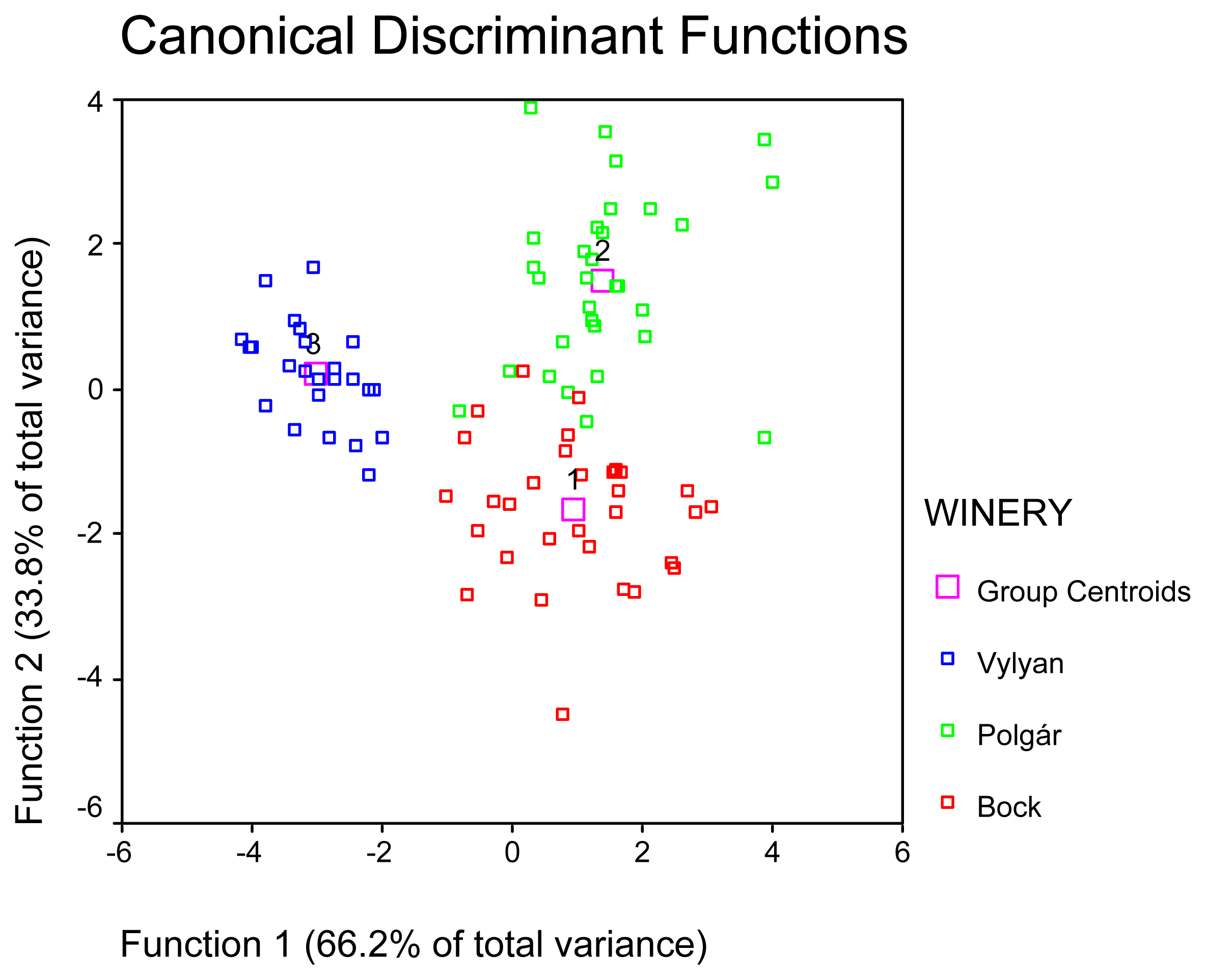
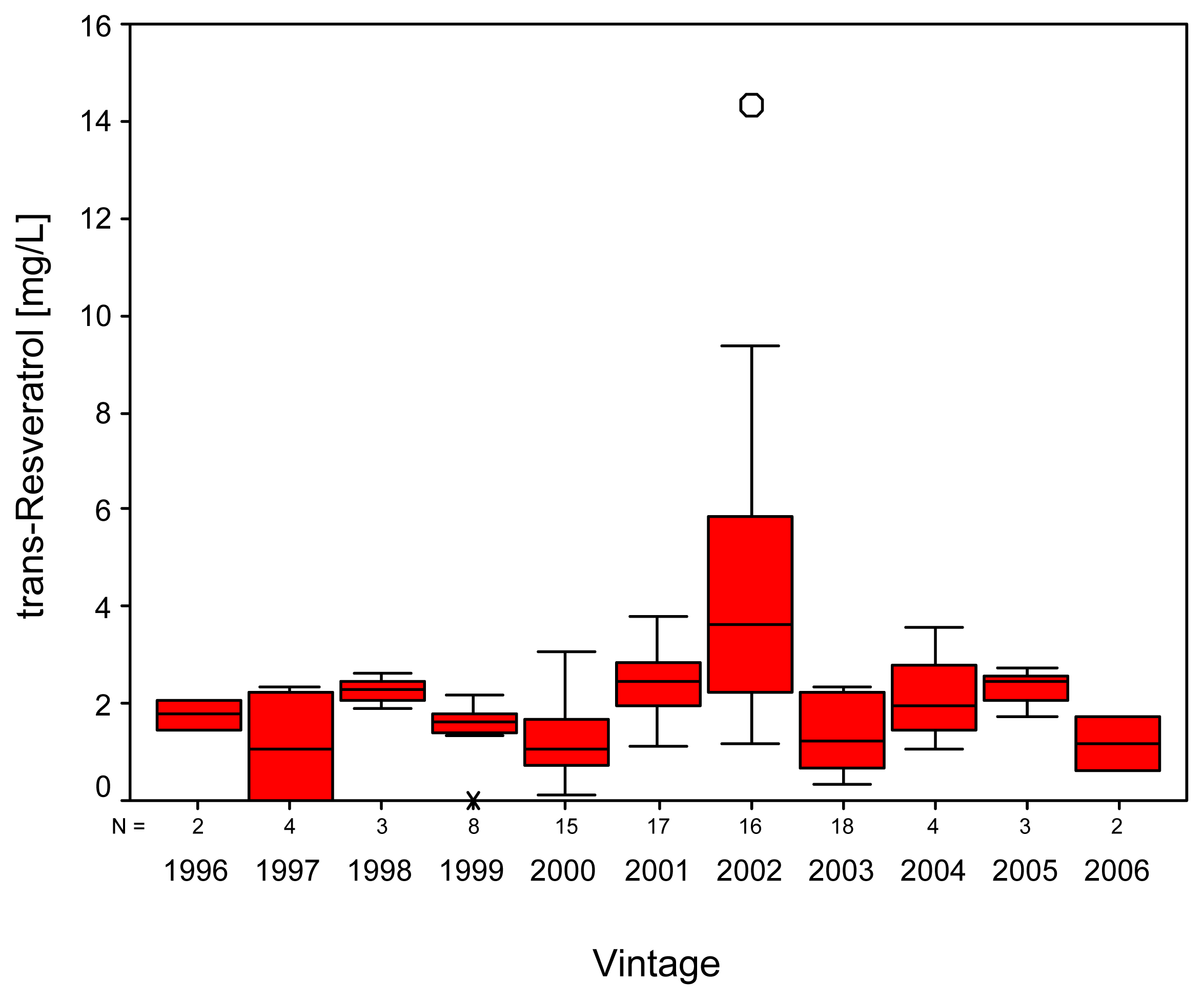
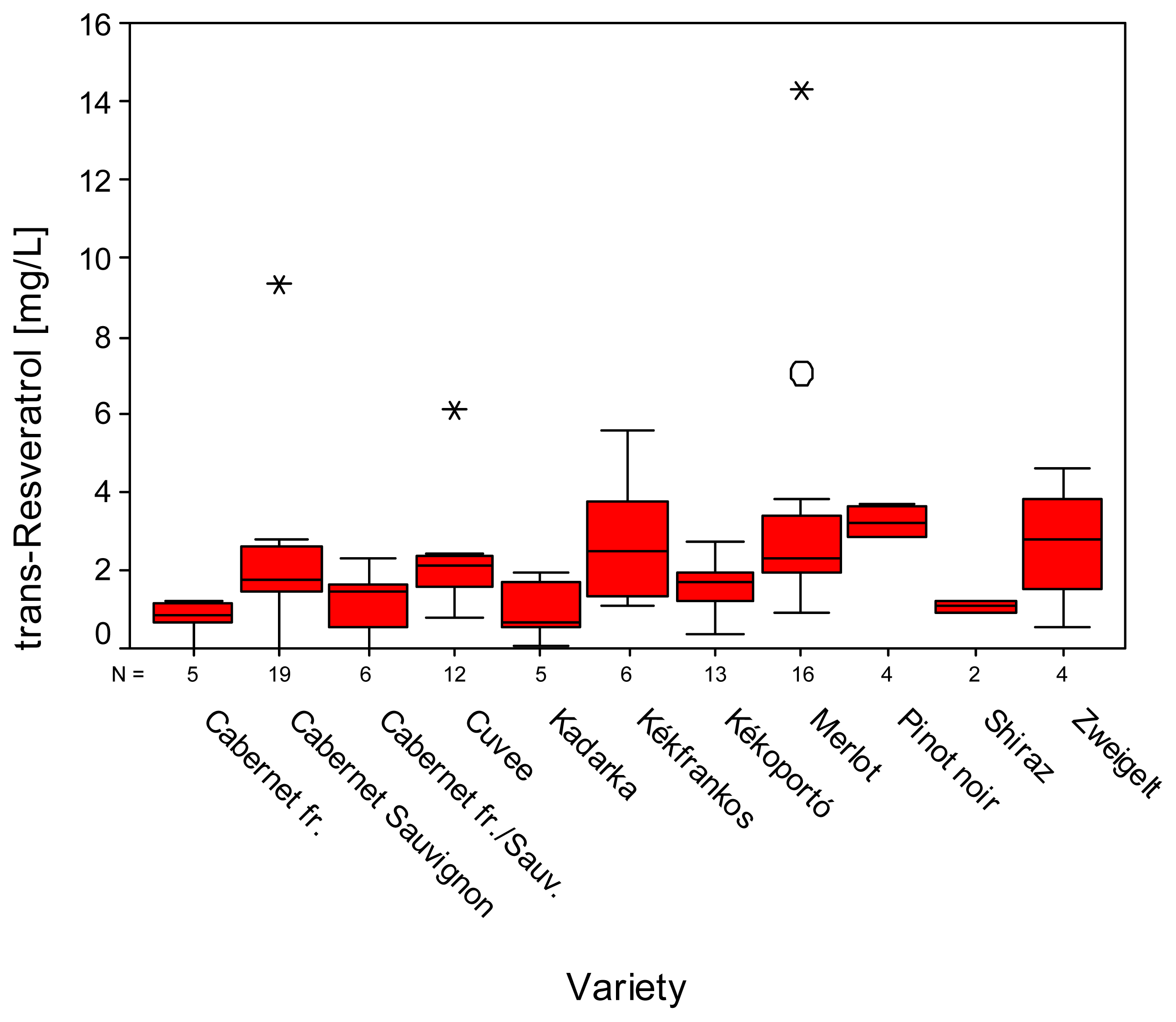
2. Results and Discussion
Materials and Methods
Chemicals & wine samples
Polyphenol analysis
Resveratrol analysis
Statistical analysis
| Variety | N | Gallicacid | Tyrosol | Caftaric acid | (+)-Catechin | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Std.dev. | Mean | Std.dev. | Mean | Std.dev. | Mean | Std.dev. | ||
| Cabernet franc | 5 | 45.3 | 33.2 | 46.5 | 47.5 | 42.1 | 11.2 | 62.1 | 29.1 |
| Cabernet Sauvignon | 19 | 40.0 | 29.4 | 63.0 | 55.6 | 40.0 | 20.4 | 63.7 | 39.5 |
| Cabernet Sauv/fr | 6 | 70.9 | 22.6 | 54.9 | 33.9 | 54.8 | 13.0 | 69.0 | 29.9 |
| Cuvée | 12 | 62.5 | 16.4 | 78.4 | 35.6 | 55.2 | 14.6 | 74.7 | 23.9 |
| Kadarka | 5 | 32.1 | 24.7 | 33.5 | 43.0 | 45.5 | 19.1 | 82.5 | 42.9 |
| Kékfrankos | 6 | 46.0 | 10.1 | 82.9 | 63.2 | 87.0 | 26.7 | 71.5 | 33.0 |
| Kékoportó | 13 | 24.0 | 18.3 | 48.3 | 51.0 | 44.0 | 21.7 | 81.7 | 47.0 |
| Merlot | 16 | 51.0 | 27.3 | 62.7 | 47.2 | 38.9 | 23.0 | 72.1 | 41.0 |
| Pinot noir | 4 | 45.2 | 15.3 | 116.5 | 65.5 | 55.5 | 25.5 | 102.9 | 46.4 |
| Shiraz | 2 | 50.0 | 8.6 | 84.3 | 14.3 | 40.4 | 2.7 | 68.2 | 5.4 |
| Zweigelt | 4 | 58.3 | 11.4 | 86.7 | 77.7 | 77.5 | 7.5 | 73.4 | 50.5 |
| Total | 92 | 46.1 | 25.8 | 64.9 | 50.9 | 49.1 | 23.1 | 73.1 | 37.6 |
| Variety | N | GRP | Caffeic acid | p-Coutaric acid | (−)-Epicatechin | p-Coumaric acid | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Std.dev. | Mean | Std.dev. | Mean | Std.dev. | Mean | Std.dev. | Mean | Std.dev. | ||
| Cabernet franc | 5 | 3.5 | 4.9 | 14.6 | 14.3 | 0.0 | 0.0 | 92.0 | 76.5 | 9.2 | 6.7 |
| Cabernet Sauvignon | 19 | 0.8 | 2.6 | 13.9 | 11.7 | 4.5 | 4.3 | 63.6 | 62.8 | 4.6 | 5.8 |
