Modeling the Colchicum autumnale Tubulin and a Comparison of Its Interaction with Colchicine to Human Tubulin
Abstract
:1. Introduction
2. Results
2.1. Colchicum autumnale and Human Tubulin Sequences
2.2. Homology Models
2.3. Free Energy of Binding Calculations
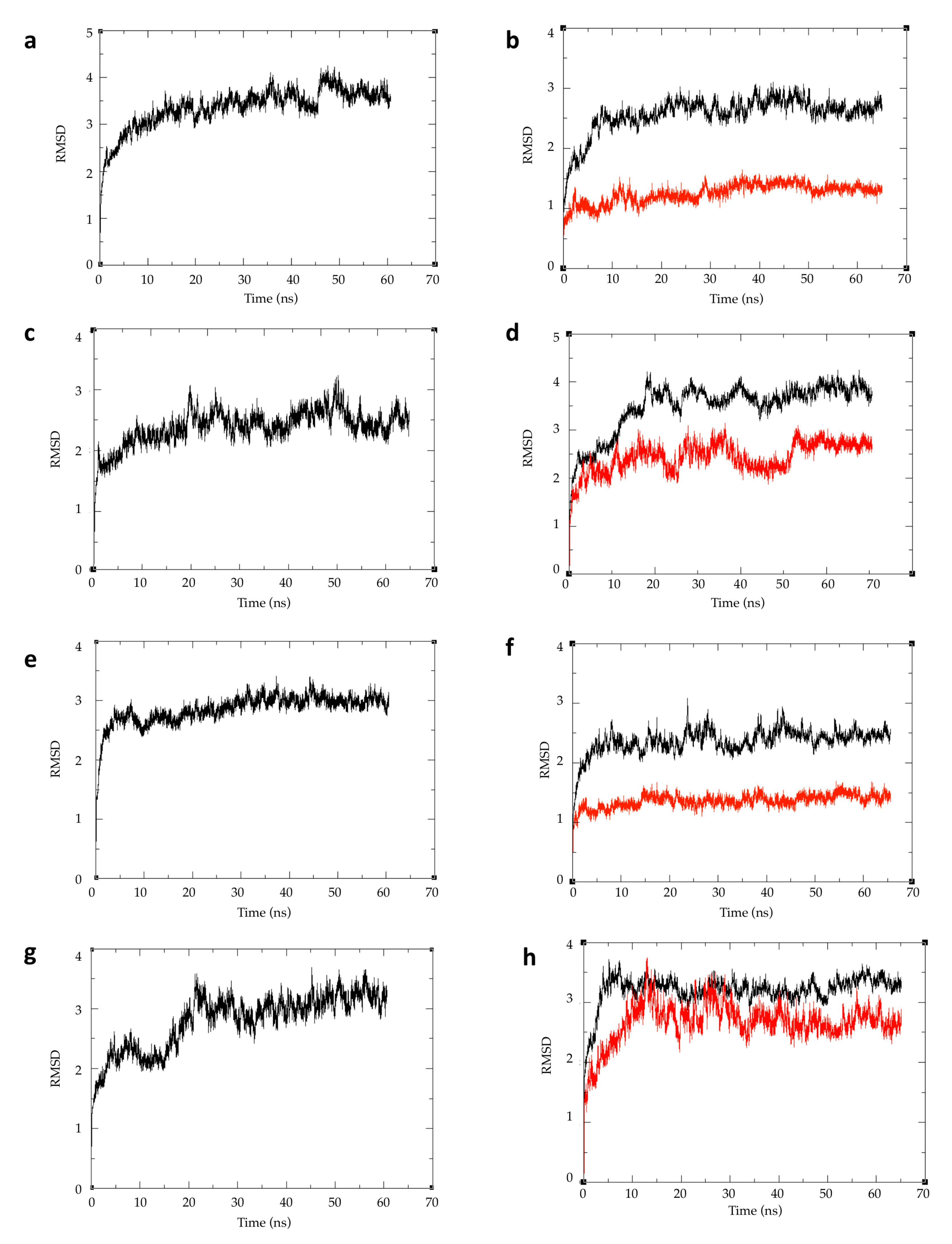
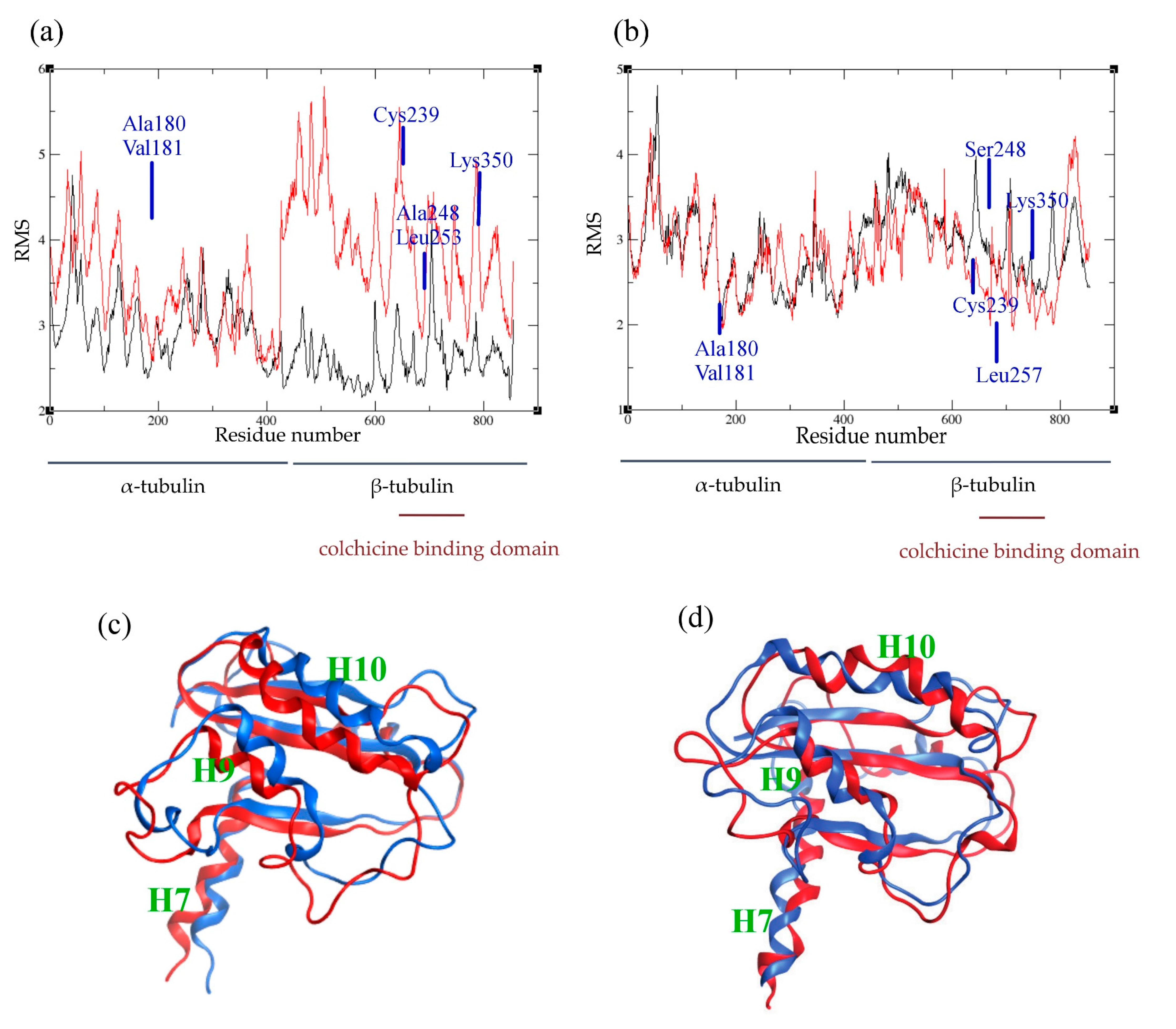
2.4. Atomic Fluctuations
2.5. Conformational Changes
3. Discussion
4. Materials and Methods
4.1. Sequence Assembly
4.2. Homology Modeling
4.3. Molecular Dynamics Simulations
4.4. Binding Free Energy Calculations
4.5. Atomic Fluctuation
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| APBS | Adaptive Poisson–Boltzmann Solver |
| BLAST | Basic Local Alignment Search Tools |
| CLN | Colchicine |
| GDP | Guanosine Diphosphate |
| GTP | Guanosine Triphosphate |
| MD | Molecular Dynamics |
| MM/PBSA | Molecular Mechanics Poisson–Boltzmann Surface Area |
| MM/GBSA | Molecular Mechanics Generalized-Born Surface Area |
| MT | Microtubule |
| RMSD | Root-Mean-Square Deviation |
