Plant Lectins Targeting O-Glycans at the Cell Surface as Tools for Cancer Diagnosis, Prognosis and Therapy
Abstract
:1. Introduction
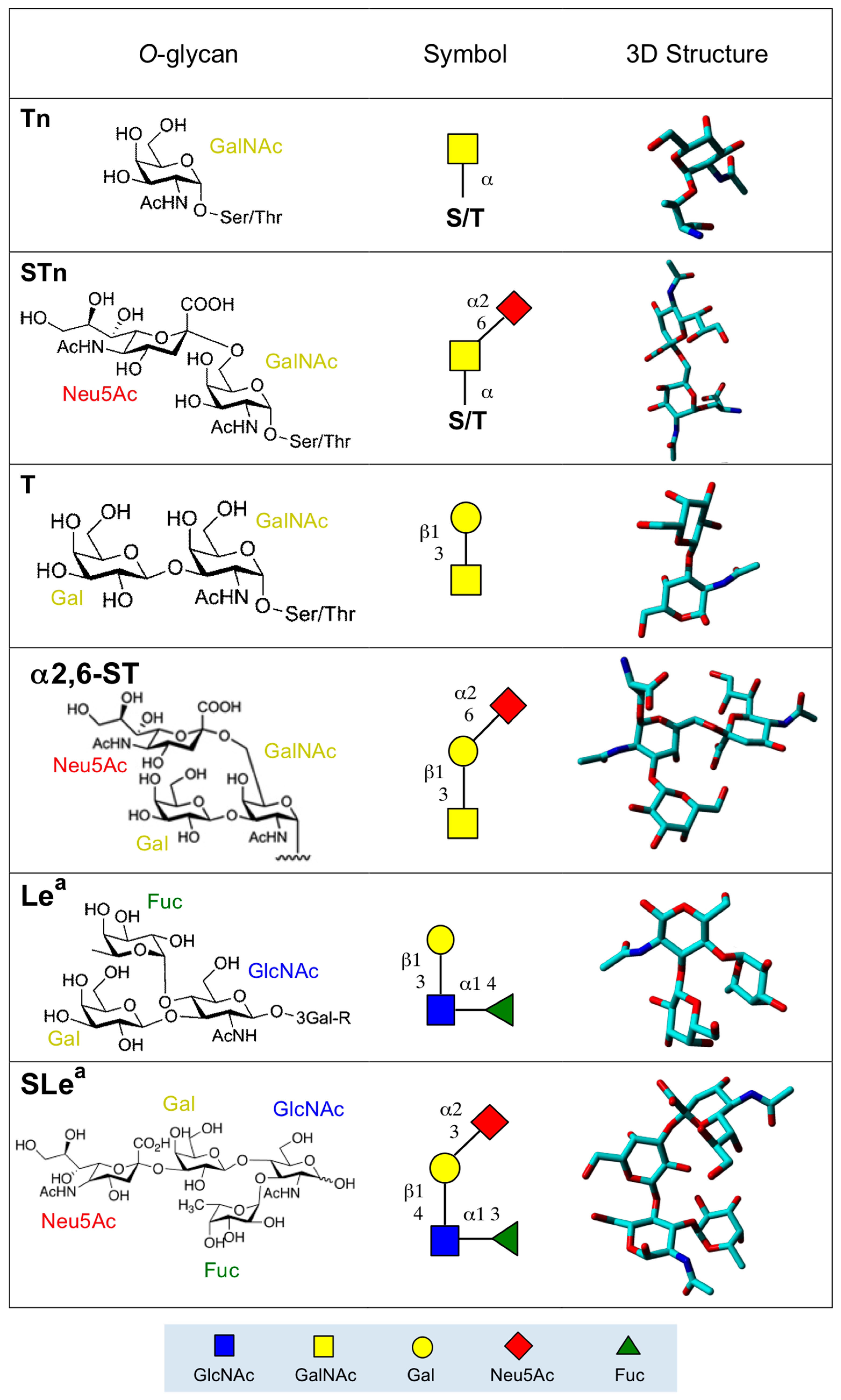
2. Altered O-Glycan Patterns Expressed by Cancer Cells
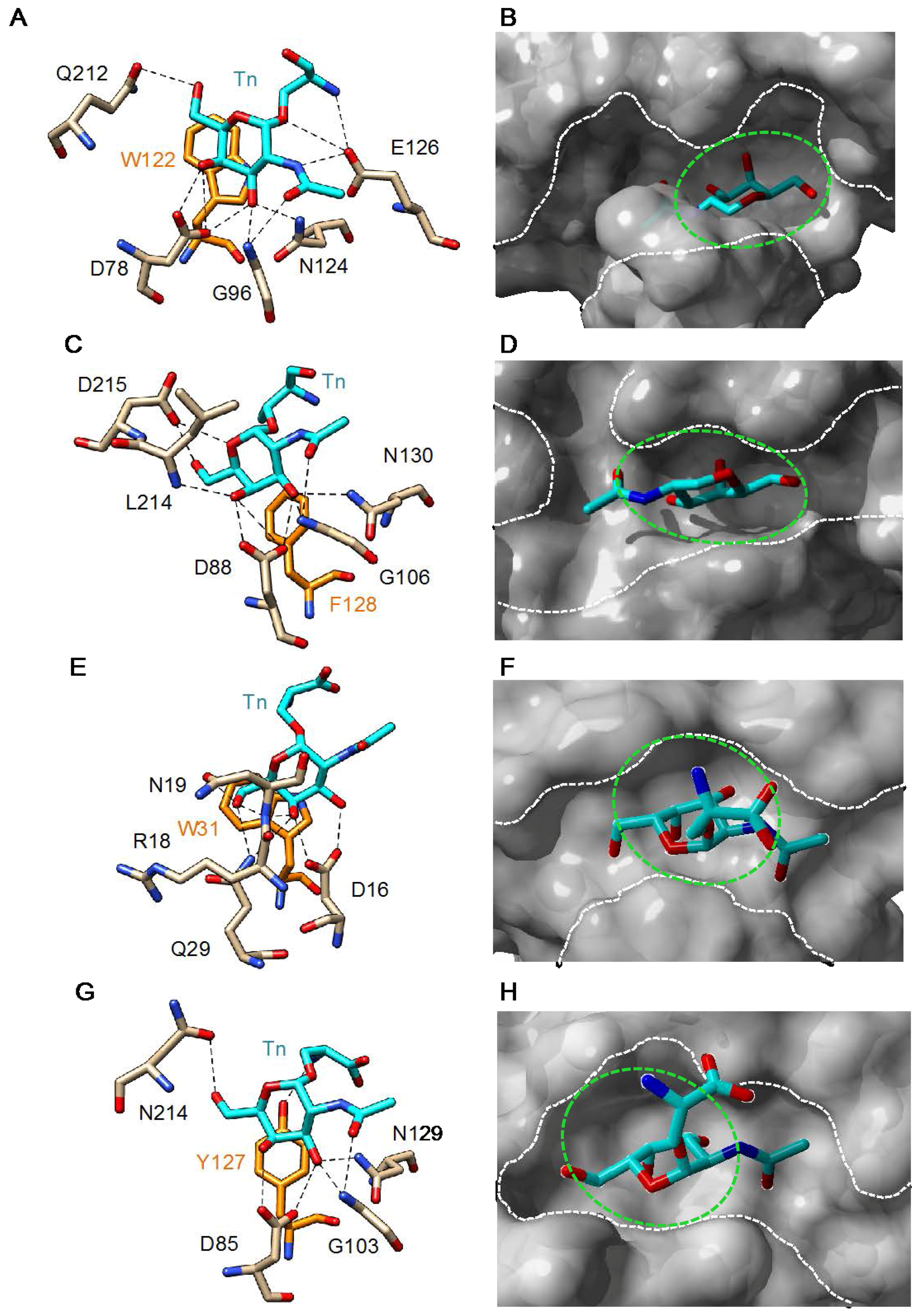
3. Plant Lectins Specific for T and Tn Antigens
4. Tn/T-Specific Lectins for Cancer Diagnosis/Prognosis
5. Toxic Effects of Tn/T-Specific Lectins on Cancer Cells
6. Tn/T-Specific Lectins as Targeting Aids for the Photodynamic Treatment of Tumors
7. Conclusions
Author Contributions
Conflicts of Interest
References
- Hakomori, S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv. Cancer Res. 1989, 52, 257–331. [Google Scholar] [PubMed]
- Cazet, A.; Julien, S.; Bobowski, M.; Burchell, J.; Delannoy, P. Tumor-associated carbohydrate antigens in breast cancer. Breast Cancer Res. 2010, 12, 204. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Ju, T.; Cummings, R.D. Protein glycosylation in cancer. Annu. Rev. Pathol. 2015, 10, 473–510. [Google Scholar] [CrossRef] [PubMed]
- Springer, G.F.; Desai, P.R. Tn epitopes, immunoreactive with ordinary anti-Tn antibodies, on normal, desialylated human erythrocytes and on Thomsen-Friedenreich antigen isolated therefrom. Mol. Immunol. 1985, 22, 1303–1310. [Google Scholar] [CrossRef]
- Itzkowitz, S.; Bloom, E.; Lau, T.; Kim, Y. Mucin associated Tn and sialosyl-Tn antigen expression in colorectal polyps. Gut 1992, 33, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Springer, G.F.; Desai, P.R.; Ghazizadeh, M.; Tegtmeyer, H. T/Tn pancarcinoma autoantigens: Fundamental, diagnostic, and prognostic aspects. Cancer Detect. Prev. 1995, 19, 173–182. [Google Scholar] [PubMed]
- Terasawa, K.; Furumoto, H.; Kamada, M.; Aono, T. Expression of Tn and sialyl-Tn antigens in the neoplastic transformation of uterine cervical epithelial cells. Cancer Res. 1996, 56, 2229–2232. [Google Scholar] [PubMed]
- Cao, Y.; Stosiek, P.; Springer, G.F.; Karsten, U. Thomsen-Friedenreich-related carbohydrate antigens in normal adult human tissue: A systematic and comparative study. Histochem. Cell Biol. 1996, 106, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Hanisch, F.G.; Baldusn, S.E. The Thomsen-Friedenreich (TF) antigen: A critical review on the structural, biosynthetic and histochemical aspects of a pancarcinoma-associated antigen. Histol. Histopathol. 1997, 12, 263–281. [Google Scholar] [PubMed]
- Cao, Y.; Merling, A.; Karsten, U.; Goletz, S.; Punzel, M.; Kraft, R.; Butschak, G.; Schwartz-Albiez, R. Expression of CD175 (Tn), CD175s (sialosyl-Tn) and CD176 (Thomsen-Friedenreich antigen) on malignant human hematopoietic cells. Int. J. Cancer 2008, 123, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.M.; Karsten, U.; Goletz, S.; Cheng, R.C.; Cao, Y. Co-expression of CD173 (H2) and CD174 (Lewis Y) with CD44 suggests that fucosylated histo-blood group antigens are markers of breast cancer-initiating cells. Virchows Arch. 2010, 456, 403–409. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.M.; Karsten, U.; Goletz, S.; Cheng, R.C.; Cao, Y. Expression of CD176 (Thomsen-Friedenreich antigen) on lung, breast and liver cancer-initiating cells. Int. J. Exp. Pathol. 2011, 92, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.M. Usefulness of novel tumour markers. Ann. Oncol. 1999, 10, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Van Elssen, C.H.; Frings, P.W.; Bot, F.J.; van de Vijver, K.K.; Huls, M.B.; Meek, B.; Hupperets, P.; Germeraad, W.T.; Bos, G.M. Expression of aberrantly glycosylated mucin-1 in ovarian cancer. Histopathology 2010, 57, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Fuster, M.M.; Esko, J.D. The sweet and sour of cancer: Glycans as novel therapeutic targets. Nat. Rev. Cancer 2005, 5, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Gemeiner, P.; Mislovicová, D.; Tkác, J.; Svitel, J.; Pätoprstý, V.; Hrabárová, E.; Kogan, G.; Kozár, T. Lectinomics II. A highway to biomedical/clinical diagnostics. Biotechnol. Adv. 2009, 27, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Glavey, S.V.; Huynh, D.; Reagan, M.R.; Manier, S.; Moschetta, M.; Kawano, Y.; Roccaro, A.M.; Ghobrial, I.M.; Joshi, L.; O’Dwyer, M.E. The cancer glycome: Carbohydrates as mediators of metastasis. Blood Rev. 2015, 29, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Feizi, T. Demonstration by monoclonal antibodies that carbohydrate structures of glycoproteins and glycolipids are onco-developmental antigens. Nature 1985, 314, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Roxby, D.J.; Morley, A.A.; Burpee, M. Detection of the Tn antigen in leukemia using monoclonal anti-Tn antibody and Immunohistochemistry. Br. J. Haematol. 1987, 67, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Springer, G.F.; Chendrasekaran, E.V.; Desai, P.R.; Tegtmeyer, H. Blood Tn-active macromolecules from human carcinomas and erythrocytes: Characterization of and specific reactivity with mono- and poly-clonal anti-Tn antibodies induced by various immunogens. Carbohydr. Res. 1988, 178, 271–292. [Google Scholar] [CrossRef]
- Kjeldsen, T.; Clausen, H.; Hirohashi, S.; Ogawa, T.; Iijima, H.; Hakomori, S. Preparation and characterization of monoclonal antibodies directed to the tumor-associated O-linked sialosyl-2→6α-N-acetylgalactosaminyl (sialosyl-Tn) epitope. Cancer Res. 1988, 48, 2214–2220. [Google Scholar] [PubMed]
- Siddiki, B.; Ho, J.J.; Huang, J.; Byrd, J.C.; Lau, E.; Yuan, M.; Kim, Y.S. Monoclonal antibody directed against colon cancer mucin has high specificity for malignancy. Int. J. Cancer 1993, 54, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Rougé, P.; Peumans, W.J.; van Damme, E.J.M.; Barre, A.; Singh, T.; Wu, J.H.; Wu, A.M. Glycotope structures and intramolecular affinity factors of plant lectins for Tn/T antigens. Adv. Exp. Med. Biol. 2011, 705, 143–154. [Google Scholar] [PubMed]
- Kobayashi, Y.; Masuda, K.; Banno, K.; Kobayashi, N.; Umene, K.; Nogami, Y.; Tsuji, K.; Ueki, A.; Nomura, H.; Sato, K.; et al. Glycan profiling of gestational choriocarcinoma using a lectin microarray. Oncol. Rep. 2014, 31, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Bandyopadhyay, D. MUC1: A target molecule for cancer therapy. Cancer Biol. Ther. 2007, 6, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.M.; Singh, T.; Liu, J.H.; André, S.; Lensch, M.; Siebert, H.C.; Krzeminski, M.; Bonvin, A.M.J.J.; Kaltner, H.; Wu, J.H.; et al. Adhesion/growth-relgulatory galectins: Insights into their ligand selectivity using natural glycoproteins and glycotopes. Adv. Exp. Med. Biol. 2011, 705, 117–141. [Google Scholar] [PubMed]
