Evaluation of a New Survivin ELISA and UBC® Rapid for the Detection of Bladder Cancer in Urine
Abstract
:1. Introduction
2. Results
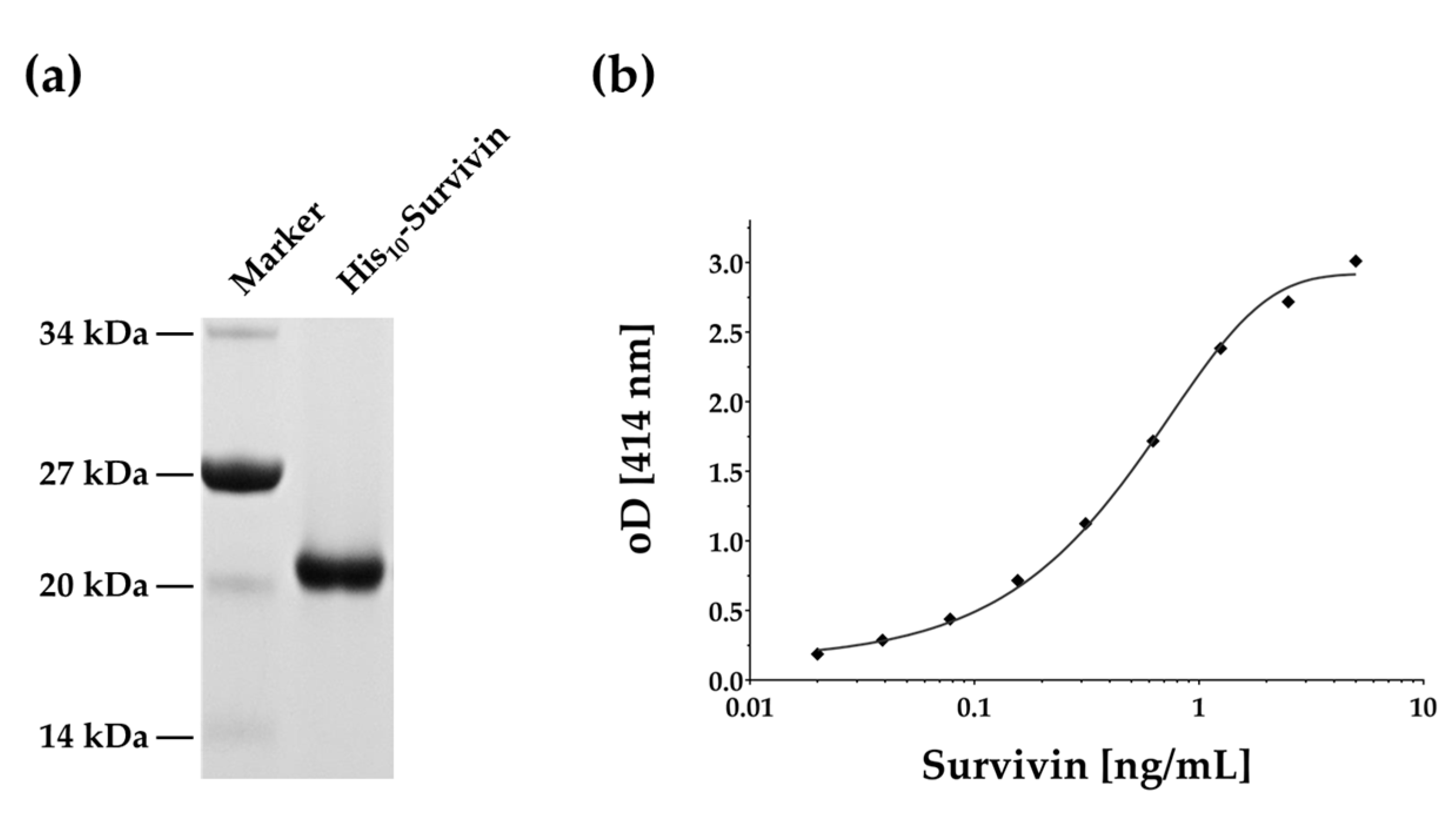
2.1. Survivin Production and ELISA Development
2.2. Study Group
2.3. Survivin in Urine of Bladder Cancer Patients
2.4. Performance of UBC® Rapid
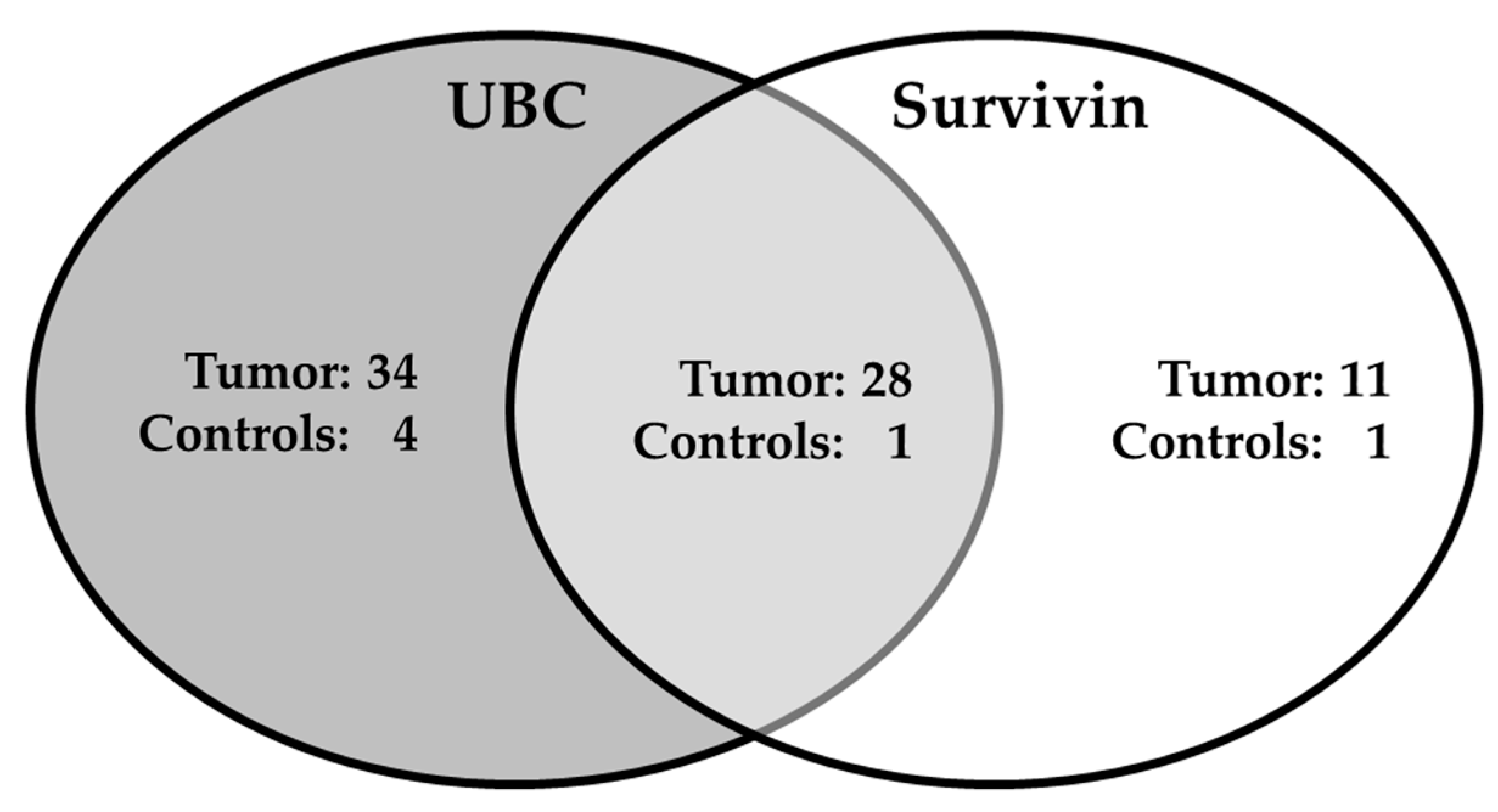
2.5. Combination of Survivin and UBC® Rapid
2.6. Possible Influence of Microhematuria
3. Discussion
4. Materials and Methods
4.1. Study Population
4.2. Urine Sample Collection
4.3. UBC® Rapid Test
4.4. Expression and Purification of Recombinant Survivin
4.5. Immunization and Antibody Purification
4.6. Biotinylation of Anti-Survivin Antibody
4.7. Survivin ELISA
4.8. Statistics
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| CV | Coefficient of variation |
| LoD | Limit of detection |
| ELISA | Enzyme-linked immunosorbent assay |
| IQR | Interquartile range |
| ROC | Receiver operating characteristic |
References
- Knowles, M.A.; Hurst, C.D. Molecular biology of bladder cancer: New insights into pathogenesis and clinical diversity. Nat. Rev. Cancer 2015, 15, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Schmitz-Drager, B.J.; Droller, M.; Lokeshwar, V.B.; Lotan, Y.; Hudson, M.L.A.; van Rhijn, B.W.; Marberger, M.J.; Fradet, Y.; Hemstreet, G.P.; Malmstrom, P.-U.; et al. Molecular markers for bladder cancer screening, early diagnosis, and surveillance: The WHO/ICUD consensus. Urol. Int. 2015, 94, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Tilki, D.; Burger, M.; Dalbagni, G.; Grossman, H.B.; Hakenberg, O.W.; Palou, J.; Reich, O.; Roupret, M.; Shariat, S.F.; Zlotta, A.R. Urine markers for detection and surveillance of non-muscle-invasive bladder cancer. Eur. Urol. 2011, 60, 484–492. [Google Scholar] [CrossRef] [PubMed]
- Smith, Z.L.; Guzzo, T.J. Urinary markers for bladder cancer. F1000prime Rep. 2013, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Miyake, M.; Goodison, S.; Giacoia, E.G.; Rizwani, W.; Ross, S.; Rosser, C.J. Influencing factors on the NMP-22 urine assay: An experimental model. BMC Urol. 2012, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Chou, R.; Gore, J.L.; Buckley, D.; Fu, R.; Gustafson, K.; Griffin, J.C.; Grusing, S.; Selph, S. Urinary Biomarkers for Diagnosis of Bladder Cancer: A Systematic Review and Meta-analysis. Ann. Intern. Med. 2015, 163, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Huber, S.; Schwentner, C.; Taeger, D.; Pesch, B.; Nasterlack, M.; Leng, G.; Mayer, T.; Gawrych, K.; Bonberg, N.; Pelster, M.; et al. Nuclear matrix protein-22: A prospective evaluation in a population at risk for bladder cancer. Results from the UroScreen study. BJU Int. 2012, 110, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Mowatt, G.; Zhu, S.; Kilonzo, M.; Boachie, C.; Fraser, C.; Griffiths, T.R.L.; N’Dow, J.; Nabi, G.; Cook, J.; Vale, L. Systematic review of the clinical effectiveness and cost-effectiveness of photodynamic diagnosis and urine biomarkers (FISH, ImmunoCyt, NMP22) and cytology for the detection and follow-up of bladder cancer. Health Technol. Assess. 2010, 14, 1–331. [Google Scholar] [CrossRef] [PubMed]
- Hajdinjak, T. UroVysion FISH test for detecting urothelial cancers: Meta-analysis of diagnostic accuracy and comparison with urinary cytology testing. Urol. Oncol. 2008, 26, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Banek, S.; Schwentner, C.; Täger, D.; Pesch, B.; Nasterlack, M.; Leng, G.; Gawrych, K.; Bonberg, N.; Johnen, G.; Kluckert, M.; et al. Prospective evaluation of fluorescence-in situ-hybridization to detect bladder cancer: Results from the UroScreen-Study. Urol. Oncol. 2013, 31, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Pesch, B.; Taeger, D.; Johnen, G.; Gawrych, K.; Bonberg, N.; Schwentner, C.; Wellhausser, H.; Kluckert, M.; Leng, G.; Nasterlack, M.; et al. Screening for bladder cancer with urinary tumor markers in chemical workers with exposure to aromatic amines. Int. Arch. Occup. Environ. Health 2014, 87, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Bonberg, N.; Taeger, D.; Gawrych, K.; Johnen, G.; Banek, S.; Schwentner, C.; Sievert, K.-D.; Wellhäußer, H.; Kluckert, M.; Leng, G.; et al. Chromosomal instability and bladder cancer: The UroVysion(TM) test in the UroScreen study. BJU Int. 2013, 112, E372–E382. [Google Scholar] [CrossRef] [PubMed]
- Unruhe, B.; Schroder, E.; Wunsch, D.; Knauer, S.K. An Old Flame Never Dies: Survivin in Cancer and Cellular Senescence. Gerontology 2016, 62, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, P.K.; Goel, A.; Mittal, R.D. Survivin: A molecular biomarker in cancer. Indian J. Med. Res. 2015, 141, 389–397. [Google Scholar] [PubMed]
