In Vitro Influence of Extracts from Snail Helix aspersa Müller on the Colon Cancer Cell Line Caco-2
Abstract
1. Introduction
2. Results
2.1. Red-Ox State Indicators
2.2. Proximate Composition
2.3. Analysis of Molecular Weights of Proteins
2.4. Analysis of Amino Acids
2.5. Composition of Fatty Acids
2.6. Analysis of Minerals
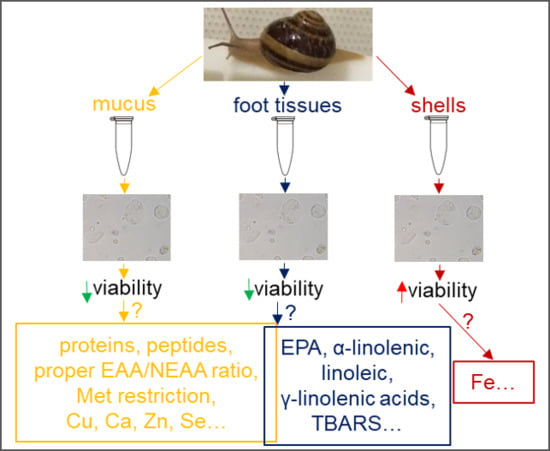
2.7. Influence of Extracts on Cell Viability
2.8. Influence of Fractions of Extracts on Cell Viability
3. Discussion
4. Materials and Methods
4.1. Animal Material and Preparation of Samples
4.2. Red-Ox State Indicators
4.2.1. Ferric-Reducing Antioxidant Power
4.2.2. Scavenging Activity of 2.2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic Acid) Radical Cation (ABTS·+)
4.2.3. Scavenging Activity of 2.2-Diphenyl-1-picrylhydrazyl Radical (DPPH·)
4.2.4. Peroxidation of Lipids
4.3. Proximate Composition
4.4. Analysis of Molecular Weights of Proteins
4.5. Analysis of Amino Acids
4.6. Composition of Fatty Acids
4.7. Analysis of Minerals
4.8. Preparation and Fractionation of Extracts for Cell Viability Tests
4.9. Caco-2 Cell Culture
4.10. Influence of Extracts on Cell Viability
4.11. Influence of Fractions of Extracts on Cell Viability
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global patterns and trends in colorectal cancer incidence and mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Bathen, T.F.; Holmgren, K.; Lundemo, A.G.; Hjelstuen, M.H.; Krokan, H.E.; Gribbestad, I.S.; Schønberg, S.A. Omega-3 fatty acids suppress growth of SW620 human colon cancer xenografts in nude mice. Anticancer Res. 2008, 28, 3717–3723. [Google Scholar] [PubMed]
- Cai, F.; Sorg, O.; Granci, V.; Lecumberri, E.; Miralbell, R.; Dupertuis, Y.M.; Pichard, C. Interaction of ω-3 polyunsaturated fatty acids with radiation therapy in two different colorectal cancer cell lines. Clin. Nutr. 2015, 33, 164–170. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Xing, R.; Wang, X.; Peng, Q.; Li, P. Proximate composition, amino acid and fatty acid profiles of marine snail Rapana venosa meat, visceral mass and operculum. J. Sci. Food Agric. 2017, 97, 5361–5368. [Google Scholar] [CrossRef] [PubMed]
- El Ouar, I.; Braicu, C.; Naimi, D.; Irimie, A.; Berindan-Neagoe, I. Effect of Helix aspersa extract on TNFα, NF-κB and some tumor suppressor genes in breast cancer cell line Hs578T. Pharmacogn. Mag. 2017, 13, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Teerasak, E.; Thongararm, P.; Roytrakul, S.; Meesuk, L.; Chumnanpuen, P. Prediction of anticancer peptides against MCF-7 breast cancer cells from the peptidomes of Achatina fulica mucus fractions. Comput. Struct. Biotechnol. J. 2016, 14, 49–57. [Google Scholar]
- Stabili, L.; Schirosi, R.; Parisi, M.G.; Piraino, S.; Cammarata, M. The mucus of Actinia equina (Anthozoa, Cnidaria): An unexplored resource for potential applicative purposes. Mar. Drugs 2015, 13, 5276–5296. [Google Scholar] [CrossRef] [PubMed]
- Dolashka, P.; Dolashki, A.; Velkova, L.; Stevanovic, S.; Molin, L.; Traldi, P.; Velikova, R.; Voelter, W. Bioactive compounds isolated from garden snails. J. BioSci. Biotechnol. 2015, SE, 147–155. [Google Scholar]
- Antonova, O.; Toncheva, D.; Rammensee, H.G.; Floetenmeyer, M.; Stevanovic, S.; Dolashka, P. In vitro antiproliferative effect of Helix aspersa hemocyanin on multiple malignant cell lines. Z. Naturforsch. C 2014, 69, 325–334. [Google Scholar] [CrossRef] [PubMed]