| Cabernet Sauv/fr | 6 | 0.0 | 0.0 | 20.0 | 14.5 | 0.0 | 0.0 | 113.0 | 40.2 | 9.2 | 7.2 |
| Cuvée | 12 | 0.0 | 0.0 | 26.4 | 11.3 | 0.9 | 2.2 | 101.3 | 30.9 | 7.4 | 5.5 |
| Kadarka | 5 | 1.9 | 2.5 | 7.2 | 4.9 | 2.6 | 2.2 | 49.5 | 38.2 | 1.8 | 2.3 |
| Kékfrankos | 6 | 0.0 | 0.0 | 37.0 | 21.8 | 3.9 | 8.1 | 125.6 | 104.0 | 9.5 | 5.3 |
| Kékoportó | 13 | 1.7 | 3.8 | 20.5 | 17.4 | 4.8 | 4.9 | 55.0 | 59.6 | 3.5 | 4.4 |
| Merlot | 16 | 1.8 | 6.3 | 12.7 | 11.6 | 4.6 | 7.7 | 87.9 | 69.1 | 7.1 | 9.0 |
| Pinot noir | 4 | 0.0 | 0.0 | 28.9 | 16.4 | 0.7 | 1.4 | 64.6 | 68.5 | 8.9 | 8.7 |
| Shiraz | 2 | 0.0 | 0.0 | 24.4 | 2.3 | 4.8 | 6.9 | 99.7 | 35.6 | 8.3 | 3.8 |
| Zweigelt | 4 | 3.4 | 6.8 | 30.6 | 14.3 | 3.0 | 4.3 | 110.6 | 51.4 | 8.0 | 8.2 |
| Total | 92 | 1.2 | 3.7 | 19.4 | 15.1 | 3.2 | 5.1 | 82.4 | 62.8 | 6.4 | 6.6 |
| Variety | N | Fertaric acid | Ferulic acid | trans-Resveratrol | Quercetin | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Std.dev. | Mean | Std.dev. | Mean | Std.dev. | Mean | Std.dev. | ||
| Cabernet franc | 5 | 2.5 | 4.3 | 0.0 | 0.0 | 0.8 | 0.5 | 3.7 | 5.2 |
| Cabernet Sauvignon | 19 | 3.1 | 7.4 | 0.3 | 1.3 | 2.2 | 1.9 | 5.1 | 3.7 |
| Cabernet Sauv/fr | 6 | 0.9 | 2.3 | 0.0 | 0.0 | 1.2 | 0.8 | 2.4 | 3.1 |
| Cuvée | 12 | 3.3 | 6.7 | 0.3 | 1.2 | 2.2 | 1.3 | 6.1 | 4.9 |
| Kadarka | 5 | 1.3 | 3.0 | 0.8 | 0.8 | 1.0 | 0.8 | 5.7 | 4.0 |
| Kékfrankos | 6 | 0.0 | 0.0 | 0.0 | 0.0 | 2.8 | 1.7 | 11.3 | 5.0 |
| Kékoportó | 13 | 4.1 | 10.3 | 0.3 | 0.6 | 1.5 | 0.7 | 7.1 | 5.6 |
| Merlot | 16 | 2.4 | 6.3 | 3.0 | 5.9 | 3.4 | 3.2 | 8.4 | 10.0 |
| Pinot noir | 4 | 8.3 | 12.2 | 0.0 | 0.0 | 3.2 | 0.5 | 7.5 | 2.0 |
| Shiraz | 2 | 0.0 | 0.0 | 2.0 | 2.8 | 1.1 | 0.2 | 13.4 | 1.8 |