- Akhtar, M.; Gallagher, L.; Rohan, S. Survivin: Role in diagnosis, prognosis, and treatment of bladder cancer. Adv. Anat. Pathol. 2006, 13, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Shariat, S.F.; Casella, R.; Khoddami, S.M.; Hernandez, G.; Sulser, T.; Gasser, T.C.; Lerner, S.P. Urine detection of survivin is a sensitive marker for the noninvasive diagnosis of bladder cancer. J. Urol. 2004, 171, 626–630. [Google Scholar] [CrossRef] [PubMed]
- Altieri, D.C. Survivin—The inconvenient IAP. Semin. Cell Dev. Boil. 2015, 39, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Groner, B.; Weiss, A. Targeting survivin in cancer: Novel drug development approaches. BioDrugs 2014, 28, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Altieri, D.C. Targeting survivin in cancer. Cancer Lett. 2013, 332, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Duan, N.; Zhang, C.; Zhang, W. Survivin and Tumorigenesis: Molecular Mechanisms and Therapeutic Strategies. J. Cancer 2016, 7, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Ku, J.H.; Godoy, G.; Amiel, G.E.; Lerner, S.P. Urine survivin as a diagnostic biomarker for bladder cancer: A systematic review. BJU Int. 2012, 110, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Johnen, G.; Gawrych, K.; Bontrup, H.; Pesch, B.; Taeger, D.; Banek, S.; Kluckert, M.; Wellhausser, H.; Eberle, F.; Nasterlack, M.; et al. Performance of survivin mRNA as a biomarker for bladder cancer in the prospective study UroScreen. PLoS ONE 2012, 7, e35363. [Google Scholar] [CrossRef] [PubMed]
- Jeon, C.; Kim, M.; Kwak, C.; Kim, H.H.; Ku, J.H. Prognostic role of survivin in bladder cancer: A systematic review and meta-analysis. PLoS ONE 2013, 8, e76719. [Google Scholar] [CrossRef] [PubMed]
- Chatziharalambous, D.; Lygirou, V.; Latosinska, A.; Stravodimos, K.; Vlahou, A.; Jankowski, V.; Zoidakis, J. Analytical Performance of ELISA Assays in Urine: One More Bottleneck towards Biomarker Validation and Clinical Implementation. PLoS ONE 2016, 11, e0149471. [Google Scholar] [CrossRef] [PubMed]
- Pesch, B.; Nasterlack, M.; Eberle, F.; Bonberg, N.; Taeger, D.; Leng, G.; Feil, G.; Johnen, G.; Ickstadt, K.; Kluckert, M.; et al. The role of hematuria in bladder cancer screening among men with former occupational exposure to aromatic amines. BJU Int. 2011, 108, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-T.; Duymich, C.E.; Weisenberger, D.J.; Liang, G. Genetic and Epigenetic Alterations in Bladder Cancer. Int. Neurourol. J. 2016, 20, S84–S94. [Google Scholar] [CrossRef] [PubMed]