- Gesheva, V.; Chausheva, S.; Mihaylova, N.; Manoylov, I.; Doumanova, L.; Idakieva, K.; Tchorbanov, A. Anti-cancer properties of gastropodan hemocyanins in murine model of colon carcinoma. BMC Immunol. 2014, 15, 34. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.A. Helix pomatia and prognosis of breast cancer. Br. J. Cancer 1993, 68, 453–454. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lescar, J.; Sanchez, J.F.; Audfray, A.; Coll, J.L.; Breton, C.; Mitchell, E.P.; Imberty, A. Structural basis for recognition of breast and colon cancer epitopes Tn antigen and Forssman disaccharide by Helix pomatia lectin. Glycobiology 2007, 17, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yu, S.; Ma, G.; Chen, S.; Shi, Y.; Yang, Y. Comparative study of proximate composition and amino acid in farmed and wild Pseudobagrus ussuriensis muscles. Int. J. Food Sci. Technol. 2014, 49, 983–989. [Google Scholar] [CrossRef]
- Cai, F.; Dupertuis, Y.M.; Pichard, C. Role of polyunsaturated fatty acids and lipid peroxidation on colorectal cancer risk and treatments. Curr. Opin. Clin. Nutr. Metab. Care 2012, 15, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Ak, T.; Gülçin, İ. Antioxidant and radical scavenging properties of curcumin. Chem.-Biol. Interact. 2008, 174, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Floegel, A.; Kim, D.O.; Chung, S.J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Walker, R.B.; Everette, J.D. Comparative reaction rates of various antioxidants with ABTS radical cation. J. Agric. Food Chem. 2009, 57, 1156–1161. [Google Scholar] [CrossRef] [PubMed]
- Williams, P. Nutritional composition of red meat. Nutr. Dietet. 2007, 64. [Google Scholar] [CrossRef]
- Domaradzki, P.; Stanek, P.; Litwińczuk, Z.; Skałecki, P.; Florek, M. Slaughter value and meat quality of suckler calves: A review. Meat Sci. 2017, 134, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Consultation, F.E. Dietary Protein Quality Evaluation in Human Nutrition; FAO Food and Nutrition Paper, 92; FAO: Rome, Italy, 2011. [Google Scholar]
- Ho, V.W.; Leung, K.; Hsu, A.; Luk, B.; Lai, J.; Shen, S.Y.; Minchinton, A.I.; Waterhouse, D.; Bally, M.B.; Lin, W.; et al. A low carbohydrate, high protein diet slows tumor growth and prevents cancer initiation. Cancer Res. 2011, 71, 4484–4493. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Fats and Fatty Acids in Human Nutrition; Report of an Expert Consultation; FAO Food Nutrition Paper; FAO: Rome, Italy, 2010; pp. 1–166. [Google Scholar]
- Bonfili, L.; Cecarini, V.; Cuccioloni, M.; Angeletti, M.; Flati, V.; Corsetti, G.; Pasini, E.; Dioguardi, F.; Eleuteri, A.M. Essential amino acid mixtures drive cancer cells to apoptosis through proteasome inhibition and autophagy activation. FEBS J. 2017, 284, 1726–1737. [Google Scholar] [CrossRef] [PubMed]
- FAO/WHO. Protein Quality Evaluation; FAO Food and Nutrition Paper 51; Food and Agriculture Organization of the United Nation: Rome, Italy, 1991. [Google Scholar]
- Cavuoto, P.; Fenech, M.F. A review of methionine dependency and the role of methionine restriction in cancer growth control and life-span extension. Cancer Treat. Rev. 2012, 38, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, M.R.; Vander Heiden, M.G. When cancer needs what's non-essential. Nat. Cell Biol. 2017, 19, 418–420. [Google Scholar] [CrossRef] [PubMed]
- Maddocks, O.D.; Athineos, D.; Cheung, E.C.; Lee, P.; Zhang, T.; van den Broek, N.J.; Mackay, G.M.; Labuschagne, C.F.; Gay, D.; Kruiswijk, F.; et al. Modulating the therapeutic response of tumours to dietary serine and glycine starvation. Nature 2017, 544, 372–376. [Google Scholar] [CrossRef] [PubMed]