| Zweigelt | 4 | 7.9 | 9.3 | 1.9 | 2.3 | 2.7 | 1.7 | 6.0 | 6.3 |
| Total | 92 | 3.1 | 7.2 | 0.8 | 2.8 | 2.2 | 1.9 | 6.6 | 6.1 |
| Variety | N | Delphinidin-3-gluc | Cyanidin-3-gluc | Petunidin-3-gluc | Peonidin-3-gluc | Malvidin-3-gluc | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Std.dev. | Mean | Std.dev. | Mean | Std.dev. | Mean | Std.dev. | Mean | Std.dev. | ||
| Cabernet franc | 5 | 63.4 | 72.8 | 0.0 | 0.0 | 73.0 | 96.5 | 39.3 | 44.4 | 656.2 | 696.2 |
| Cabernet Sauvignon | 19 | 53.5 | 62.1 | 2.2 | 6.4 | 41.9 | 46.2 | 24.8 | 27.1 | 314.0 | 381.4 |
| Cabernet Sauv/fr | 6 | 24.3 | 26.2 | 0.0 | 0.0 | 18.8 | 18.6 | 11.4 | 10.2 | 129.4 | 129.1 |
| Cuvée | 12 | 46.7 | 34.2 | 0.0 | 0.0 | 41.8 | 27.2 | 27.5 | 17.6 | 323.3 | 202.7 |
| Kadarka | 5 | 25.1 | 35.1 | 0.0 | 0.0 | 26.1 | 35.5 | 24.8 | 32.4 | 259.9 | 341.0 |
| Kékfrankos | 6 | 64.4 | 49.4 | 0.0 | 0.0 | 72.4 | 64.6 | 82.3 | 65.6 | 796.7 | 670.7 |
| Kékoportó | 13 | 39.1 | 42.0 | 0.0 | 0.0 | 53.8 | 56.2 | 34.9 | 38.6 | 638.0 | 752.2 |
| Merlot | 16 | 39.2 | 48.6 | 3.3 | 7.3 | 35.6 | 42.0 | 27.3 | 33.5 | 191.2 | 234.2 |
| Pinot noir | 4 | 43.0 | 22.8 | 0.0 | 0.0 | 39.9 | 24.6 | 48.1 | 35.6 | 316.1 | 156.2 |
| Shiraz | 2 | 151.8 | 43.4 | 0.0 | 0.0 | 220.2 | 109.3 | 127.9 | 42.7 | 1810.0 | 923.0 |
| Zweigelt | 4 | 71.5 | 74.6 | 0.0 | 0.0 | 88.0 | 84.8 | 61.7 | 61.8 | 753.9 | 867.2 |
| Total | 92 | 48.3 | 51.0 | 1.0 | 4.3 | 49.6 | 57.0 | 35.5 | 39.8 | 426.5 | 539.6 |
| WINERY | Mean | N | Std. Deviation | Minimum | Maximum |
|---|---|---|---|---|---|
| Bock | 1.87 | 37 | 1.1985 | .00 | 7.03 |
| Polgár | 2.90 | 31 | 2.8447 | .10 | 14.32 |
| Vylyan | 1.72 | 24 | 0.9414 | .00 | 3.81 |