- Singh, R.; Subramanian, S.; Rhodes, J.M.; Campbell, B.J. Peanut lectin stimulates proliferation of colon cancer cells by interaction with glycosylated CD44v6 isoforms and consequential activation of c-Met and MAPK: Functional implications for disease-associated glycosylation changes. Glycobiology 2006, 16, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Peumans, W.J.; Hao, Q.; van Damme, E.J.M. Ribosome-inactivating proteins from plants: More than RNA N-glycosidases? FASEB J. 2001, 15, 1493–1506. [Google Scholar] [CrossRef] [PubMed]
- Kumar, G.S.; Appukuttan, P.S.; Basu, D. α-d-Galactose-specific lectin from jack fruit (Artocarpus integra) seed. J. Biosci. 1982, 4, 257–261. [Google Scholar] [CrossRef]
- Ahmed, H.; Chatterjee, B.P. Further characterization and immunochemical studies on the carbohydrate specificity of jackfruit (Artocarpus integrifolia) lectin. J. Biol. Chem. 1989, 264, 9365–9372. [Google Scholar] [PubMed]
- Jeyaprakash, A.A.; Katiyar, S.; Swaminathan, C.P.; Sekar, K.; Surolia, A.; Vijayan, M. Structural basis of the carbohydrate specificitiesof jacalin: An X-ray and modeling study. J. Mol. Biol. 2003, 332, 217–228. [Google Scholar] [CrossRef]
- Wu, A.M.; Shen, F.; Herp, A.; Wu, J.H. Interaction of hamster submaxillary sialyl-Tn and Tn glycoproteins with Gal, GalNAc and GlcNAc specific lectins. Mol. Immunol. 1994, 31, 485–490. [Google Scholar] [CrossRef]
- Irazoqui, F.J.; Vides, M.A.; Nores, G.A. Structural requirements of carbohydrates to bind Agaricus bisporus lectin. Glycobiology 1999, 9, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Carrizo, M.E.; Capaldi, S.; Perduca, M.; Irazoqui, F.J.; Nores, G.A.; Monaco, H.L. The antineoplastic lectin of the common edible mushroom (Agaricus bisporus) has two binding sites, each specific for a different configuration at a single epimeric hydroxyl. J. Biol. Chem. 2005, 280, 10614–10623. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Sun, H.; Tong, X.; Qi, Y. An antitumour lectin from edible mushroom Agrocybe aegerita. Biochem. J. 2003, 374, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Cammue, B.; Stinissen, H.M.; Peumans, W.J. A new type of cereal lectin from leaves of couch grass (Agropyrum repens). Eur. J. Biochem. 1985, 148, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Transue, T.R.; Smith, A.K.; Mo, H.; Goldstein, I.J.; Saper, M.A. Structure of benzyl T-antigen disaccharide bound to Amaranthus caudatus agglutinin. Nat. Struct. Biol. 1997, 4, 779–783. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.M.; Wu, J.H.; Yang, Z.; Singh, T.; Goldstein, I.J.; Sharon, N. Differential contributions of recognition factors of two plant lectins, Amaranthus caudatus lectin and Arachis hypogaea agglutinin, reacting with Thomsen-Friedenreich disaccharide (Galβ1–3GalNAcα1-Ser/Thr). Biochimie 2008, 90, 1769–1780. [Google Scholar] [CrossRef] [PubMed]
- Zenteno, E.; Lascurain, R.; Montaño, L.F.; Vazquez, L.; Debray, H.; Montreuil, J. Specificity of Amaranthus leucocarpus lectin. Glycoconj. J. 1992, 9, 204–208. [Google Scholar] [CrossRef] [PubMed]
- Lotan, R.; Skutelsky, E.; Danon, D.; Sharon, N. The purification, composition, and specificity of the anti-T lectin from peanut (Arachis hypogaea). J. Biol. Chem. 1975, 250, 8518–8523. [Google Scholar] [PubMed]
- Ravishankar, R.; Ravindran, M.; Suguna, K.; Surolia, A.; Vijayan, M. The specificity of peanut agglutinin for Thomsen-Friedenreich antigen is mediated by water-bridges. Curr. Sci. 1997, 72, 855–861. [Google Scholar]
- Moreira, R.A.; Castelo-Branco, C.C.; Monteiro, A.C.; Tavares, R.O.; Beltramini, L.M. Isolation and partial characterization of a lectin from Artocarpus incisa L. seeds. Phytochemistry 1998, 47, 1183–1188. [Google Scholar] [CrossRef]
- Tachibana, K.; Nakamura, S.; Wang, H.; Iwasaki, H.; Tachibana, K.; Maebara, K.; Cheng, L.; Hirabayashi, J.; Narimatsu, H. Elucidation of binding specificity of jacalin toward O-glycosylated peptides: Quantitative analysis by frontal affinity chromatography. Glycobiology 2006, 16, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Muthusamy, A.; Abdul-Rahman, P.S.; Bhavanandan, V.P.; Hashim, O.H. An improved lectin-based method for the detection of mucin-type O-glycans in biological samples. Analyst 2013, 138, 3522–3529. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Chatterjee, U.; Wu, J.H.; Chatterjee, B.P.; Wu, A.M. Carbohydrate recognition factors of a Tα (Galβ1→3GalNAcα1→Ser/Thr) and Tn (GalNAcα1→Ser/Thr) specific lectin isolated from the seeds of Artocarpus lakoocha. Glycobiology 2005, 15, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Lubkowski, J.; Durbin, S.V.C.; Silva, M.C.; Farnsworth, D.; Gildersleeve, J.C.; Oliva, M.L.; Wlodawer, A. Structural analysis and unique molecular recognition properties of a Bauhinia forficata lectin that inhibits cancer cell growth. FEBS J. 2017, 284, 429–450. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.M.; Wu, J.H.; Liu, J.H.; Singh, T. Recognition profile of Bauhinia purpurea agglutinin (BPA). Life Sci. 2004, 74, 1763–1779. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.M.; Wu, J.H.; Chen, Y.Y.; Tsai, M.S.; Herp, A. Forssman pentasaccharide and polyvalent Galβ1→4GlcNAc as major ligands with affinity for Caragana arborescens agglutinin. FEBS Lett. 1999, 463, 225–230. [Google Scholar] [CrossRef]
- Wu, A.M.; Song, S.C.; Chang, S.C.; Wu, J.H.; Chang, K.S.; Kabat, E.A. Further characterization of the binding properties of a GalNAc specific lectin from Codium fragile subspecies tomentosoides. Glycobiology 1997, 7, 1061–1066. [Google Scholar] [CrossRef] [PubMed]
- Piller, V.; Piller, F.; Cartron, J.P. Comparison of the carbohydrate-binding specificities of seven N-acetyl-d-galactosamine-recognizing lectins. Eur. J. Biochem. 1990, 191, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Wu, J.H.; Peumans, W.J.; Rougé, P.; van Damme, E.J.M.; Alvarez, R.A.; Blixt, O.; Wu, A.M. Carbohydrate specificity of an insecticidal lectin isolated from the leaves of Glechoma hederacea (ground ivy) towards mammalian glycoconjugates. Biochem. J. 2006, 393, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Sletmoen, M.; Dam, T.K.; Gerken, T.A.; Stokke, B.T.; Brewer, C.F. Single-molecule pair studies of the interaction of the α-GalNAc (Tn-antigen) form of porcine submaxillary mucin with soybean agglutinin. Biopolymers 2009, 91, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.M.; Wu, J.H.; Song, S.C.; Kabat, E.A. Bandeiraea (Griffonia) simplicifolia lectin I, isolectin A4, reacting with (GalNAcα1→Ser/Thr) ou galabiose (Galα1→4Gal) containing ligands. FEBS Lett. 1996, 398, 183–186. [Google Scholar] [PubMed]