- De Mejia, E.G.; Dia, V.P. The role of nutraceutical proteins and peptides in apoptosis, angiogenesis, and metastasis of cancer cells. Cancer Metast. Rev. 2010, 29, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, G.; Flati, V.; Sanità, P.; Pasini, E.; Dioguardi, F.S. Protect and Counter-attack: Nutritional Supplementation with Essential Amino acid Ratios Reduces Doxorubicin-induced Cardiotoxicity in vivo and promote Cancer Cell Death in vitro. J. Cytol. Histol. 2015, 6, 354. [Google Scholar] [CrossRef]
- Fauser, J.K.; Prisciandaro, L.D.; Cummins, A.G.; Howarth, G.S. Fatty acids as potential adjunctive colorectal chemotherapeutic agents. Cancer Biol. Ther. 2011, 11, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Sasazuki, S.; Inoue, M.; Iwasaki, M.; Sawada, N.; Shimazu, T.; Yamaji, T.; Takachi, R.; Tsugane, S. Intake of n-3 and n-6 polyunsaturated fatty acids and development of colorectal cancer by subsite: Japan Public Health Center-based prospective study. Int. J. Cancer 2011, 129, 1718–1729. [Google Scholar] [CrossRef] [PubMed]
- Correia-da-Silva, M.; Sousa, E.; Pinto, M.M.M.; Kijjoa, A. Anticancer and cancer preventive compounds from edible marine organisms. Semin. Cancer Biol. 2017, 46, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Zhang, X.; Meyerhardt, J.A.; Giovannucci, E.L.; Ogino, S.; Fuchs, C.S.; Chan, A.T. Marine omega-3 polyunsaturated fatty acid intake and survival after colorectal cancer diagnosis. Gut 2017, 66, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Yu, H.; Shen, Y.; Ni, X.; Shen, S.; Das, U.N. Polyunsaturated fatty acids trigger apoptosis of colon cancer cells through a mitochondrial pathway. Arch. Med. Sci. 2015, 11, 1081–1094. [Google Scholar] [PubMed]
- Yang, T.; Fang, S.; Zhang, H.X.; Xu, L.X.; Zhang, Z.Q.; Yuan, K.T.; Xue, C.L.; Yu, H.L.; Zhang, S.; Li, Y.F.; et al. N-3 PUFAs have antiproliferative and apoptotic effects on human colorectal cancer stem-like cells in vitro. J. Nutr. Biochem. 2013, 24, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Kuan, C.Y.; Walker, T.H.; Luo, P.G.; Chen, C.F. Long-chain polyunsaturated fatty acids promote paclitaxel cytotoxicity via inhibition of the MDR1 gene in the human colon cancer Caco-2 cell line. J. Am. Coll. Nutr. 2011, 30, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Majewska, D.; Jakubowska, M.; Ligocki, M.; Tarasewicz, Z.; Szczerbińska, D.; Karamucki, T.; Sales, J. Physicochemical characteristics, proximate analysis and mineral composition of ostrich meat as influenced by muscle. Food Chem. 2009, 117, 207–211. [Google Scholar] [CrossRef]
- Tomovic, V.; Jokanovic, M.; Sojic, B.; Skaljac, S.; Tasic, T.; Ikonic, P. Minerals in pork meat and edible offal. Procedia Food Sci. 2015, 5, 293–295. [Google Scholar] [CrossRef]
- World Health Organization. Sodium Intake for Adults and Children; WHO: Geneva, Switzerland, 2012. [Google Scholar]
- Zhang, X.; Giovannucci, E. Calcium, vitamin D and colorectal cancer chemoprevention. Best Pract. Res. Clin. Gastroenterol. 2011, 25, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Kesse, E.; Boutron-Ruault, M.C.; Norat, T.; Riboli, E.; Clavel-Chapelon, F. Dietary calcium, phosphorus, vitamin D, dairy products and the risk of colorectal adenoma and cancer among French women of the E3N-EPIC prospective study. Int. J. Cancer 2005, 117, 137–144. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guideline: Potassium Intake for Adults and Children; World Health Organization: Geneva, Switzerland, 2012. [Google Scholar]
- Key, T.J.; Appleby, P.N.; Masset, G.; Brunner, E.J.; Cade, J.E.; Greenwood, D.C.; Stephen, A.M.; Kuh, D.; Bhaniani, A.; Powell, N.; et al. Vitamins, minerals, essential fatty acids and colorectal cancer risk in the United Kingdom Dietary Cohort Consortium. Int. J. Cancer 2012, 131, E320–E325. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization, Food and Agricultural Organization of the United Nations. Vitamin and Mineral Requirements in Human Nutrition; WHO: Geneva, Switzerland, 2005. [Google Scholar]