| Total | 2.18 | 92 | 1.9319 | .00 | 14.32 |
Acknowledgements
References and Notes
- Kalea, A.Z.; Lamari, F.N.; Theocharis, A.D.; Cordopatis, P.; Schuschke, D.A.; Karamanos, N.K.; Klimis-Zacas, D.J. Wild blueberry (Vaccinium angustifolium) consumption affects the composition and structure of glycosaminoglycans in Sprague-Dawley rat aorta. J. Nutr. Biochem 2006, 17, 109–116. [Google Scholar]
- Kahle, K.; Kraus, M.; Scheppach, W.; Ackermann, M.; Ridder, F.; Richling, E. Studies on apple and blueberry fruit constituents: do the polyphenols reach the colon after ingestion? Mol. Nutr. Food Res 2006, 50, 418–423. [Google Scholar]
- Vinson, J.A.; Zubik, L.; Bose, P.; Samman, N.; Proch, J. Dried fruits: excellent in vitro and in vivo antioxidants. J. Am. Coll. Nutr 2005, 24, 44–50. [Google Scholar]
- Vrhovsek, U.; Rigo, A.; Tonon, D.; Mattivi, F. Quantitation of polyphenols in different apple varieties. J. Agric. Food Chem 2004, 52, 6532–6538. [Google Scholar]
- Jefremov, V.; Zilmer, M.; Zilmer, K.; Bogdanovic, N.; Karelson, E. Antioxidative effects of plant polyphenols: from protection of G protein signaling to prevention of age-related pathologies. Ann. N Y Acad. Sci 2007, 1095, 449–457. [Google Scholar]
- Schaefer, S.; Baum, M.; Eisenbrand, G.; Dietrich, H.; Will, F.; Janzowski, C. Polyphenolic apple juice extracts and their major constituents reduce oxidative damage in human colon cell lines. Mol Nutr. Food Res 2006, 50(1), 24–33. [Google Scholar]
- Chun, O.K.; Kim, D.O.; Lee, C.Y. Superoxide radical scavenging activity of the major polyphenols in fresh plums. J. Agric. Food. Chem 2003, 51(27), 8067–8072. [Google Scholar]
- Weisburger, J.H. Mechanism of action of antioxidants as exemplified in vegetables, tomatoes and tea. Food Chem. Toxicol 1999, 37, 943–948. [Google Scholar]