- Chen, Y.F.; Boland, C.R.; Kraus, E.R.; Goldstein, I.J. The lectin Griffonia simplicifolia I-A4 (GS I-A4) specifically recognizes terminal α-linked N-acetylgalactosaminyl groups and is cytotoxic to the human colon cancer cell lines LS174t and SW1116. Int. J. Cancer 1994, 57, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Guillot, J.; Giollant, M.; Damez, M.; Dusser, M. Isolation and characterization of a lectin from the mushroom, Lactarius deliciosus. J. Biochem. 1991, 109, 840–845. [Google Scholar] [CrossRef] [PubMed]
- Giollant, M.; Guillot, J.; Damez, M.; Dusser, M.; Didier, P.; Didier, E. Characterization of a lectin from Lactarius deterrimus. Research on the possible involvement of the fungal lectin in recognition between mushroom and spruce during the early stages of mycorrhizae formation. Plant Physiol. 1993, 101, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Zenteno, R.; Chávez, R.; Portugal, D.; Páez, A.; Lascurain, R.; Zenteno, E. Purification of a N-acetyl-d-galactosamine specific lectin from the orchid Laelia autumnalis. Phytochemistry 1995, 40, 651–655. [Google Scholar] [CrossRef]
- Thompson, A.; Zhang, Z.; Ton-that, H.; Biesterfeldt, J.; Ogata, C.; Xu, L.; Johnston, R.A.; Young, N.M. Structure of the complex of Maclura pomifera agglutinin and the T-antigen disaccharide, Galβ1,3GalNAc. J. Biol. Chem. 1998, 273, 6312–6318. [Google Scholar]
- Wu, A.M. Polyvalent GalNAcα1→Ser/Thr (Tn) and Galβ1→3GalNAcα1→Ser/Thr (Tα) as the most potent recognition factors involved in Maclura pomifera agglutinin-glycan interactions. J. Biomed. Sci. 2005, 12, 135–152. [Google Scholar] [CrossRef] [PubMed]
- Thurnher, M.; Clausen, H.; Sharon, N.; Berger, E.G. Use of O-glycosylation-defective human lymphoid cell lines and flow cytometry to delineate the specificity of Moluccella laevis lectin and monoclonal antibody 5F4 for the Tn antigen (GalNAcα1-O-Set/Thr). Immunol. Lett. 1993, 36, 239–243. [Google Scholar] [CrossRef]
- Teneberg, S.; Leonardsson, I.; Angström, J.; Ehrlich-Rogozinski, S.; Sharon, N. Characterization of the specificity of binding of Moluccella laevis lectin to glycosphingolipids. Glycoconj. J. 1994, 11, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Chandran, T.; Sharma, A.; Vijayan, M. Structural studies on a non-toxic homologue of type II RIPs from bitter gourd: Molecular basis of non-toxicity, conformational selection and glycan structure. J. Biosci. 2015, 40, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Wu, J.H.; Peumans, W.J.; Rougé, P.; van Damme, E.J.M.; Wu, A.M. Recognition profile of Morus nigra agglutinin (Morniga G) expressed by monomeric ligands, simple clusters and mammalian polyvalent glycotopes. Mol. Immunol. 2007, 44, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, A.; Berois, N.; Incerti, M.; Bay, S.; Franco Fraguas, L.; Osinaga, E. A Tn antigen binding lectin from Myrsine coriacea displays toxicity in human cancer cell lines. J. Nat. Med. 2013, 67, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, K.A.; Sinha, S.; Katiyar, S.; Surolia, A.; Vijayan, M.; Suguna, K. Structural basis for the specificity of basic winged bean lectin for the Tn-antigen: A crystallographic, thermodynamic and modelling study. FEBS Lett. 2005, 579, 6775–6780. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.M.; Wu, J.H.; Singh, T.; Hwang, P.Y.; Tsai, M.S.; Herp, A. Lectinochemical studies on the binding properties of a toxic lectin (ricin) isolated from the seeds of Ricinus communis. Chang Gung Med. J. 2005, 28, 530–542. [Google Scholar] [PubMed]
- Wu, A.M.; Wu, J.H.; Singh, T.; Lai, L.J.; Yang, Z.; Herp, A. Recognition factors of Ricinus communis agglutinin I (RCA). Mol. Immunol. 2006, 43, 1700–1715. [Google Scholar] [CrossRef] [PubMed]
- Vega, N.; Pérez, G. Isolation and characterization of a Salvia bogotensis seed lectin specific for the Tn antigen. Phytochemistry 2006, 67, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Piller, V.; Piller, F.; Cartron, J.P. Isolation and characterization of an N-acetylgalactosamine specific lectin from Salvia sclarea seeds. J. Biol. Chem. 1986, 261, 14069–14075. [Google Scholar] [PubMed]
- Medeiros, A.; Bianchi, S.; Calvete, J.J.; Balter, H.; Bay, S.; Robles, A.; Cantacuzène, D.; Nimtz, M.; Alzari, P.M.; Osinaga, E. Biochemical and functional characterization of the Tn-specific lectin from Salvia sclarea seeds. Eur. J. Biochem. 2000, 267, 1434–1440. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.M. Lectinochemical studies on the glyco-recognition factors of a Tn (GalNAcα1→Ser/Thr) specific lectin isolated from the seeds of Salvia sclarea. J. Biomed. Sci. 2005, 12, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Moore, B.P.L.; Marsh, S.; Laschinger, C.; Simpson, S. Mixed-field polyagglutinability due to Tn: A further example. Transfusion 1975, 15, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, E.J.M.; Barre, A.; Rougé, P.; van Leuven, F.; Peumans, W.J. The NeuAc(α-2,6)-Gal/GalNAc-binding lectin from elderberry (Sambucus nigra) bark, a type-2 ribosome-inactivating protein with an unusual specificity and structure. Eur. J. Biochem. 1996, 235, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Maveyraud, L.; Niwa, H.; Guillet, V.; Svergun, D.I.; Konarev, P.V.; Palmer, R.A.; Peumans, W.J.; Rougé, P.; van Damme, E.J.M.; Reynolds, C.D.; et al. Structural basis for sugar recognition, including the Tn carcinoma antigen, by the lectin SNA-II from Sambucus nigra. Proteins 2009, 75, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Peppa, V.I.; Venkat, H.; Kantsadi, A.L.; Inamdar, S.R.; Bhat, G.G.; Eligar, S.; Shivanand, A.; Chachadi, V.B.; Satisha, G.J.; Swamy, B.M.; et al. Molecular cloning, carbohydrate specificity and the crystal structure of two Sclerotium rolfsii lectin variants. Molecules 2015, 20, 10848–10865. [Google Scholar] [CrossRef] [PubMed]
- Yadav, P.; Shahane, G.; Ramasamy, S.; Sengupta, D.; Gaikwad, S. Structural-functional insights and studies on saccharide binding of Sophora japonica seed lectin. Int. J. Biol. Macromol. 2016, 91, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.M.; Wu, J.H.; Song, S.C.; Tsai, M.S.; Herp, A. Studies on the binding of wheat germ agglutinin (Triticum vulgaris) to O-glycans. FEBS Lett. 1998, 440, 315–319. [Google Scholar] [CrossRef]
- Natsuka, S.; Kawaguchi, M.; Wada, Y.; Ichikawa, A.; Ikura, K.; Hase, S. Characterization of wheat germ agglutinin ligand on soluble glycoproteins in Caenothabditis elegans. J. Biochem. 2005, 135, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Sousa, B.L.; Silva Filho, J.C.; Kumar, P.; Pereira, R.I.; Łyskowski, A.; Rocha, B.A.; Delatorre, P.; Bezerra, G.A.; Nagano, C.S.; Gruber, K.; et al. High-resolution strycture of a new Tn antigen-binding lectin from Vatairea macrocarpa and a comparative analysis of Tn-binding legume lectins. Int. J. Biochem. Cell Biol. 2015, 59, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Uchide, N.; Ohyama, K.; Yamakawa, T.; Ohkuma, S. Presence of Vicia graminea lectin- or Vicia unijuga lectin-binding (Vgu) glycoproteins, Vgu glycoproteins with Thomsen-Friedenreich (T) activity and T-reactive glycoproteins in human meconium. Int. J. Biochem. Cell Biol. 1995, 27, 319–327. [Google Scholar] [CrossRef]