| SASA | Solvent Accessible Surface Area |
References
- Hyams, J.S.; Lloyd, C.W. Microtubules; Wiley-Liss: New York, NY, USA, 1994. [Google Scholar]
- Mitchison, T.; Kirschner, M. Dynamic instability of microtubule growth. Nature 1984, 312, 237–242. [Google Scholar] [CrossRef]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Wallace, S.L. Colchicine. Colchicine 1974, 3, 369–381. [Google Scholar] [CrossRef]
- Weisenberg, R.C.; Borisy, G.G.; Taylor, E.W. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry 1968, 7, 4466–4479. [Google Scholar] [CrossRef] [PubMed]
- Ravelli, R.B.; Gigant, B.; Curmi, P.A.; Jourdain, I.; Lachkar, S.; Sobel, A.; Knossow, M. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature 2004, 428, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Luis, L.; Serrano, M.L.; Hidalgo, M.; Mendoza-Leon, A. Comparative analyses of the β-tubulin gene and molecular modeling reveal molecular insight into the colchicine resistance in kinetoplastids organisms. BioMed Res. Int. 2013, 2013, 843748. [Google Scholar] [CrossRef] [PubMed]
- Mane, J.Y.; Klobukowski, M.; Huzil, J.T.; Tuszynski, J. Free energy calculations on the binding of colchicine and its derivatives with the α/β-tubulin isoforms. J. Chem. Inf. Model. 2008, 48, 1824–1832. [Google Scholar] [CrossRef] [PubMed]
- Gigant, B.; Cormier, A.; Dorleans, A.; Ravelli, R.B.; Knossow, M. Microtubule-destabilizing agents: Structural and mechanistic insights from the interaction of colchicine and vinblastine with tubulin. Top. Curr. Chem. 2009, 286, 259–278. [Google Scholar] [PubMed]
- Tuszynski, J.A.; Craddock, T.J.; Mane, J.Y.; Barakat, K.; Tseng, C.Y.; Gajewski, M.; Winter, P.; Alisaraie, L.; Patterson, J.; Carpenter, E.; et al. Modeling the yew tree tubulin and a comparison of its interaction with paclitaxel to human tubulin. Pharm. Res. 2012, 29, 3007–3021. [Google Scholar] [CrossRef] [PubMed]
- MOE-Molecular_Operating_Environment. Available online: https://www.chemcomp.com/announcements/2013-10-23-MOE2013.08.pdf (accessed on 23 August 2013).