- Pace-Asciak, C.R.; Hahn, S.; Diamandis, E.P.; Soleas, G.; Goldberg, D.M. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: Implications for protection against coronary heart disease. Clin. Chim. Acta 1995, 235(2), 207–219. [Google Scholar]
- Singh, I.; Mok, M.; Christensen, A.M.; Turner, A.H.; Hawley, J.A. The effects of polyphenols in olive leaves on platelet function Nutrition. Metabolism and Cardiovascular Diseases 2007. In Press, Corrected Proof, Available online 7 March 2007. [Google Scholar]
- Rusak, G.; Gutzeit, H.O.; Müller, J.L. Structurally related flavonoids with antioxidative properties differentially affect cell cycle progression and apoptosis of human acute leukemia cells. Nutr. Res 2005, 25(2), 143–155. [Google Scholar]
- Yi, W.; Fischer, J.; Akoh, C.C. Study of anticancer activities of muscadine grape phenolics in vitro. J. Agric. Food Chem 2005, 53(22), 8804–8812. [Google Scholar]
- Mokni, M.; Limam, F.; Elkahoui, S.; Amri, M.; Aouani, E. Strong cardioprotective effect of resveratrol, a red wine polyphenol, on isolated rat hearts after ischemia/reperfusion injury. Arch. Bioch. Biophsy 2007, 457(1), 1–6. [Google Scholar]
- Castillo-Sánchez, J.X.; García-Falcón, M.S.; Garrido, J.; Martínez-Carballo, E.; Martins-Dias, L.R.; Mejuto, X.C. Phenolic compounds and colour stability of Vinhão wines: Influence of winemaking protocol and fining agents. Food Chem 2007. In Press, Accepted Manuscript, Available online 13 May 2007. [Google Scholar]
- Stervbo, U.; Vang, O.; Bonnesen, C. A review of the content of the putative chemopreventive phytoalexin resveratrol in red wine. Food Chem 2007, 101(2), 449–457. [Google Scholar]