- Chen, G.C.; Pang, Z.; Liu, Q.F. Magnesium intake and risk of colorectal cancer: A meta-analysis of prospective studies. Eur. J. Clin. Nutr. 2012, 66, 1182–1186. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Bhattacharjee, P.; Guha, D.; Kajal, K.; Khan, P.; Chakraborty, S.; Mukherjee, S.; Paul, S.; Manchanda, R.; Khurana, A.; et al. Sulphur alters NFκB-p300 cross-talk in favour of p53-p300 to induce apoptosis in non-small cell lung carcinoma. Int. J. Oncol. 2015, 47, 573–582. [Google Scholar] [CrossRef] [PubMed]
- Mates, J.M.; Segura, J.A.; Alonso, F.J.; Marquez, J. Sulphur-containing non enzymatic antioxidants: Therapeutic tools against cancer. Front. Biosci. 2012, 4, 722–748. [Google Scholar] [CrossRef]
- Davis, C.D.; Johnson, W.T. Dietary copper affects azoxymethane-induced intestinal tumor formation and protein kinase C isozyme protein and mRNA expression in colon of rats. J. Nutr. 2002, 132, 1018–1025. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xiao, Y.; Zhai, Q.; Wang, G.; Liu, X.; Zhao, J.; Tian, F.; Zhang, H.; Chen, W. Metabolomics analysis reveals heavy metal copper-induced cytotoxicity in HT-29 human colon cancer cells. RSC Adv. 2016, 6, 78445–78456. [Google Scholar] [CrossRef]
- Zödl, B.; Zeiner, M.; Marktl, W.; Steffan, I.; Ekmekcioglu, C. Pharmacological levels of copper exert toxic effects in Caco-2 cells. Biol. Trace Elem. Res. 2003, 96, 143–152. [Google Scholar] [CrossRef]
- Davis, C.D.; Feng, Y. Dietary copper, manganese and iron affect the formation of aberrant crypts in colon of rats administered 3, 2′-dimethyl-4-aminobiphenyl. J. Nutr. 1999, 129, 1060–1067. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Torti, S.V.; Torti, F.M. Iron and cancer: More ore to be mined. Nat. Rev. Cancer 2013, 13, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Fonseca-Nunes, A.; Jakszyn, P.; Agudo, A. Iron and cancer risk—A systematic review and meta-analysis of the epidemiological evidence. Cancer Epidemiol. Biomark. Prev. 2013, 23, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Senesse, P.; Méance, S.; Cottet, V.; Faivre, J.; Boutron-Ruault, M.C. High dietary iron and copper and risk of colorectal cancer: A case-control study in Burgundy, France. Nutr. Cancer 2004, 49, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Xu, J.; Shi, Y.; Ye, Y.; Chen, K.; Yang, J.; Wu, Y. Association between zinc intake and risk of digestive tract cancers: A systematic review and meta-analysis. Clin. Nutr. 2014, 33, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Chadha, V.D.; Garg, M.L.; Dhawan, D. Influence of extraneous supplementation of zinc on trace elemental profile leading to prevention of dimethylhydrazine-induced colon carcinogenesis. Toxicol. Mech. Methods 2010, 20, 493–497. [Google Scholar] [CrossRef] [PubMed]
- John, S.; Briatka, T.; Rudolf, E. Diverse sensitivity of cells representing various stages of colon carcinogenesis to increased extracellular zinc: Implications for zinc chemoprevention. Oncol. Rep. 2011, 25, 769–780. [Google Scholar] [CrossRef] [PubMed]
- Brookes, M.J.; Hughes, S.; Turner, F.E.; Reynolds, G.; Sharma, N.; Ismail, T.; Berx, G.; McKie, A.T.; Hotchin, N.; Anderson, G.J.; et al. Modulation of iron transport proteins in human colorectal carcinogenesis. Gut 2006, 55, 1449–1460. [Google Scholar] [CrossRef] [PubMed]