- Tollefsen, S.; Kornfeld, R. The B4 lectin from Vicia villosa seeds Interacts with N-acetylgalactosamine residues α-linked to serine or threonine residues in cell surface glycoproteins. J. Biol. Chem. 1983, 258, 5172–5176. [Google Scholar] [PubMed]
- Wu, A.M.; Song, S.C.; Hwang, P.Y.; Wu, J.H.; Pfüller, U. Interaction of mistletoe toxic lectin-I with sialoglycoproteins. Biochem. Biophys. Res. Commun. 1995, 214, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Haji-Ghassemi, O.; Gilbert, M.; Spence, J.; Schur, M.J.; Parker, M.J.; Jenkins, M.L.; Burke, J.E.; van Faassen, H.; Young, N.M.; Evans, S.V. Molecular basis for recognition of cancer glycobiomarker, LacdiNAc (GalNAc(β1→4)GlcNAc), by Wisteria floribunda agglutinin. J. Biol. Chem. 2016, 291, 24085–24095. [Google Scholar] [CrossRef] [PubMed]
- Damian, L.; Fournier, D.; Winterhalter, M.; Paquereau, L. Determination of thermodynamic parameters of Xerocomus chrysenteron lectin interactions with N-acetylgalactosamine and Thomsen-Friedenreich antigen by isothermal titration calorimetry. BMC Biochem. 2005, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Adwan, H.; Bayer, H.; Pervaiz, A.; Sagini, M.; Berger, M.R. Riproximin is a recently discovered type II ribosome inactivating protein with potential for treating cancer. Biotechnol. Adv. 2014, 32, 1077–1090. [Google Scholar] [CrossRef] [PubMed]
- Osinaga, E.; Bay, S.; Tello, D.; Babino, A.; Pritsch, O.; Assemat, K.; Cantacuzene, D.; Nakada, H.; Alzari, P. Analysis of the fine specificity of Tn-binding proteins using synthetic glycopeptide epitopes and a biosensor based on surface plasmon resonance spectroscopy. FEBS Lett. 2000, 469, 24–28. [Google Scholar] [CrossRef]
- Madariaga, D.; Martínez-Sáez, N.; Somovilla, V.J.; García-García, L.; Berbis, M.Á.; Valero-Gónzalez, J.; Martín-Santamaria, S.; Hurtado-Guerrero, R.; Asension, J.L.; Jiménez-Barbero, J.; et al. Serine versus threonine glycosylation with α-O-GalNAc: Unexpected selectivity in their molecular recognition with lectins. Chemistry 2014, 20, 12616–12627. [Google Scholar] [CrossRef] [PubMed]
- Delbaere, L.T.; Vandonselaar, M.; Prasad, L.; Quail, J.W.; Wilson, K.S.; Dauter, Z. Structures of the lectin IV of Griffonia simplicifolia and its complex with the Lewis b human blood group determinant at 2.0 Å resolution. J. Mol. Biol. 1993, 230, 950–965. [Google Scholar] [CrossRef] [PubMed]
- Madariaga, D.; Martinez-Sáez, N.; Somovilla, V.J.; Coelho, H.; Valero-González, J.; Castro-López, J.; Asension, J.L.; Jiménez-Barbero, J.; Busto, J.H.; Avenoza, A.; et al. Detection of tumor-associated glycopeptides by lectins: The peptide context modulates carbohydrate recognition. ACS Chem. Biol. 2015, 10, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Babino, A.; Tello, D.; Rojas, A.; Bay, S.; Osinaga, E.; Alzari, P.M. The crystal structure of a plant lectin in complex with the Tn antigen. FEBS Lett. 2003, 536, 106–110. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Jeyaprakash, A.A.; Geetha Rani, P.; Banuprakash Reddy, G.; Banumathi, S.; Betzel, C.; Sekar, K.; Surolia, A.; Vijayan, M. Crystal structure of the jacalin-T-antigen complex and a comparative study of lectin-T-antigen complexes. J. Mol. Biol. 2002, 321, 637–645. [Google Scholar] [CrossRef]
- Nagae, M.; Nishi, N.; Nakamura-Tsuruta, S.; Hirabayashi, J.; Wakatsuki, S.; Kato, R. Structural analysis of the human galectin-9 N-terminal carbohydrate recognition domain reveals unexpected properties that differ from the mouse orthologue. J. Mol. Biol. 2007, 375, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Springer, G.F. T and Tn pancarcinoma markers: Autoantigenic adhesion molecules in pathogenesis, prebiopsy carcinoma-detection, and long-term breast carcinoma immunotherapy. Crit. Rev. Oncog. 1995, 6, 57–85. [Google Scholar] [CrossRef] [PubMed]
- Springer, G.F. Immunoreactive T and Tn epitopes in cancer diagnosis, prognosis, and immunotherapy. J. Mol. Med. 1997, 75, 594–602. [Google Scholar] [CrossRef] [PubMed]
- Desai, P.R. Immunoreactive T and Tn antigens in malignancy: Role in carcinoma diagnosis, prognosis, and immunotherapy. Transfus. Med. Rev. 2000, 14, 312–325. [Google Scholar] [CrossRef] [PubMed]
- Campo, E.; Condom, E.; Palacín, A.; Quesada, E.; Cardesa, A. Lectin binding patterns in normal and neoplastic colonic mucosa. Dis. Colon Rectum 1988, 31, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Calderó, J.; Campo, E.; Ascaso, C.; Ramos, J.; Panadés, M.J.; Reñé, J.M. Regional distribution of glycoconjugates in normal, transitional and neoplastic human colonic mucosa. A histochemical study using lectins. Virchows Arch. A Pathol. Anat. Histopathol. 1989, 415, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Boland, C.R.; Martin, M.A.; Goldstein, I.J. Lectin reactivities as intermediate biomarkers in premalignant colorectal epithelium. J. Cell Biochem. Suppl. 1992, 16G, 103–109. [Google Scholar] [CrossRef] [PubMed]
- Fucci, L.; Valentini, A.M.; Caruso, M.L. Can peanut agglutinin distinguish between pseudo and true invasion in coloic adenomas? Eur. J. Histochem. 1993, 37, 335–344. [Google Scholar] [PubMed]
- Mazumdar, S.; SenGupta, S.K.; Param, R.; Sinha, S.N. Binding pattern of eight different lectins in healthy subjects and patients with dysplastic and malignant lesions of the oral cavity. Int. J. Oral Maxillofac. Surg. 1993, 22, 301–305. [Google Scholar] [CrossRef]
- Brooks, S.A.; Leathem, A.J. Expression of N-acetyl galactosaminylated and sialylated glycans by metastases arising from primary breast cancer. Invasion Metastasis 1998, 18, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Springer, G.F. Tn epitope (N-acetyl-d-galactosamine-α-O-serine/threonine) density in primary breast carcinoma: A functional predictor of aggressiveness. Mol. Immunol. 1989, 26, 1–5. [Google Scholar] [CrossRef]
- Yu, L.G. The oncofetal Thomsen-Fiedenreich carbohydrate antigen in cancer progession. Glycoconj. J. 2007, 24, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Sobrinho-Simões, M.; Damjanov, I. Lectin histochemistry of papillary and follicular carcinoma of the thyroid gland. Arch. Pathol. Lab. Med. 1986, 110, 722–729. [Google Scholar] [PubMed]
- Fritz, P.; Dippon, J.; Kierschke, T.; Siegle, I.; Möhring, A.; Moisa, A.; Mürdter, T.E. Impact of mistletoe lectin binding in breast cancer. Anticancer Res. 2004, 24, 1187–1192. [Google Scholar] [PubMed]