- Matasci, N.; Hung, L.H.; Yan, Z.; Carpenter, E.J.; Wickett, N.J.; Mirarab, S.; Nguyen, N.; Warnow, T.; Ayyampalayam, S.; Barker, M.; et al. Data access for the 1000 plants (1kp) project. GigaScience 2014, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped blast and psi-blast: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal w and clustal x version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.; Li, H.; Downing, K.H.; Nogales, E. Refined structure of α β-tubulin at 3.5 a resolution. J. Mol. Biol. 2001, 313, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Krebs, A.; Goldie, K.N.; Hoenger, A. Structural rearrangements in tubulin following microtubule formation. EMBO Rep. 2005, 6, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Kollman, P.A.; Massova, I.; Reyes, C.; Kuhn, B.; Huo, S.; Chong, L.; Lee, M.; Lee, T.; Duan, Y.; Wang, W.; et al. Calculating structures and free energies of complex molecules: Combining molecular mechanics and continuum models. Acc. Chem. Res. 2000, 33, 889–897. [Google Scholar] [CrossRef] [PubMed]
- Genheden, S.; Ryde, U. The MM/PBSA and MM/GBSA methods to estimate ligand-binding affinities. Expert Opin. Drug Discov. 2015, 10, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, B.; Panda, D.; Gupta, S.; Banerjee, M. Anti-mitotic activity of colchicine and the structural basis for its interaction with tubulin. Med. Res. Rev. 2008, 28, 155–183. [Google Scholar] [CrossRef] [PubMed]
- Hastie, S.B. Interactions of colchicine with tubulin. Pharmacol. Ther. 1991, 51, 377–401. [Google Scholar] [CrossRef]
- Jolliffe, I.T.; Cadima, J. Principal component analysis: A review and recent developments. Philos. Trans. A Math. Phys. Eng. Sci. 2016, 374, 20150202. [Google Scholar] [CrossRef] [PubMed]
- UniProt, C. Update on activities at the universal protein resource (uniprot) in 2013. Nucleic Acids Res. 2013, 41, D43–D47. [Google Scholar]
- Nogales, E.; Wolf, S.G.; Downing, K.H. Structure of the α β tubulin dimer by electron crystallography. Nature 1998, 391, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Pearlman, D.A.; Case, D.A.; Caldwell, J.W.; Ross, W.S.; Cheatham, T.E.; DeBolt, S.; Ferguson, D.; Seibel, G.; Kollman, P. Amber, a package of computer programs for applying molecular mechanics, normal mode analysis, molecular dynamics and free energy calculations to simulate the structural and energetic properties of molecules. Comput. Phys. Commun. 1995, 91, 1–41. [Google Scholar] [CrossRef]