- Komada, H.; Kise, Y.; Nakagawa, M.; Yamamura, M.; Hioki, K.; Yamamoto, M. Effect of dietary molybdenum on esophageal carcinogenesis in rats induced by N-methyl-N-benzylnitrosamine. Cancer Res. 1990, 50, 2418–2422. [Google Scholar] [PubMed]
- Odukanmi, O.A.; Salami, A.T.; Koda, K.; Morakinyo, O.L.; Olaleye, S.B. Trivalent Chromium Promotes Healing of Experimental Colitis in Mice by Suppression of Inflammation and Oxidative Stress. J. Biosci. Med. 2017, 5, 108–126. [Google Scholar] [CrossRef][Green Version]
- Kopec, A.K.; Kim, S.; Forgacs, A.L.; Zacharewski, T.R.; Proctor, D.M.; Harris, M.A.; Haws, L.C.; Thompson, C.M. Genome-wide gene expression effects in B6C3F1 mouse intestinal epithelia following 7 and 90 days of exposure to hexavalent chromium in drinking water. Toxicol. Appl. Pharmacol. 2012, 259, 13–26. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Ren, T.; Xiao, C.; Li, H.; Wu, T. Nickel promotes the invasive potential of human lung cancer cells via TLR4/MyD88 signaling. Toxicology 2011, 285, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Nolfo, F.; Rametta, S.; Marventano, S.; Grosso, G.; Mistretta, A.; Drago, F.; Gangi, S.; Basile, F.; Biondi, A. Pharmacological and dietary prevention for colorectal cancer. BMC Surg. 2013, 13 (Suppl. S2), S16. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.H.; Li, J.G.; Zhao, H.; Shi, J.; Huang, J.Q.; Wang, K.N.; Xia, X.J.; Li, L.; Lei, X.G. Porcine Serum Can Be Biofortified with Selenium to Inhibit Proliferation of Three Types of Human Cancer Cells–3. J. Nutr. 2013, 143, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Oyaizu, M. Studies on products of browning reactions: Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Sun, Y.; Hayakawa, S.; Ogawa, M.; Izumori, K. Antioxidant properties of custard pudding dessert containing rare hexose, d-psicose. Food Control 2007, 18, 220–227. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, L.; Wang, W. Formation of aldehyde and ketone compounds during production and storage of milk powder. Molecules 2012, 17, 9900–9911. [Google Scholar] [CrossRef] [PubMed]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef]
- AOAC International. Official Methods of Analysis of AOAC International, 19th ed.; AOAC International: Gaithersburg, MD, USA, 2012. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Szkudzińska, K.; Smutniak, I.; Rubaj, J.; Korol, W.; Bielecka, G. Method validation for determination of amino acids in feed by UPLC. Accredit. Qual. Assur. 2017, 22, 247–252. [Google Scholar] [CrossRef]
- Commission Regulation (EC) No. 152/2009 of 27 January 2009; Annex III G; European Union: Brussels, Belgium, 2009.
- Oser, B.L. An integrated essential amino acid index for predicting biological value of proteins. In Protein and Amino Acid Nutritional; Academic Press: New York, NY, USA, 1959; pp. 295–311. [Google Scholar]
- Analytical Procedure of the Analytical Centre of Warsaw University of Life Sciences (Warsaw, Poland); PB 52 wydanie 5 z dnia 08.03.2017 r; Warsaw University of Life Sciences: Warsaw, Poland, 2017.
- Analytical Procedure of the Analytical Centre of Warsaw University of Life Sciences (Warsaw, Poland); PB 34 wydanie 7 z dnia 08.03.2017 r; Warsaw University of Life Sciences: Warsaw, Poland, 2017.
- Analytical Procedure of the Central Laboratory of Agroecology of the University of Life Sciences in Lublin (Poland); CLA/ESA/5/2014 wersja 2 z dnia 03.03.2014 r; University of Life Sciences in Lublin: Lublin, Poland, 2014.
- Analytical Procedure of the Central Laboratory of Agroecology of the University of Life Sciences in Lublin (Poland); CLA/ESA/3/2014 wersja 1 z dnia 03.03.2014 r; University of Life Sciences in Lublin: Lublin, Poland, 2014.