- Santaella-Verdejo, A.; Gallegos, N.; Pérez-Campos, E.; Hernández, P.; Zenteno, E. Use of Amaranthus leucocarpus lectin to differentiate cervical dysplasia (CIN). Prep. Biochem. Biotechnol. 2007, 37, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Mo, C.; Peng, Q.; Kang, X.; Sun, C.; Jiang, K.; Huang, L.; Lu, Y.; Sui, J.; Qin, X.; et al. Cell surface glycan alterations in epithelial mesenchymal transition process of Huh7 hepatocellular carcinoma cell. PLoS ONE 2013, 8, e71273. [Google Scholar] [CrossRef] [PubMed]
- Feizi, T.; Fazio, F.; Chai, W.; Wong, C.H. Carbohydrate microarrays—A new set of technologies at the frontiers of glycomics. Curr. Opin. Struct. Biol. 2003, 13, 637–645. [Google Scholar] [CrossRef] [PubMed]
- Angeloni, S.; Ridet, J.L.; Kusy, N.; Gao, H.; Crevoisier, F.; Guinchard, S.; Kochhar, S.; Sigrist, H.; Sprenger, N. Glycoprofiling with micro-arrays of glycoconjugates and lectins. Glycobiology 2005, 15, 31–41. [Google Scholar] [CrossRef] [PubMed]
- Pilobello, K.T.; Krishnamoorthy, L.; Slawek, D.; Mahal, L.K. Development of a lectin microarray for the rapid analysis of protein glycopatterns. ChemBioChem 2005, 6, 985–989. [Google Scholar] [CrossRef] [PubMed]
- Kuno, A.; Uchiyama, N.; Koseki-Kuno, S.; Ebe, Y.; Takashima, S.; Yamada, M.; Hirabayashi, J. Evanescent-field fluorescence-assisted lectin microarray: A new strategy for glycan profiling. Nat. Methods 2005, 2, 851–856. [Google Scholar] [CrossRef] [PubMed]
- Manimala, J.C.; Li, Z.; Jain, A.; VedBrat, S.; Gildersleeve, J.C. Carbohydrate array analysis of anti-Tn antibodies and lectins reveals unexpected specificities: Implications for diagnostic and vaccine development. ChemBioChem 2005, 6, 2229–2241. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Patwa, T.H.; Lubman, D.M.; Simeone, M. Protein biomarkers in cancer: Natural glycoprotein microarray approaches. Curr. Opin. Mol. Ther. 2008, 10, 602–610. [Google Scholar] [PubMed]
- Syed, P.; Gidwani, K.; Kekki, H.; Leivo, J.; Pettersson, K.; Lamminmäki, U. Role of lectin microarrays in cancer diagnosis. Proteomics 2016, 16, 1257–1265. [Google Scholar] [CrossRef] [PubMed]
- Dan, X.; Liu, W.; Ng, T.B. Development and applications of lectins as biological tools in biomedical research. Med. Res. Rev. 2016, 36, 221–247. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K.; Inomata, M.; Iha, H.; Hiratsuka, T.; Etoh, T.; Shiraishi, N.; Kashima, K.; Kitano, S. Establishment of new predictive markers for distant reccurence of colorectal cancer using lectin microarray analysis. Cancer Med. 2015, 4, 293–302. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Patwa, T.H.; Xu, L.; Shedden, K.; Misek, D.E.; Tuck, M.; Jin, G.; Ruffin, M.T.; Turgeon, D.K.; Synal, S.; et al. Plasma glycoprotein profiling for colorectal cancer biomarker identification by lectin glycoarray and lectin blot. J. Proteome Res. 2008, 7, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Patwa, T.H.; Qiu, W.; Shedden, K.; Hinderer, R.; Misek, D.E.; Anderson, M.A.; Simeone, D.M.; Lubman, D.M. Glycoprotein microarrays with multi-lectin detection: Unique lectin binding patterns as a tool for classifying normal, chronic pancreatitis and pancreatic cancer sera. J. Proteome Res. 2007, 6, 1864–1874. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Harris, L.E.; Palmer-Toy, D.E.; Hancock, W.S. Multilectin affinity chromatography for characterization of multiple glycoprotein biomarker candidates in serum from breast cancer patients. Clin. Chem. 2006, 52, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Guan, F.; Li, D.; Tan, Z.; Yang, G.; Wu, Y.; Huang, Z. Identification of aberrantly expressed glycans in gastric cancer by integrated lectin microarray and mass spectrometric analyses. Oncotarget 2016, 52, 87284–87300. [Google Scholar] [CrossRef] [PubMed]
- Nishijima, Y.; Toyoda, M.; Yamazaki-Inoue, M.; Sugiyama, T.; Miyazawa, M.; Muramatsu, T.; Nakamura, K.; Narimatsu, H.; Umezawa, A.; Mikami, M. Glycan profiling of endometrial cancers using lectin microarray. Genes Cells 2012, 17, 826–836. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.L.; Li, Y.G.; Lv, Y.C.; Guan, X.H.; Ji, H.F.; Chi, B.R. Use of lectin microarray to differentiate gastric cancer from gastric ulcer. World J. Gastroenterol. 2014, 20, 5474–5482. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, A.; Kuno, A.; Nakagawa, T.; Ikehara, Y.; Irimura, T.; Yamamoto, M.; Nakanuma, Y.; Miyoshi, E.; Nakamori, S.; Nakanishi, H.; et al. Lectin microarray-based sero-biomarker verification targeting aberrant O-linked glycosylation on mucin 1. Anal. Chem. 2015, 87, 7274–7281. [Google Scholar] [CrossRef] [PubMed]
- Šunderić, M.; Šedivá, A.; Robajac, D.; Miljuš, G.; Gemeiner, P.; Nedicć, O.; Katrlík, J. Lectin-based protein microarray analysis of differences in serum α2-macroglobulin glycosylation between patients with colorectal cancer and persons without cancer. Biotechnol. Appl. Biochem. 2016, 63, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Hirao, Y.; Matsuzaki, H.; Iwaki, J.; Kuno, A.; Kaji, H.; Ohkura, T.; Togayachi, A.; Abe, M.; Nomura, M.; Noguchi, M.; et al. Glycoproteomics approach for identifying glycobiomarker canditate molecules for tissue type classification of non-small cell lung carcinoma. J. Proteome Res. 2014, 13, 4705–4716. [Google Scholar] [CrossRef] [PubMed]
- Roy, B.; Chattopadhyay, G.; Misrha, D.; Das, T.; Chakraborty, S.; Maiti, T.K. On-chip lectin microarray for glycoprofiling of different gastritis types and gastric cancer. Biomicrofluidics 2014, 8, 034107. [Google Scholar] [CrossRef] [PubMed]
- Fry, S.; Afrough, B.; Lomax-Browne, H.J.; Timms, J.F.; Velentzis, L.S.; Leathem, A.J.C. Lectin microarray profiling of metastatic breast cancer. Glycobiology 2011, 21, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Fry, S.; Afrough, B.; Leathem, A.; Dwek, M. Lectin array-based strategies for identifying metastasis-associated changes in glycosylation. Methods Mol. Biol. 2012, 878, 267–272. [Google Scholar] [PubMed]
- He, J.; Liu, Y.; Xie, X.; Zhu, T.; Soules, M.; DiMeco, F.; Vescovi, A.L.; Fan, X.; Lubman, D.M. Identification of cell surface glycoprotein markers for glioblastoma-derived stem-like cells using a lectin microarray and LC-MS/MS approach. J. Proteome Res. 2010, 9, 2565–2572. [Google Scholar] [CrossRef] [PubMed]