- Sondergaard, C.R.; Olsson, M.H.; Rostkowski, M.; Jensen, J.H. Improved treatment of ligands and coupling effects in empirical calculation and rationalization of pka values. J. Chem. Theory Comput. 2011, 7, 2284–2295. [Google Scholar] [CrossRef] [PubMed]
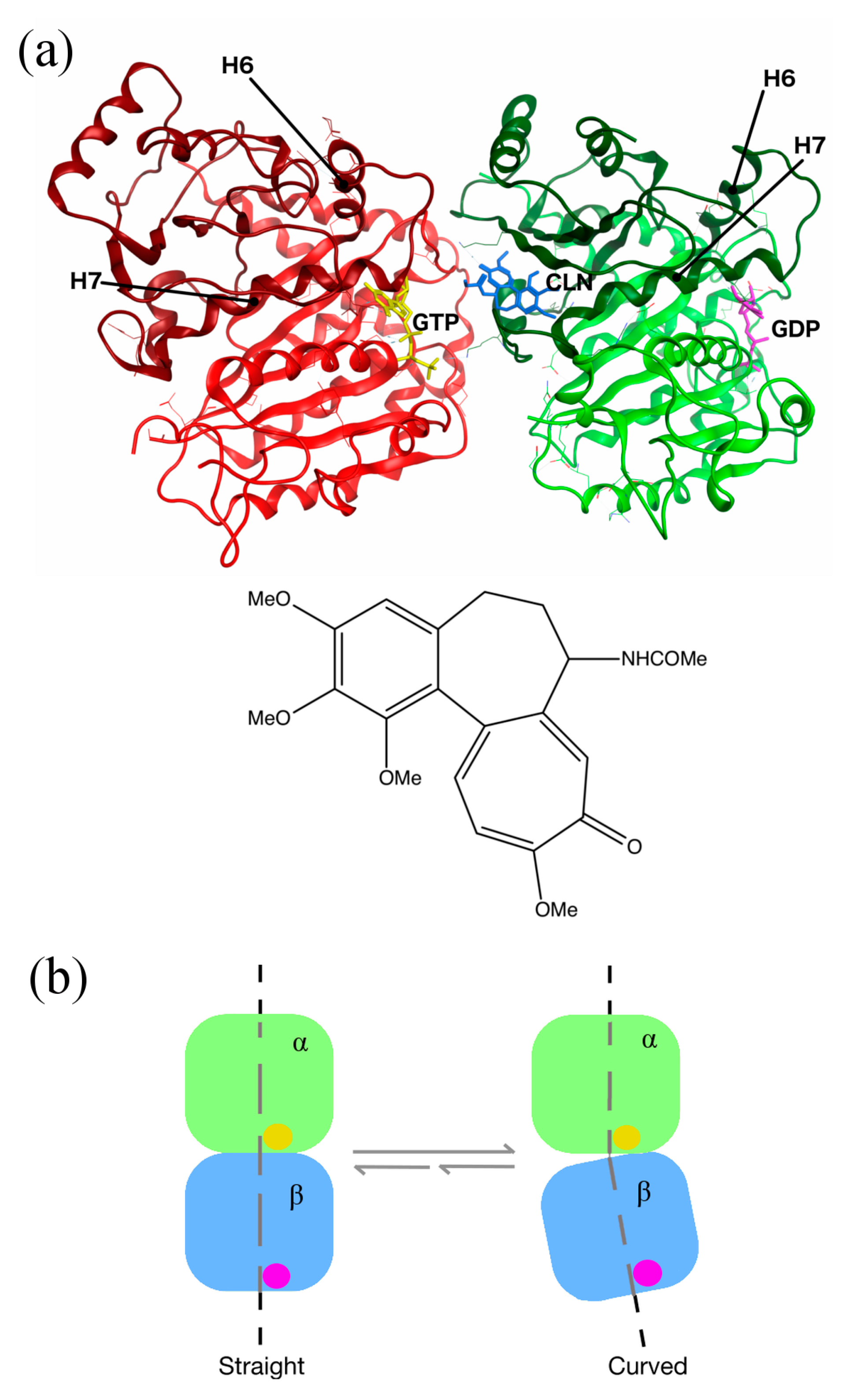
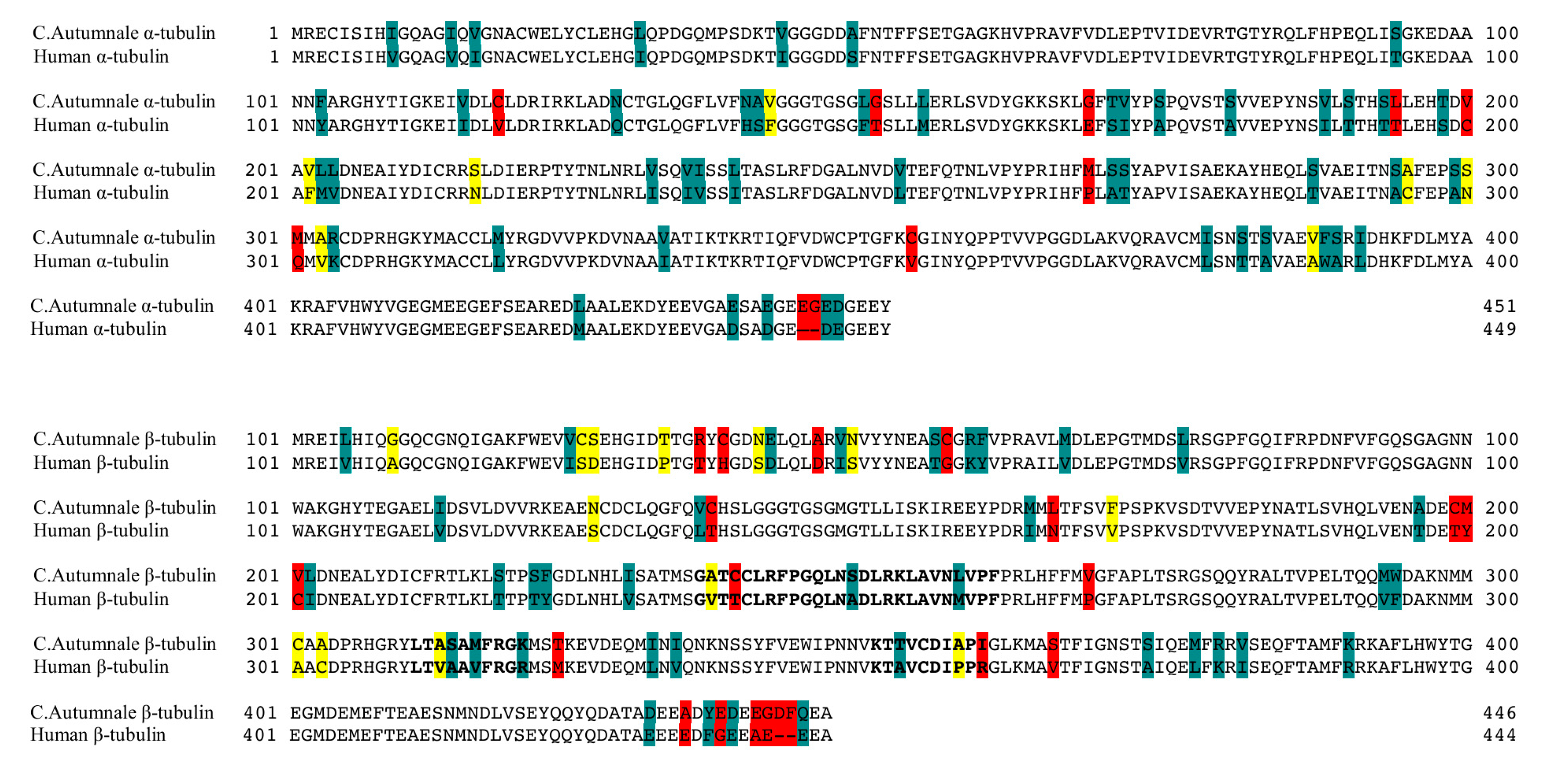
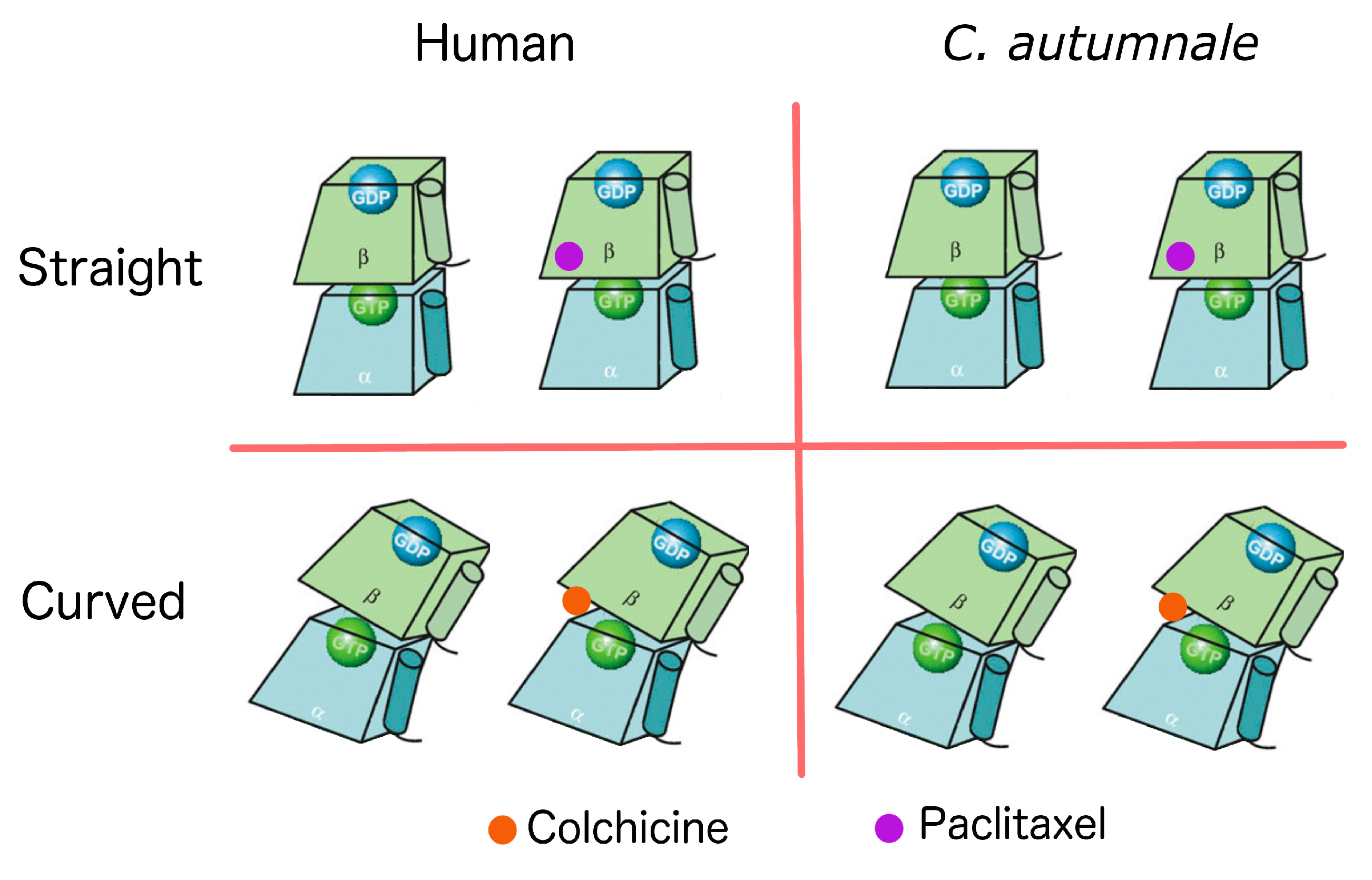
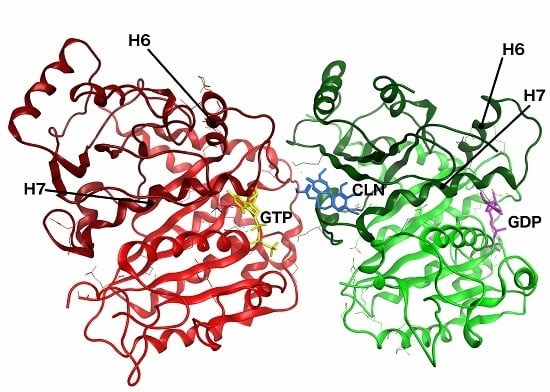
| Species | Ligand | Generalized Born ΔG (kcal/mol) | Poisson–Boltzman ΔG (kcal/mol) |
|---|---|---|---|
| Homo Sapiens | Paclitaxel | −23.25 | −43.30 |
| C. autumnale | Paclitaxel | −20.78 | −44.81 |
| Homo Sapiens | Colchicine | −51.79 | −76.68 |
| C .autumnale | Colchicine | −43.44 | −64.71 |
| Ligand | Species | Residue | ΔE vdw | ΔE elec | ΔE Polar | ΔE Non p | ΔG Total |
|---|---|---|---|---|---|---|---|
| Paclitaxel | Homo Sapiens | βLeu215 | −2.90 | 0.48 | 0.43 | −0.36 | −2.36 |
| βThr274 | −0.48 | −2.91 | 2.07 | −0.05 | −1.37 | ||
| βSer275 | −1.69 | −2.00 | 1.77 | −0.14 | −2.07 | ||