- Goldfinger, T.M.. Beyond the French paradox: the impact of moderate beverage alcohol and wine consumption in the prevention of cardiovascular disease. Clin. Cardiol 2003, 21(3), 449–457. [Google Scholar]
- Iijima, K.; Yoshizumi, M.; Ouchi, Y. Effect of red wine polyphenols on vascular smooth muscle cell function—molecular mechanism of the ‘French paradox’. Mech. Ageing Dev 2002, 123(8), 1033–1039. [Google Scholar]
- Sun, A.Y.; Simonyi, A.; Sun, G.Y. The “French paradox” and beyond: neuroprotective effects of polyphenols. Free Rad. Biol. Med 2002, 32(4), 314–318. [Google Scholar]
- Casavecchia, C.; Magnisi, R.; La Pera, L.; Maisano, R.; Dugo, G. Classification of Sicilian Red Wines from Autochthonous and Allochthonous Cultivars According to Anthocyanin Pattern. Am. J. Enol. Vitic 2007, 58, 286–290. [Google Scholar]
- Picque, D.; Cattenoz, T.; Tréléa, C.; Cuinier, C.; Corrieu, G. Classification géographique de vins rouges par analyse de leur extrait sec en spectroscopie moyen infrarouge à transmission. Bull. de l’O.I.V 2002, 75, 809–822. [Google Scholar]
- Edelmann, A.; Diewok, J.; Schuster, K.C.; Lendl, B. Rapid Method for the Discrimination of Red Wine Cultivars Based on Mid-Infrared Spectroscopy of Phenolic Wine Extracts. J. Agric. Food Chem 2001, 49, 1139–1145. [Google Scholar]
- Pour Nikfardjam, M.S.; Márk, L.; Avar, P.; Figler, M.; Ohmacht, R. Polyphenols, anthocyanins, and trans-resveratrol in red wines from the Hungarian Villány region. Food Chem 2006, 98(3), 453–462. [Google Scholar]
- White, M.A.; Diffenbaugh, N.S.; Jones, G.V.; Pal, J.S.; Giorgi, F. Extreme heat reduces and shifts United States premium wine production in the 21st century. Proc. Natl. Acad. Sci. U S A 2006, 103(30), 11217–11222. [Google Scholar]