- Antushevich, H.; Krawczynska, A.; Kapica, M.; Herman, A.P.; Zabielski, R. Effect of apelin on mitosis, apoptosis and DNA repair enzyme OGG 1/2 expression in intestinal cell lines IEC-6 and Caco-2. Folia Histochem. Cytobiol. 2014, 52, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Tada, H.; Shiho, O.; Kuroshima, K.I.; Koyama, M.; Tsukamoto, K. An improved colorimetric assay for interleukin 2. J. Immunol. Methods 1986, 93, 157–165. [Google Scholar] [CrossRef]




| Compound (% of Lyophilisate) | Mucus | Foot Tissues | Shells |
|---|---|---|---|
| Crude protein | 55.84 | 80.74 | 1.98 |
| Crude fat | 0.35 | 3.67 | 0.17 |
| Crude ash | 30.60 | 8.03 | 91.43 |
| Amino Acids | Mucus | Foot Tissues | Shells |
|---|---|---|---|
| Essential Amino Acids (EAA) * | |||
| Isoleucine | 37.82 ± 0.02 | 36.11 ± 0.03 | 109.96 ± 1.01 |
| Leucine | 75.42 ± 0.28 | 62.03 ± 0.17 | 164.41 ± 1.31 |
| Lysine | 40.79 ± 1.32 | 53.10 ± 2.31 | 129.76 ± 3.13 |
| Methionine | 4.05 ± 0.06 | 14.28 ± 0.24 | 33.92 ± 0.56 |
| Phenylalanine | 40.46 ± 1.31 | 34.23 ± 1.35 | 106.81 ± 0.08 |
| Threonine | 44.29 ± 0.18 | 39.41 ± 0.37 | 106.38 ± 1.77 |
| Valine | 45.05 ± 0.19 | 40.36 ± 0.28 | 160.57 ± 2.68 |
| Tryptophan | 8.87 ± 0.12 | 7.88 ± 0.17 | 22.38 ± 1.06 |
| Half-Essential Amino Acids (HEAA) * | |||
| Arginine | 40.84 ± 0.85 | 70.95 ± 1.91 | 126.66 ± 0.79 |
| Histidine | 18.08 ± 0.59 | 15.54 ± 0.29 | 32.86 ± 1.29 |
| Non-Essential Amino Acids (NEAA) * | |||
| Cysteine | 25.79 ± 0.57 | 10.31 ± 0.21 | 27.66 ± 0.17 |
| Aspartic acid # | 87.80 ± 1.52 | 89.03 ± 1.70 | 231.24 ± 3.44 |
| Glycine # | 63.75 ± 0.71 | 64.04 ± 1.73 | 225.28 ± 1.57 |
| Glutamic acid # | 82.74 ± 0.69 | 132.56 ± 2.24 | 271.57 ± 3.46 |
| Alanine # | 33.67 ± 0.39 | 46.61 ± 0.47 | 117.79 ± 1.21 |
| Serine | 42.37 ± 0.23 | 47.90 ± 0.66 | 119.31 ± 3.74 |
| Proline | 32.80 ± 0.10 | 41.41 ± 0.14 | 93.50 ± 0.46 |
| Tyrosine | 33.85 ± 1.40 | 30.86 ± 1.36 | 50.89 ± 1.64 |
| Amino Acid Groups and Ratios | |||
| Total amino acids (TAA) | 758.42 ± 2.32 | 836.56 ± 0.00 | 2103.24 ± 24.37 |
| Essential amino acids (EAA) | 296.73 ± 0.48 | 287.37 ± 1.46 | 834.18 ± 6.80 |
| Half-essential amino acids (HEAA) | 58.92 ± 1.44 | 86.49 ± 2.19 | 159.51 ± 2.07 |
| Non-essential amino acids (NEAA) | 402.77 ± 0.40 | 462.70 ± 0.73 | 1109.55 ± 15.51 |
| Delicious amino acids (DAA) | 267.97 ± 1.90 | 332.23 ± 2.68 | 845.87 ± 9.68 |
| EAA/TAA | 0.39 | 0.34 | 0.40 |
| EAA/NEAA | 0.74 | 0.62 | 0.75 |
| DAA/TAA | 0.35 | 0.40 | 0.40 |
| Amino Acids | AAS | CS | ||||
|---|---|---|---|---|---|---|
| Mucus | Foot Tissues | Shells | Mucus | Foot Tissues | Shells | |
| Histidine | 0.95 | 0.82 | 1.73 | 0.82 | 0.71 | 1.49 |
| Isoleucine | 1.35 | 1.29 | 3.93 | 0.70 | 0.67 | 2.04 |