- Di Meo, A.; Bartlett, J.; Cheng, Y.; Pasic, M.D.; Yousef, G.M. Liquid biopsy: A step forward towards precision medicine in urologic malignancies. Mol. Cancer 2017, 16, 80. [Google Scholar] [CrossRef] [PubMed]
- Burger, M.; Catto, J.W.F.; Dalbagni, G.; Grossman, H.B.; Herr, H.; Karakiewicz, P.; Kassouf, W.; Kiemeney, L.A.; la Vecchia, C.; Shariat, S.; et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013, 63, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Öge, Ö.; Kozaci, D.; Gemalmaz, H. The BTA Stat Test is Nonspecific for Hematuria: An Experimental Hematuria Model. J. Urol. 2002, 167, 1318–1320. [Google Scholar] [CrossRef]
- Shariat, S.F.; Karam, J.A.; Lotan, Y.; Karakiewizc, P.I. Critical evaluation of urinary markers for bladder cancer detection and monitoring. Rev. Urol. 2008, 10, 120–135. [Google Scholar] [PubMed]
- Findlay, J.W.A.; Dillard, R.F. Appropriate calibration curve fitting in ligand binding assays. AAPS J. 2007, 9, E260–E267. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.K.; Singh, P.K.; Srivastava, K.; Singh, D.; Dalela, D.; Rath, S.K.; Goel, M.M.; Bhatt, M.L.B. Diagnostic Role of Survivin in Urinary Bladder Cancer. Asian Pac. J. Cancer Prev. 2013, 14, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Fichorova, R.N.; Richardson-Harman, N.; Alfano, M.; Belec, L.; Carbonneil, C.; Chen, S.; Cosentino, L.; Curtis, K.; Dezzutti, C.S.; Donoval, B.; et al. Biological and technical variables affecting immunoassay recovery of cytokines from human serum and simulated vaginal fluid: A multicenter study. Anal. Chem. 2008, 80, 4741–4751. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, Y.-D.; Gu, W. Urinary proteomics as a novel tool for biomarker discovery in kidney diseases. J. Zhejiang Univ. Sci. B 2010, 11, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Zoidakis, J.; Makridakis, M.; Zerefos, P.G.; Bitsika, V.; Esteban, S.; Frantzi, M.; Stravodimos, K.; Anagnou, N.P.; Roubelakis, M.G.; Sanchez-Carbayo, M.; et al. Profilin 1 is a potential biomarker for bladder cancer aggressiveness. Mol. Cell. Proteom. 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Adachi, J.; Kumar, C.; Zhang, Y.; Olsen, J.V.; Mann, M. The human urinary proteome contains more than 1500 proteins, including a large proportion of membrane proteins. Genome Biol. 2006, 7, R80. [Google Scholar] [CrossRef] [PubMed]