- Bavaresco, L. Role of viticultural factors on stilbene concentrations of grapes and wine. Drugs Exp. Clin. Res 2003, 29(5–6), 181–187. [Google Scholar]
- Roldán, A.; Palacios, V.; Caro, I.; Pérez, L. Resveratrol content of palomino fino grapes: influence of vintage and fungal infection. J. Agric. Food Chem 2003, 51, 1464–1468. [Google Scholar]
- Cheynier, V.; Osse, C.; Rigaud, J. Oxidation of Grape juice phenolic compounds in model solutions. J. Food Sci 1988, 53, 1729–1732. [Google Scholar]
- Kilmartin, P.A.; Zou, H.; Waterhouse, A.L. Correlation of Wine Phenolic Composition versus Cyclic Voltametry Response. Am. J. Enol. Vitic 2002, 53(4), 294–302. [Google Scholar]
- Rodríguez-Delgado, M.A.; Malovaná, S.; Pérez, J.P.; Borges, T.; García Montelongo, F.J. Separation of phenolic compounds by high-performance liquid chromatography with absorbance and fluorometric detection. J. Chromatogr. A 2001, 912, 249–257. [Google Scholar]
- Orbán, N.; Kiss, A.; Drávucz, M.; Gál, L.; Orbán, S. Comparative study on selected polyphenol content in red wines of Eger (Hungary). Acta Alim 2006, 35(4), 465–477. [Google Scholar]
- Ibern-Gómez, M.; Andrés-Lacueva, C.; Lamuela-Raventós, R.M.; Waterhouse, A.L. Rapid HPLC Analysis of Phenolic Compounds in Red Wines. Am. J. Enol. Vitic 2002, 53(3), 218–221. [Google Scholar]
- Frankel, E.; Waterhouse, A. L.; Teissedre, P. L. Principal phenolic phytochemicals in selected California wines and their antioxidant activity in inhibiting oxidation of human low-density lipoproteins. J. Agric. Food Chem 1995, 43, 890–894. [Google Scholar]