| βArg276 | −2.69 | −4.00 | 4.75 | −0.43 | −2.28 | ||
| PTX | −18.28 | −15.35 | 22.19 | 0.19 | −14.40 | ||
| Paclitaxel | C. autumnale | βLeu272 | −2.10 | 0.19 | 0.72 | −0.28 | −1.84 |
| βThr274 | −1.02 | 0.53 | 0.11 | −0.11 | −0.49 | ||
| βIle358 | −2.28 | −1.22 | 2.22 | −0.50 | −1.78 | ||
| βLeu360 | −1.65 | −0.24 | 0.46 | −0.29 | −1.72 | ||
| PTX | −18.23 | −15.11 | 22.93 | −3.36 | −12.77 | ||
| Colchicine | Homo Sapiens | αAla180 | −1.53 | −2.19 | 0.74 | −0.14 | −3.13 |
| αVal181 | −0.89 | −2.52 | 1.00 | −0.055 | −2.46 | ||
| βCys239 | −0.80 | −0.76 | 0.52 | −0.10 | −1.12 | ||
| βLeu246 | −2.71 | −1.08 | 1.00 | −0.28 | −3.07 | ||
| βAla248 | −1.77 | −0.26 | 0.48 | −0.13 | −1.67 | ||
| βLeu253 | −2.68 | −0.30 | −0.089 | −0.28 | −3.36 | ||
| βAla314 | −1.24 | −0.32 | −0.080 | −0.18 | −1.82 | ||
| βLys350 | −2.24 | −0.25 | 0.89 | 0.17 | −1.77 | ||
| CLN | −32.12 | −14.78 | 23.65 | −4.96 | −28.22 | ||
| Colchicine | C. autumnale | αSer180 | −1.43 | −1.53 | 0.43 | −0.19 | −2.72 |
| αVal181 | −1.19 | −2.35 | 0.93 | −0.058 | −2.66 | ||
| βLeu246 | −2.58 | −0.55 | 0.75 | −0.52 | −2.90 | ||
| βLeu253 | −2.25 | −0.57 | 0.50 | −0.22 | −2.57 | ||