- Konska, G.; Guerry, M.; Caldefie-Chezet, F.; de Latour, M.; Guillot, J. Study of the expression of Tn antigen in different types of human breast cancer cells using VVA-B4 lectin. Oncol. Rep. 2006, 15, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, T.; Takazawa, H.; Imai, S.; Morimoto, J.; Watanabe, T.; Kanno, M.; Igarashi, S. Expression of Vicia villosa agglutinin (VVA)-binding glycoprotein in primary breast cancer in relation to lymphatic metastasis: Is atypical MUC1 bearing Tn antigen a receptor of VVA? Breast Cancer Res. Treat. 2006, 98, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Campbell, B.J.; Yu, L.G.; Fernig, D.G.; Milton, J.D.; Goodlad, R.A.; FitzGerald, A.J.; Rhodes, J.M. Cell surface-expressed Thomsen-Friedenreich antigen in colon cancer is predominantly carried on high molecular weight splice variants of CD44. Glycobiology 2001, 11, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Shio, Y.; Suzuki, H.; Kawaguchi, T.; Ohsugi, J.; Higuchi, M.; Fujiu, K.; Kanno, R.; Ohishi, A.; Gotoh, M. Carbohydrate status detecting by PNA is changeable through cancer prognosis from primary to metastatic nodal site: A possible prognostic factor in patient with node-positive lung adenocarcinoma. Lung Cancer 2007, 57, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Futsukaichi, T.; Etoh, T.; Nakajima, K.; Daa, T.; Shiroshita, H.; Shiraishi, N.; Kitano, S.; Inomata, M. Decreased expression of Bauhinia purpurea lectin is a predictor of gastric cancer recurrence. Surg. Today 2015, 45, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Kuno, A.; Ikehata, Y.; Katada, N.; Hirabayashi, J.; Narimatsu, H.; Watanabe, M. Lectin microarray technology identifies specific lectins related to lymp node metastasis of advanced gastric cancer. Gastric Cancer 2016, 19, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Chen, H.; Zhang, H.B.; Jin, Y.X.; Guo, H.Q.; Chen, X.G.; Sun, H. Lectin from Agrocybe aegerita as a glycophenotype probe for evaluation of progression and survival in colorectal cancer. Asian Pac. J. Cancer Prev. 2014, 15, 5601–5605. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.M.; Cheng, L.; Guo, S.J.; Wang, Y.; Czajkowsky, D.M.; Gao, H.; Hu, X.F.; Tao, S.C. Lectin RECA-I specifically binds to metastasis-associated cell surface glycan in triple-negative breast cancer. Breast Cancer Res. 2015, 17, 36. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Simeone, D.M.; Brenner, D.E.; Anderson, M.A.; Shedden, K.A.; Ruffin, M.T.; Lubman, D.M. Pancreatic cancer serum detection using a lectin/glyco-antibody array method. J. Proteome Res. 2009, 8, 483–492. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, R.; Yamasaki, K.; Abiru, S.; Komori, A.; Nagaoka, S.; Saeki, A.; Hashimoto, S.; Bekki, S.; Kugiyama, Y.; Kuno, A.; et al. Serum Wisteria floribunda agglutinin-positive Mac-2 binding protein values predict the development of hepatocellular carcinoma among patients with chronic hepatitis C after sustained virological response. PLoS ONE 2015, 10, e129053. [Google Scholar] [CrossRef] [PubMed]
- Lyu, S.Y.; Choi, S.H.; Park, W.B. Korean mistletoe lectin-induced apoptosis in hepatocarcinoma cells is associated with inhibition of telomerase via mitochondrial controlled pathway idependent of p53. Arch. Pharm. Res. 2002, 25, 93–101. [Google Scholar] [CrossRef] [PubMed]
- De Mejía, E.G.; Prisecaru, V.I. Lectins as bioactive plant proteins: A potential in cancer treatment. Crit. Rev. Food Sci. Nutr. 2005, 45, 425–445. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.L.; Zhou, C.C.; Yao, S.; Yu, J.Y.; Liu, B.; Bao, J.K. Plant lectins: Targeting programmed cell death pathways as antitumor agents. Int. J. Biochem. Cell Biol. 2011, 43, 1442–1449. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Dan, X.; Ng, C.C.W.; Ng, T.B. Lectins with potential for anti-cancer therapy. Molecules 2015, 20, 3791–3810. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.L.; Zhang, S.; Tian, M.; Zhang, S.Y.; Xie, T.; Chen, D.Y.; Chen, Y.J.; He, J.; Liu, J.; Ouyang, L.; et al. Plant lectins, from ancient sugar-binding proteins to emerging anti-cancer drugs in apoptosis and autophagy. Cell Prolif. 2015, 48, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Shang, C.; Chen, Q.; Dell, A.; Haslam, S.M.; de Vos, W.H.; van Damme, E.J.M. The cytotoxicity of elderberry ribosome-inactivating proteins is not solely determined by their protein translation inhibition activity. PLoS ONE 2015, 10, e0132389. [Google Scholar] [CrossRef] [PubMed]
- De Virgilio, M.; Lombardi, A.; Caliandro, R.; Fabbrini, M.S. Ribosome-inactivating proteins: From plant defense to tumor attack. Toxins 2010, 2, 2699–2737. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Kumar, M.S.; Karande, A.A. Inhibition of protein synthesis leading to unfolded protein response is the major event in abrin-mediated apoptosis. Mol. Cell Biochem. 2015, 403, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Li, L.N.; Zhang, H.D.; Zhi, R.; Yuan, S.J. Down-regulation of some miRNA by degrading their precursors contributes to anti-cancer effect of mistletoe lectin-I. Br. J. Pharmacol. 2011, 162, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.L.; Zhao, X.; Xu, H.L.; Wen, X.; Wang, S.Y.; Liu, B.; Bao, J.K.; Wei, Y.Q. Identification of microRNA-regulated autophagic pathways in plant lectin-induced cancer cell death. Cell Prolif. 2012, 45, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Barkeer, S.; Guha, N.; Hothpet, V.; Saligrama Adavigowda, D.; Hegde, P.; Padmanaban, A.; Yu, L.G.; Swamy, B.M.; Inamdar, S.R. Molecular mechanism of anticancer effect of Sclerotium rolfsii lectin in HT29 cells involves differential expression of genes associated with multiple signaling pathways: A microarray analysis. Glycobiology 2015, 25, 1375–1391. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Sun, R.; Yu, T.; Liu, R.; Cheng, L.J.; Bao, J.K.; Zou, L.; Tang, Y. Identification of novel pathways in plant lectin-induced cancer cell apoptosis. Int. J. Mol. Sci. 2016, 17, 228. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Fernig, D.G.; Smith, J.A.; Milton, J.D.; Rhodes, J.M. Reversible inhibition of proliferation of epithelial cell lines by Agaricus bisporus (edible mushroom) lectin. Cancer Res. 1993, 53, 4627–4632. [Google Scholar] [PubMed]
- Loréa, P.; Goldschmidt, D.; Darro, F.; Salmon, I.; Bovin, N.; Gabius, H.J.; Kiss, R.; Danguy, A. In vitro characterization of lectin-induced alterations on the proliferative activity of three human melanoma cell lines. Melanoma Res. 1997, 7, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Ng, T.B.; Ooi, V.E.C.; Liu, W.K. Effects of lectins with different carbohydrate-binding specificities on hepatoma, choriocarcinoma, melanoma and osteosarcoma cell lines. Int. J. Biochem. Cell Biol. 2000, 32, 365–372. [Google Scholar] [CrossRef]