- Reverberi, R.; Reverberi, L. Factors affecting the antigen-antibody reaction. Blood Transfus. 2007, 5, 227–240. [Google Scholar] [PubMed]
- Khan, S.; Bennit, H.F.; Wall, N.R. The emerging role of exosomes in survivin secretion. Histol. Histopathol. 2015, 30, 43–50. [Google Scholar] [PubMed]
- Nong, R.Y.; Di, W.; Yan, J.; Hammond, M.; Gu, G.J.; Kamali-Moghaddam, M.; Landegren, U.; Darmanis, S. Solid-phase proximity ligation assays for individual or parallel protein analyses with readout via real-time PCR or sequencing. Nat. Protoc. 2013, 8, 1234–1248. [Google Scholar] [CrossRef] [PubMed]
- Darmanis, S.; Nong, R.Y.; Hammond, M.; Gu, J.; Alderborn, A.; Vanelid, J.; Siegbahn, A.; Gustafsdottir, S.; Ericsson, O.; Landegren, U.; et al. Sensitive plasma protein analysis by microparticle-based proximity ligation assays. Mol. Cell. Proteom. 2010, 9, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, I.; Nishimura, T.; Kondo, Y.; Kimura, G.; Satoh, M.; Matsuzawa, I.; Hamasaki, T.; Ohta, S. Detection of urine survivin in 40 patients with bladder cancer. J. Nippon Med. Sch. 2004, 71, 379–383. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Xu, J.; Zhang, Q. Sandwich ELISA for detecting urinary Survivin in bladder cancer. Chin. J. Cancer Res. 2013, 25, 375–381. [Google Scholar] [PubMed]
- Eissa, S.; Badr, S.; Barakat, M.; Zaghloul, A.; Mohanad, M. The Diagnostic Efficacy of Urinary Survivin and Hyaluronidase mRNA as Urine Markers in Patients with Bladder Cancer. Clin. Lab. 2013, 59, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Garg, M. Urothelial cancer stem cells and epithelial plasticity: Current concepts and therapeutic implications in bladder cancer. Cancer Metastasis Rev. 2015, 34, 691–701. [Google Scholar] [CrossRef] [PubMed]
- Adamowicz, J.; Pokrywczynska, M.; Tworkiewicz, J.; Wolski, Z.; Drewa, T. The relationship of cancer stem cells in urological cancers. Cent. Eur. J. Urol. 2013, 66, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Ecke, T.; Arndt, C.; Stephan, C.; Hallmann, S.; Lux, O.; Otto, T.; Ruttloff, J.; Gerullis, H. Preliminary Results of a Multicentre Study of the UBC® Rapid Test for Detection of Urinary Bladder Cancer. Anticancer Res. 2015, 2015, 2651–2656. [Google Scholar]