- Goldberg, D. M.; Yan, J.; Ngu, E.; Diamandis, E. P.; Karumanchiri, A.; Soleas, G.; Waterhouse, A. L. A global survey of trans-resveratrol concentrations in commercial wines. Am. J. Enol. Vitic 1995, 46(2), 159–165. [Google Scholar]
- Kontkanen, D.; Reynolds, A.G.; Cliff, M.A.; King, M. Canadian terroir: sensory characterization of Bordeaux-style red wine varieties in the Niagara Peninsula. Food Res. Int 2005, 38(4), 417–425. [Google Scholar]
- Fischer, U.; Roth, D.; Christmann, M. The impact of geographic origin, vintage and wine estate on sensory properties of Vitis vinifera cv. Riesling wines. In Food Qual. Pref; 1999; Volume 10, 4–5, pp. 281–288. [Google Scholar]
- Márk, L.; Pour Nikfardjam, M.S.; Avar, P.; Ohmacht, R. “Validated HPLC Method for Quantitative Analysis of trans-Resveratrol and trans-Piceid in Hungarian Wines”. J. Chrom. Sci 2005, 43(9), 445–449. [Google Scholar]
© 2007 by MDPI Reproduction is permitted for noncommercial purposes.
Share and Cite
Avar, P.; Nikfardjam, M.S.P.; Kunsági-Máté, S.; Montskó, G.; Szabó, Z.; Böddi, K.; Ohmacht, R.; Márk, L. Investigation of Phenolic Components of Hungarian Wines. Int. J. Mol. Sci. 2007, 8, 1028-1038. https://doi.org/10.3390/i8101028
Avar P, Nikfardjam MSP, Kunsági-Máté S, Montskó G, Szabó Z, Böddi K, Ohmacht R, Márk L. Investigation of Phenolic Components of Hungarian Wines. International Journal of Molecular Sciences. 2007; 8(10):1028-1038. https://doi.org/10.3390/i8101028
Chicago/Turabian StyleAvar, Péter, Martin S. Pour Nikfardjam, Sándor Kunsági-Máté, Gergely Montskó, Zoltán Szabó, Katalin Böddi, Róbert Ohmacht, and László Márk. 2007. "Investigation of Phenolic Components of Hungarian Wines" International Journal of Molecular Sciences 8, no. 10: 1028-1038. https://doi.org/10.3390/i8101028