| Leucine | 1.14 | 0.94 | 2.49 | 0.88 | 0.72 | 1.91 |
| Lysine | 0.70 | 0.92 | 2.24 | 0.58 | 0.76 | 1.85 |
| Threonine | 1.30 | 1.16 | 3.13 | 0.94 | 0.84 | 2.26 |
| Tryptophan | 0.81 | 0.72 | 2.03 | 0.52 | 0.46 | 1.32 |
| Valine | 1.29 | 1.15 | 4.59 | 0.68 | 0.61 | 2.43 |
| Methionine + cysteine | 1.19 | 0.98 | 2.46 | 0.52 | 0.43 | 1.08 |
| Phenylalanine + tyrosine | 1.18 | 1.03 | 2.50 | 0.80 | 0.70 | 1.70 |
| EAAI | 107.80 | 98.63 | 266.65 | 70.18 | 64.21 | 173.59 |
| Fatty Acids (g/100 g FAME) | Foot Tissues | Shells | p |
|---|---|---|---|
| C14:0 (myristic) | - | 0.227 ± 0.004 | |
| C15:0 (pentadecanoic) | - | 0.201 ± 0.009 | |
| C16:0 (palmitic) | 5.02 ± 0.33 | 5.20 ± 0.40 | 0.7457 |
| C16:1 cis-9 (palmitoleic) | 0.059 ± 0.011 a | 0.253 ± 0.047 b | 0.0154 |
| C17:0 (margaric) | 1.317 ± 0.073 B | 0.243 ± 0.010 A | 0.0001 |
| C18:0 (stearic) | 16.27 ± 0.03 B | 4.71 ± 0.22 A | 0.0000 |
| C18:1 cis-9 (oleic) | 11.6 ± 0.2 A | 30.0 ± 1.3 B | 0.0002 |
| C18:1 cis-11 (cis-vaccenic) | 0.416 ± 0.018 A | 1.337 ± 0.087 B | 0.0005 |
| C18:2 all trans-9,12 (linolelaidic) | 0.198 ± 0.039 b | 0.076 ± 0.003 a | 0.0356 |
| C18:2 trans isomer | 0.147 ± 0.000 B | 0.099 ± 0.006 A | 0.0010 |
| C18:2 all cis-9,12 (linoleic), n-6 | 16.4 ± 0.5 A | 28.1 ± 0.6 B | 0.0001 |
| C18:3 all cis-6,9,12 (γ-linolenic), n-6 | 0.091 ± 0.018 | 0.101 ± 0.007 | 0.6287 |
| C18:3 trans isomer | 0.130 ± 0.025 a | 0.261 ± 0.030 b | 0.0279 |
| C18:3 all cis-9, 12, 15 (α-linolenic), n-3 | 2.74 ± 0.21 a | 3.63 ± 0.09 b | 0.0169 |
| C20:0 (arachidic) | 0.495 ± 0.012 B | 0.312 ± 0.010 A | 0.0003 |
| C20:1 cis-11 (gondoic) | 1.127 ± 0.023 B | 0.653 ± 0.026 A | 0.0002 |
| C20:2 all cis-11, 14 (eicosadienoic), n-6 | 8.92 ± 0.46 B | 2.62 ± 0.07 A | 0.0002 |
| C20:3 all cis-8, 11, 14 (dihomo-γ-linolenic), n-6 | 0.977 ± 0.198 B | 0.609 ± 0.006 A | 0.0095 |
| C20:3 all cis-11, 14, 17 (eicosatrienoic), n-3 | 0.584 ± 0.114 B | 0.053 ± 0.006 A | 0.0097 |
| C20:5 all cis-5, 8, 11, 14, 17 (eicosapentaenoic), n-3 | 3.323 ± 0.141 B | 0.169 ± 0.009 A | 0.0000 |
| C21:0 (heneicosylic) | - | 0.093 ± 0.006 | |
| C22:0 (behenic) | 0.193 ± 0.065 | 0.175 ± 0.009 | 0.7984 |
| C22:1 cis-13 (erucic) | 13.77 ± 0.45 B | 3.09 ± 0.21 A | 0.0000 |
| C22:2 all cis-13, 16 (docosadienoic), n-6 | 0.068 ± 0.013 | - | |
| C23:0 (tricosylic) | 0.122 ± 0.048 | 0.077 ± 0.004 | 0.4129 |
| C24:0 (lignoceric) | 2.463 ± 0.147 B | 0.665 ± 0.043 A | 0.0003 |
| Fatty acid groups and ratios | |||
| Saturated fatty acids (SFA) | 25.9 ± 0.4 B | 11.9 ± 0.8 A | 0.0001 |
| Monounsaturated fatty acids (MUFA) | 27.0 ± 0.3 A | 35.3 ± 1.4 B | 0.0044 |
| Polyunsaturated fatty acids (PUFA) | 33.6 ± 0.5 a | 35.7 ± 0.6 b | 0.0481 |
| n-3 | 6.65 ± 0.20 B | 3.85 ± 0.07 A | 0.0002 |