- D’Costa, J.J.; Goldsmith, J.C.; Wilson, J.S.; Bryan, R.T.; Ward, D.G. A Systematic Review of the Diagnostic and Prognostic Value of Urinary Protein Biomarkers in Urothelial Bladder Cancer. Bladder Cancer 2016, 2, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Ritter, R.; Hennenlotter, J.; Kuhs, U.; Hofmann, U.; Aufderklamm, S.; Blutbacher, P.; Deja, A.; Hohneder, A.; Gerber, V.; Gakis, G.; et al. Evaluation of a new quantitative point-of-care test platform for urine-based detection of bladder cancer. Urol. Oncol. 2014, 32, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Hakenberg, O.W.; Fuessel, S.; Richter, K.; Froehner, M.; Oehlschlaeger, S.; Rathert, P.; Meye, A.; Wirth, M.P. Qualitative and quantitative assessment of urinary cytokeratin 8 and 18 fragments compared with voided urine cytology in diagnosis of bladder carcinoma. Urology 2004, 64, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Styrke, J.; Henriksson, H.; Ljungberg, B.; Hasan, M.; Silfverberg, I.; Einarsson, R.; Malmström, P.-U.; Sherif, A. Evaluation of the diagnostic accuracy of UBC® Rapid in bladder cancer: A Swedish multicentre study. Scand. J. Urol. 2017, 51, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Ecke, T.H.; Weiß, S.; Stephan, C.; Hallmann, S.; Barski, D.; Otto, T.; Gerullis, H. UBC® Rapid Test for detection of carcinoma in situ for bladder cancer. Tumour Biol. 2017, 39. [Google Scholar] [CrossRef] [PubMed]
- Pesch, B.; Bruning, T.; Johnen, G.; Casjens, S.; Bonberg, N.; Taeger, D.; Muller, A.; Weber, D.G.; Behrens, T. Biomarker research with prospective study designs for the early detection of cancer. Biochim. Biophys. Acta 2014, 1844, 874–883. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Carbayo, M.; Herrero, E.; Megias, J.; Mira, A.; Espasa, A.; Chinchilla, V.; Soria, F. Initial evaluation of the diagnostic performance of the new urinary bladder cancer antigen test as a tumor marker for transitional cell carcinoma of the bladder. J. Urol. 1999, 161, 1110–1115. [Google Scholar] [CrossRef]
- Larré, S.; Catto, J.W.F.; Cookson, M.S.; Messing, E.M.; Shariat, S.F.; Soloway, M.S.; Svatek, R.S.; Lotan, Y.; Zlotta, A.R.; Grossman, H.B. Screening for bladder cancer: Rationale, limitations, whom to target, and perspectives. Eur. Urol. 2013, 63, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Altznauer, F.; Martinelli, S.; Yousefi, S.; Thurig, C.; Schmid, I.; Conway, E.M.; Schoni, M.H.; Vogt, P.; Mueller, C.; Fey, M.F.; et al. Inflammation-associated cell cycle-independent block of apoptosis by survivin in terminally differentiated neutrophils. J. Exp. Med. 2004, 199, 1343–1354. [Google Scholar] [CrossRef] [PubMed]