- Sahasrabuddhe, A.A.; Ahmed, N.; Krishnasastry, M.V. Stress-induced phosphorylation of caveolin-1 and p38, and down-regulation of EGFr and ERK by the dietary lectin jacalin in two human carcinoma cell lines. Cell Stress Chaperones 2006, 11, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Kabir, S.R.; Nabi, M.M.; Nurujjaman, M.; Abu Reza, M.; Alam, A.H.; Zaman, R.; Khalid-Bin-Ferdaus, K.M.; Amin, R.; Khan, M.M.; Hossain, M.A.; et al. Momordica charantia seed lectin: Toxicity, bacterial agglutination and antitumor properties. Appl. Biochem. Biotechnol. 2015, 175, 2616–2628. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Zhang, C.Z.Y.; Ng, T.B.; Wong, J.H.; Pan, W.L.; Ya, W.J.; Chan, Y.S.; Fong, W.P. Momordica charantia lectin, a type II ribosome inactivating protein, exhibits antitumor activity toward human nasopharyngeal carcinoma cells in vitro and in vivo. Cancer Prev. Res. 2012, 5, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; He, L.; Meng, Y.; Li, G.; Li, L.; Meng, Y. A-MMC and MAP30, two ribosome-inactivating proteins extracted from Momordica charantia, induce cell cycle arrest and apoptosis in A549 human lung carcinoma cells. Mol. Med. Rep. 2015, 11, 3553–3558. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, U.; Stamouli, A.; Adam, E.; Peddie, M.; Pfüller, U. Biochemical, histochemical and cell biological investigations on the action of mistletoe lectins I, II and III with human breast cancer cell lines. Glycoconj. J. 1995, 12, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Griffin, T.W.; Yaynes, L.R.; DeMartino, J.A. Selective cytotoxicity of a ricin A-chain-anti-carcinoembryonic antigen antibody conjugate for a human colon adenocarcinoma cell line. J. Natl. Cancer Inst. 1982, 69, 799–805. [Google Scholar] [PubMed]
- Tsukazaki, K.; Hayman, E.G.; Ruoslahti, E. Effects of ricin A chain conjugates of monoclonal antibodies to human α-fetoprotein and placental alkaline phosphatase on antigen-producing tumor cells in culture. Cancer Res. 1985, 45, 1834–1838. [Google Scholar] [PubMed]
- Ohba, H.; Bakalova, R. Relationships between degree of binding, cytotoxicity and cytoagglutinating activity of plant-derived agglutinins in normal lymphocytes and cultured leukemic cell lines. Cancer Chemother. Pharmacol. 2003, 51, 451–458. [Google Scholar] [PubMed]
- Savanur, M.A.; Eligar, S.M.; Pujari, R.; Chen, C.; Mahajan, P.; Borges, A.; Shastry, P.; Ingle, A.; Kalraiya, R.D.; Swamy, B.M.; et al. Sclerotium rolfsii induces stronger inhibition of proliferation in human breast cancer cells than normal human mammary epithelial cells by induction of cell apoptosis. PLoS ONE 2014, 9, e110107. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.; Surolia, A.; Karande, A.A. Ribosome-inactivating protein and apoptosis: Abrin causes cell death via mitochondrial pathway in Jurkat cells. Biochem. J. 2004, 377, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.; Misrha, D.; Roy, B.; Devi, K.S.; Narayan, R.; Das, J.; Ghosh, S.K.; Maiti, T.K. Abrus precatorius agglutinin-derived peptides induce ROS-dependent mitochondrial apoptosis through JNK and Akt/P3/P53 pathways in HeLa cells. Chem. Biol. Interact. 2014, 222, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Yang, R.; Zhao, X.; Qin, D.; Liu, Z.; Liu, F.; Song, X.; Li, L.; Feng, R.; Gao, N. Abrin P2 suppresses proliferation and induces apoptosis of colon cancer cells via mitochondrial membrane depolarization and caspase activation. Acta Biochim. Biophys. Sin. 2016, 48, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Bhutia, S.K.; Behera, B.; Das, D.N.; Mukhopadhyay, S.; Sinha, N.; Panda, P.K.; Naik, P.P.; Patra, S.K.; Mandal, M.; Sarkar, S.; et al. Abrus agglutinin is a potent anti-proliferative and anti-angiogenic agent in human breast cancer. Int. J. Cancer 2016, 139, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Mukhopahyay, S.; Panda, P.K.; Behera, B.; Das, C.K.; Hassan, M.K.; Das, D.N.; Sinha, N.; Bissoyi, A.; Pramanik, K.; Maiti, T.K.; et al. In vitro and in vivo antitumor effects of peanut agglutinin through induction of apoptotic and autophagic cell death. Food Chem. Toxicol. 2014, 64, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.C.; de Paula, C.A.; Ferreira, J.G.; Paredes-Gamero, E.J.; Vaz, A.M.; Sampaio, M.U.; Correia, M.T.; Oliva, M.L. Bauhinia forficata lectin (BfL) induces cell death and inhibits integrin-mediated adhesion on MCF7 human breast cancer cells. Biochim. Biophys. Acta 2014, 1840, 2262–2271. [Google Scholar] [CrossRef] [PubMed]
- Panda, P.K.; Mukhopadhyay, S.; Behera, B.; Bhol, C.S.; Day, S.; Das, D.N.; Sinha, N.; Bissoyi, A.; Pramanik, K.; Maiti, T.K.; et al. Antitumor effect of soybean lectin mediated through reactive oxygen species-dependent pathway. Life Sci. 2014, 111, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.Z.; Fang, E.F.; Zhang, H.T.; Liu, L.L.; Yun, J.P. Momordica charantia lectin exhibits antitumor activity towards hepatocellular carcinoma. Investig. New Drugs 2015, 33, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.F.; Zhang, C.Z.; Wong, J.H.; Shen, J.Y.; Li, C.H.; Ng, T.B. The MAP30 protein from bitter gourd (Momordica charantia) seeds promotes apoptosis in liver cancer cells in vitro and in vivo. Cancer Lett. 2012, 324, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.R.; Ray, U.; Chatterjee, B.; Roy, S.B. Targeted apoptosis in ovarian cancer cells through mitochondrial dysfunction in response to Sambucus nigra agglutinin. Cell Death Dis. 2017, 8, e2762. [Google Scholar] [CrossRef] [PubMed]
- Zwierzina, H.; Bergmann, L.; Fiebig, H.; Aamdal, S.; Schoffski, P.; Vitthohm, K.; Leutzen, H. The preclinical and clinical activity of aviscumine: A potential anticancer drug. Eur. J. Cancer 2011, 47, 1450–1457. [Google Scholar] [CrossRef] [PubMed]
- Han, S.Y.; Hong, C.E.; Kim, H.G.; Lyu, S.Y. Anti-cancer effects of enteric-coated polymers containing mistletoe lectin in murine melanoma cells in vitro and in vivo. Mol. Cell Biochem. 2015, 408, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, N.; Tyagi, M.; Pachauri, M.; Ghosh, P.C. Potential therapeutic applications of plant toxin-ricin in cancer: Challenges and advances. Tumour Biol. 2015, 36, 8239–8246. [Google Scholar] [CrossRef] [PubMed]
- Liao, P.; Liu, W.; Li, H.; Gao, H.; Wang, H.; Li, N.; Xu, N.; Li, J.; Wan, J.; Liu, L.; et al. Morphological changes of ricin toxin-induced apoptosis in human cervical cancer cells. Toxicol. Ind. Health 2012, 28, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Horrix, C.; Raviv, Z.; Flescher, E.; Voss, C.; Berger, M.R. Plant ribosome-inactivating proteins type II induce the unfolded protein response in human cancer cells. Cell Mol. Life Sci. 2011, 68, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; So, H.S.; Lee, K.M.; Park, J.S.; Lee, J.H.; Moon, S.K.; Ryu, D.G.; Chung, S.Y.; Jung, B.H.; Kim, Y.K.; et al. Activation of caspase cascades in Korean mitletoe (Viscum album var. coloratum) lectin-II-induced apoptosis of human myeloleukemic U937 cells. Gen. Pharmacol. 2000, 34, 349–355. [Google Scholar] [CrossRef]
- Kim, M.S.; Lee, J.; Lee, K.M.; Yang, S.H.; Choi, S.; Chung, S.Y.; Kim, T.Y.; Jeong, W.H.; Park, R. Involvement of hydrogen peroxide in mistletoe lectin-II-induced apoptosis of myeloleukemic U937 cells. Life Sci. 2003, 73, 1231–1243. [Google Scholar] [CrossRef]