| βLeu257 | −2.13 | −0.21 | 0.20 | −0.19 | −2.33 | ||
| βLys350 | −2.35 | −2.20 | 1.46 | −0.30 | −3.38 | ||
| CLN | −27.14 | −12.85 | 20.86 | −4.42 | −23.55 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spasevska, I.; Ayoub, A.T.; Winter, P.; Preto, J.; Wong, G.K.-S.; Dumontet, C.; Tuszynski, J.A. Modeling the Colchicum autumnale Tubulin and a Comparison of Its Interaction with Colchicine to Human Tubulin. Int. J. Mol. Sci. 2017, 18, 1676. https://doi.org/10.3390/ijms18081676
Spasevska I, Ayoub AT, Winter P, Preto J, Wong GK-S, Dumontet C, Tuszynski JA. Modeling the Colchicum autumnale Tubulin and a Comparison of Its Interaction with Colchicine to Human Tubulin. International Journal of Molecular Sciences. 2017; 18(8):1676. https://doi.org/10.3390/ijms18081676
Chicago/Turabian StyleSpasevska, Ivana, Ahmed T. Ayoub, Philip Winter, Jordane Preto, Gane K.-S. Wong, Charles Dumontet, and Jack A. Tuszynski. 2017. "Modeling the Colchicum autumnale Tubulin and a Comparison of Its Interaction with Colchicine to Human Tubulin" International Journal of Molecular Sciences 18, no. 8: 1676. https://doi.org/10.3390/ijms18081676