- Atsumi, T.; Singh, R.; Sabharwal, L.; Bando, H.; Meng, J.; Arima, Y.; Yamada, M.; Harada, M.; Jiang, J.-J.; Kamimura, D.; et al. Inflammation amplifier, a new paradigm in cancer biology. Cancer Res. 2014, 74, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Guven Maiorov, E.; Keskin, O.; Gursoy, A.; Nussinov, R. The structural network of inflammation and cancer: Merits and challenges. Semin. Cancer Biol. 2013, 23, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Sauter, G.; Eble, J.N.; Epstein, J.I.; Sesterhenn, I.A. (Eds.) World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs; IARC Press: Lyon, France, 2004; Volume 6. [Google Scholar]
- Shen, C.; Liu, W.; Buck, A.K.; Reske, S.N. Pro-apoptosis and anti-proliferation effects of a recombinant dominant-negative survivin-T34A in human cancer cells. Anticancer Res. 2009, 29, 1423–1428. [Google Scholar] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the Areas under Two or More Correlated Receiver Operating Characteristic Curves: A Nonparametric Approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | All (n = 244) | Tumor (n = 111) | Clinical Controls (n = 133) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Gender | Male | Female | Male | Female | Male | Female | |||
| n | 175 | 69 | 83 | 28 | 92 | 41 | |||
| n | Median | IQR | n | Median | IQR | n | Median | IQR | |
| Age (years) | 244 | 73 | 63–80 | 111 | 74 | 65–80 | 133 | 71 | 60–79 |
| Body mass index | 244 | 26.5 | 24.0–29.8 | 111 | 26.9 | 24.3–30.4 | 133 | 26.3 | 23.6–29.6 |
| Urine volume (mL) | 244 | 30 | 16–40 | 111 | 20 | 14–40 | 133 | 35 | 20–50 |
| Urine retention within bladder (h) | 236 | 2.0 | 1.0–3.0 | 106 | 1.75 | 1.0–2.5 | 130 | 2.0 | 1.0–3.5 |
| Specific gravity (g/L) | 240 | 240 | 1015 | 109 | 1015 | 1015–1020 | 131 | 1020 | 1015–1020 |
| pH | 240 | 5 | 5.0–6.5 | 109 | 5 | 5–6.5 | 131 | 5 | 5–6.5 |
| Survivin (ng/mL) | 111 | 0.014 * | 0–0.528 | 133 | 0 | 0–0.249 | |||
| UBC® Rapid (mg/L) | 111 | 16.3 | 5–300 | 133 | 5 | 5–28.2 | |||
| Characteristic | Status | (n) |
|---|---|---|
| Tumor Stage | Ta | 61 |
| T1 | 14 | |
| T2 | 23 | |
| T3 | 9 | |
| Carcinoma in situ | 3 | |
| Missing | 1 | |
| Histological grade | Low | 55 |
| High | 55 | |
| Missing | 1 | |
| Recurrent | Yes | 52 |
| No | 58 | |
| Missing | 1 | |
| Gross hematuria | Yes | 66 |
| No | 45 | |
| Dysuria | Yes | 33 |