| n-6 | 26.5 ± 0.3 A | 31.4 ± 0.5 B | 0.0009 |
| n-6/n-3 | 3.98 ± 0.09 A | 8.15 ± 0.03 B | 0.0000 |
| Elements | Mucus | Foot Tissues | Shells | p |
|---|---|---|---|---|
| Macroelements (g/kg Lyophilisate) | ||||
| Na | 70.26 ± 0.19 C | 6.53 ± 0.07 B | 1.08 ± 0.01 A | 0.0000 |
| Ca | 35.50 ± 0.12 B | 15.70 ± 0.79 A | 329.67 ± 0.88 C | 0.0000 |
| K | 9.67 ± 0.39 B | 9.98 ± 0.08 B | 1.77 ± 0.02 A | 0.0000 |
| Mg | 5.60 ± 0.04 C | 1.52 ± 0.02 B | 0.64 ± 0.01 A | 0.0000 |
| P | 1.87 ± 0.01 A | 9.50 ± 0.09 C | 2.56 ± 0.00 B | 0.0000 |
| S | ND | 5.01 ± 0.02 B | 1.11 ± 0.02 A | 0.0000 |
| Cl | ND | 1.56 ± 0.05 B | 0.41 ± 0.01 A | 0.0000 |
| Microelements (mg/kg lyophilisate) | ||||
| Cu | 239.00 ± 6.11 C | 29.67 ± 0.67 B | 10.33 ± 0.18 A | 0.0000 |
| Zn | 52.80 ± 2.21 B | 65.80 ± 0.76 C | 21.53 ± 0.54 A | 0.0000 |
| B | 32.43 ± 1.04 C | 7.95 ± 0.13 B | 1.42 ± 0.01 A | 0.0000 |
| Fe | 28.17 ± 0.93 A | 101.00 ± 0.58 B | 760.00 ± 10.69 C | 0.0000 |
| Mo | 6.81 ± 0.04 C | 2.62 ± 0.01 B | 0.03 ± 0.00 A | 0.0000 |
| Mn | 5.05 ± 0.37 A | 12.23 ± 0.19 B | 24.10 ± 0.21 C | 0.0000 |
| Cr | 3.45 ± 0.09 C | 2.45 ± 0.06 B | 0.11 ± 0.01 A | 0.0000 |
| Ni | 1.64 ± 0.06 A | 9.49 ± 0.14 C | 2.33 ± 0.08 B | 0.0000 |
| Se | 0.27 ± 0.06 | 0.36 ± 0.03 | - | 0.2114 |
| Co | 0.10 ± 0.00 B | 0.07 ± 0.00 A | 0.14 ± 0.00 C | 0.0000 |
| Si | ND | 820 ± 20.82 B | 123.33 ± 3.18 A | 0.0000 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matusiewicz, M.; Kosieradzka, I.; Niemiec, T.; Grodzik, M.; Antushevich, H.; Strojny, B.; Gołębiewska, M. In Vitro Influence of Extracts from Snail Helix aspersa Müller on the Colon Cancer Cell Line Caco-2. Int. J. Mol. Sci. 2018, 19, 1064. https://doi.org/10.3390/ijms19041064
Matusiewicz M, Kosieradzka I, Niemiec T, Grodzik M, Antushevich H, Strojny B, Gołębiewska M. In Vitro Influence of Extracts from Snail Helix aspersa Müller on the Colon Cancer Cell Line Caco-2. International Journal of Molecular Sciences. 2018; 19(4):1064. https://doi.org/10.3390/ijms19041064
Chicago/Turabian StyleMatusiewicz, Magdalena, Iwona Kosieradzka, Tomasz Niemiec, Marta Grodzik, Hanna Antushevich, Barbara Strojny, and Małgorzata Gołębiewska. 2018. "In Vitro Influence of Extracts from Snail Helix aspersa Müller on the Colon Cancer Cell Line Caco-2" International Journal of Molecular Sciences 19, no. 4: 1064. https://doi.org/10.3390/ijms19041064
APA StyleMatusiewicz, M., Kosieradzka, I., Niemiec, T., Grodzik, M., Antushevich, H., Strojny, B., & Gołębiewska, M. (2018). In Vitro Influence of Extracts from Snail Helix aspersa Müller on the Colon Cancer Cell Line Caco-2. International Journal of Molecular Sciences, 19(4), 1064. https://doi.org/10.3390/ijms19041064