| No | 78 | |
| Frequent urination | Yes | 51 |
| No | 60 | |
| Bladder Stones | Yes | 12 |
| No | 99 | |
| Diabetes mellitus type II | Yes | 12 |
| No | 99 | |
| Smoking | Never | 23 |
| Former | 51 | |
| Actual | 35 | |
| Missing | 2 |
| Groups | Cut-Off | Sensitivity (%) | Specificity (%) | True-Positive (n) | True-Negative (n) | False-Positive (n) | False-Negative (n) |
|---|---|---|---|---|---|---|---|
| Survivin ELISA | |||||||
| Tumor vs. Controls | 0.033 ng/mL | 35 | 98 | 39 | 131 | 2 | 72 |
| High-grade tumor vs. Controls | 51 | 98 | 28 | 131 | 2 | 27 | |
| Low-grade tumor vs. Controls | 18 | 98 | 10 | 131 | 2 | 45 | |
| UBC® Rapid | |||||||
| Tumor vs. Controls | 10.0 mg/L | 56 | 96 | 62 | 128 | 5 | 49 |
| High-grade tumor vs. Controls | 73 | 96 | 40 | 128 | 5 | 15 | |
| Low-grade tumor vs. Controls | 40 | 96 | 22 | 128 | 5 | 33 | |
| Combination | |||||||
| Tumor vs. Controls | >Survivin or >UBC® Rapid | 66 | 95 | 73 | 127 | 6 | 38 |
| High-grade tumor vs. Controls | 82 | 95 | 45 | 127 | 6 | 10 | |
| Low-grade tumor vs. Controls | 49 | 95 | 27 | 127 | 6 | 28 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gleichenhagen, J.; Arndt, C.; Casjens, S.; Meinig, C.; Gerullis, H.; Raiko, I.; Brüning, T.; Ecke, T.; Johnen, G. Evaluation of a New Survivin ELISA and UBC® Rapid for the Detection of Bladder Cancer in Urine. Int. J. Mol. Sci. 2018, 19, 226. https://doi.org/10.3390/ijms19010226
Gleichenhagen J, Arndt C, Casjens S, Meinig C, Gerullis H, Raiko I, Brüning T, Ecke T, Johnen G. Evaluation of a New Survivin ELISA and UBC® Rapid for the Detection of Bladder Cancer in Urine. International Journal of Molecular Sciences. 2018; 19(1):226. https://doi.org/10.3390/ijms19010226
Chicago/Turabian StyleGleichenhagen, Jan, Christian Arndt, Swaantje Casjens, Carmen Meinig, Holger Gerullis, Irina Raiko, Thomas Brüning, Thorsten Ecke, and Georg Johnen. 2018. "Evaluation of a New Survivin ELISA and UBC® Rapid for the Detection of Bladder Cancer in Urine" International Journal of Molecular Sciences 19, no. 1: 226. https://doi.org/10.3390/ijms19010226
APA StyleGleichenhagen, J., Arndt, C., Casjens, S., Meinig, C., Gerullis, H., Raiko, I., Brüning, T., Ecke, T., & Johnen, G. (2018). Evaluation of a New Survivin ELISA and UBC® Rapid for the Detection of Bladder Cancer in Urine. International Journal of Molecular Sciences, 19(1), 226. https://doi.org/10.3390/ijms19010226