- Choi, S.H.; Lyu, S.Y.; Park, W.B. Mistletoe lectin induces apoptosis and telomerase inhibition in human A253 cancer cells through dephosphorylation of Akt. Arch. Pharm. Res. 2004, 27, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Twardziok, M.; Kleinsimon, S.; Rolff, J.; Jäger, S.; Eggert, A.; Seifert, G.; Delebinski, C.I. Multiple active compounds from Viscum album L. synergistically converge to promote apoptosis in Ewing sarcoma. PLoS ONE 2016, 11, e0159749. [Google Scholar] [CrossRef] [PubMed]
- Twardziok, M.; Meierhofer, D.; Börno, B.; Timmermann, B.; Jäger, S.; Boral, S.; Eggert, A.; Delebinski, C.I.; Seifert, G. Transcriptomic and proteomic insight into the effects of a defined European mistletoe extract in Ewing sarcoma cells reveals cellular stress responses. BMC Complement. Altern. Med. 2017, 17, 237. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, A.; Zepp, M.; Adwan, H.; Berger, M.R. Riproximin modulates multiple signaling cascades leading to cytostatic and apoptotic effects in human breast cancer cells. J. Cancer Res. Clin. Oncol. 2016, 142, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Ditamo, Y.; Rupil, L.L.; Sendra, V.G.; Nores, G.A.; Roth, G.A.; Irazoqui, F.J. In vivo immunomodulatory effect of the lectin from edible mushroom Agaricus bisporus. Food Funct. 2016, 7, 162–269. [Google Scholar] [CrossRef] [PubMed]
- Bocci, V. Mistletoe (Viscum album) lectins as cytokine inducers and immunoadjuvant in tumor therapy. A review. J. Biol. Regul. Homeost. Agents 1993, 7, 1–6. [Google Scholar] [PubMed]
- Hajto, T.; Hostanka, K.; Weber, K.; Zinke, H.; Fischer, J.; Mengs, U.; Lentzen, H.; Saller, R. Effect of a recombinant lectin, Viscum album agglutinin, on the secretion of interleukin-12 in cultured human peripheral blood mononuclear cells and on NK-cell-mediated cytotoxicity of rat splenocytes in vitro and in vivo. Nat. Immunol. 1998, 16, 34–46. [Google Scholar] [CrossRef]
- Hostanka, K.; Hajto, T.; Spagnoli, G.C.; Fischer, J.; Lentzen, H.; Herrmann, R. A plant lectin derived from Viscum album induces cytokine gene expression and protein production in cultures of human peripheral blood mononuclear cells. Nat. Immunol. 1995, 14, 295–304. [Google Scholar]
- Lyu, S.Y.; Park, W.B. Mistletoe lectin transport by M-cells in follicle-associated epithelium (FAE) and IL-12 secretion in dendritic cells situated below FAE in vitro. Arch. Pharm. Res. 2010, 33, 1433–1441. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.S.; Xia, L.; Ng, T.B. White kidney bean lectin exerts anti-proliferative and apoptotic effects on cancer cells. Int. J. Biol. Macromol. 2016, 85, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.A.; Carvalho, F.C.; Ruas, L.P.; Ricci-Azevedo, R.; Roque-Barreira, M.C. The immunomodulatory effects of plant lectins: A review with emphasis on artinM properties. Glycoconj. J. 2013, 30, 641–657. [Google Scholar] [CrossRef] [PubMed]
- Akhlynina, T.V.; Gulak, P.V.; Serebriakova, N.V.; Rozenkrants, A.A.; Sobolev, A.S. Photodynamic effects of a concanavalin A-chlorin e6 conjugate on human fibroblasts [Article in Russian]. Biull. Eksp. Biol. Med. 1990, 109, 150–152. [Google Scholar] [CrossRef] [PubMed]
- Komath, S.S.; Kavitha, M.; Swamy, M.J. Beyond carbohydrate binding: New directions in plant lectin research. Org. Biomol. Chem. 2006, 4, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Komath, S.S.; Bhanu, K.; Maiya, B.G.; Swamy, M.J. Binding of porphyrin by the tumor-specific lectin, jacalin [Jack fruit (Artocarpus integrifolia) agglutinin]. Biosci. Rep. 2000, 20, 265–276. [Google Scholar] [CrossRef] [PubMed]
- Komath, S.S.; Kenoth, R.; Giribabu, L.; Maiya, B.G.; Swamy, M.J. Fluorescence and absorption spectroscopic studies on the interaction of porphyrins with snake gourd (Trichosanthes anguina) seed lectin. J. Photochem. Photobiol. B Biol. 2000, 55, 49–55. [Google Scholar] [CrossRef]
- Sultan, N.A.M.; Maiya, B.G.; Swamy, M.J. Thermodynamic analysis of porphyrin binding to Momordica charantia (bitter gourd) lectin. Eur. J. Biochem. 2004, 271, 3274–3282. [Google Scholar] [CrossRef] [PubMed]
- Goel, M.; Damai, R.S.; Sethi, D.K.; Kaur, K.J.; Maiya, B.G.; Swamy, M.J.; Salunke, D.M. Crystal structure of the PNA-porphyrin complex in the presence and absence of lactose: Mapping of the conformational changes on lactose binding, interacting surfaces, and supramolecular aggregations. Biochemistry 2005, 44, 5588–5596. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Fatma, T.; Komath, S.S. Specific interaction of the legume lectins, concanavalin A and peanut agglutinin, with phycocyanin. Photochem. Photobiol. 2009, 85, 1126–1133. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, M.; Swamy, M.J. Thermodynamic studies on the interaction of water soluble porphyrins with the glucose/mannose-specific lectin from garden pea (Pisum sativum). Life 2006, 58, 720–730. [Google Scholar] [CrossRef] [PubMed]
- Pandey, G.; Fatma, T.; Cowsik, S.M.; Komath, S.S. Specific interaction of jacalin with phycocyanin, a fluorescent phycobiliprotein. J. Photochem. Photobiol. B Biol. 2009, 97, 87–93. [Google Scholar] [CrossRef] [PubMed]
- D’Auria, S.; Petrova, L.; John, C.; Russev, G.; Varriale, A.; Bogoeva, V. Tumor-specific protein human galectin-1 interacts with anticancer agents. Mol. BioSyst. 2009, 5, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
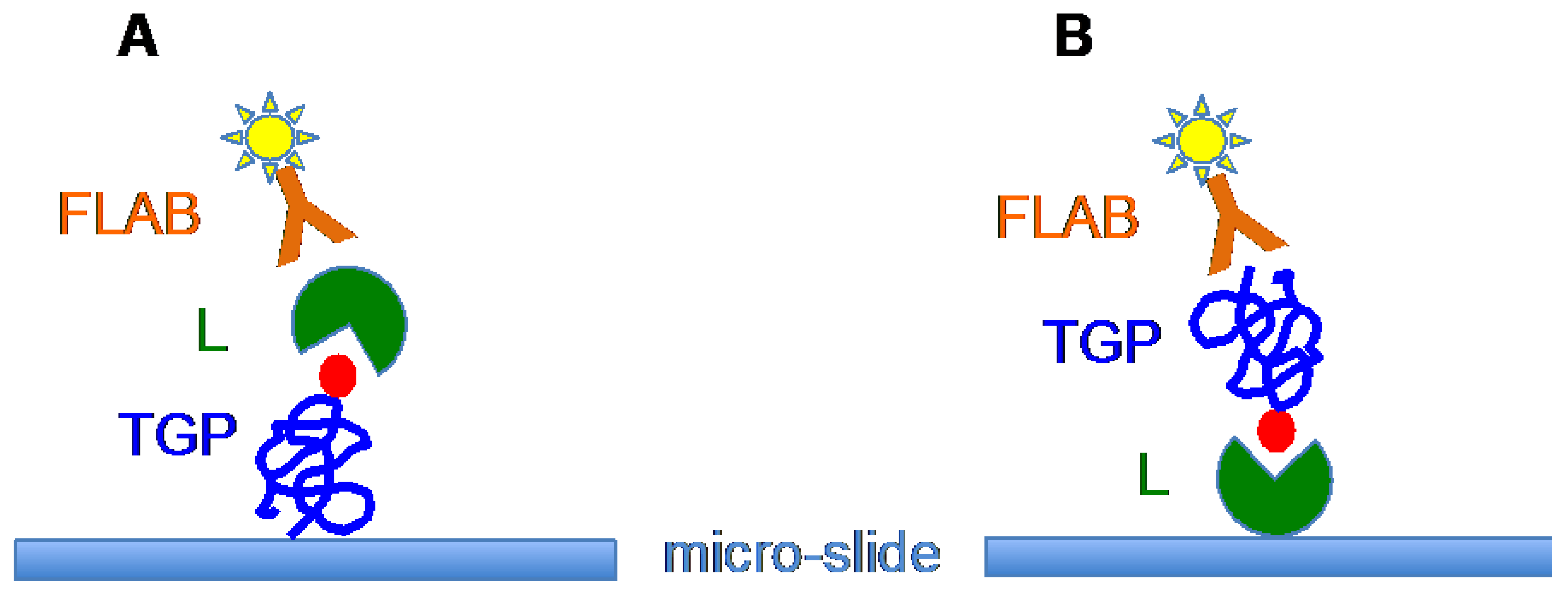
- Poiroux, G.; Pitié, M.; Culerrier, R.; Ségui, B.; van Damme, E.J.M.; Peumans, W.J.; Bernadou, J.; Levade, T.; Rougé, P.; Barre, A.; et al. Morniga G: A plant lectin as an endocytic ligand for photosensitizer molecule targeting toward tumor-associated T/Tn antigens. Photochem. Photobiol. 2011, 87, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Poiroux, G.; Pitié, M.; Culerrier, R.; Lafont, E.; Ségui, B.; van Damme, E.J.M.; Peumans, W.J.; Bernadou, J.; Levade, T.; Rougé, P.; et al. Targeting of T/Tn antigens with a plant lectin to kill human leukemia cells by photochemotherapy. PLoS ONE 2011, 6, e23315. [Google Scholar] [CrossRef] [PubMed]
- Evangelio, E.; Poiroux, G.; Culerrier, R.; Pratviel, G.; van Damme, E.J.M.; Peumans, W.J.; Barre, A.; Rougé, P.; Benoist, H.; Pitié, M. Comparative study of the phototoxicity of long-wavelength photosensitizers targeted by the Morniga G lectin. Bioconjug. Chem. 2011, 22, 1337–1344. [Google Scholar] [CrossRef] [PubMed]
- Kejík, Z.; Bříza, T.; Králová, J.; Potčková, P.; Král, A.; Martásek, P.; Král, V. Coordination conjugates of therapeutic proteins with drug carriers: A new approach for versatile advanced drug delivery. Bioorg. Med. Chem. Lett. 2011, 21, 5514–5520. [Google Scholar] [CrossRef] [PubMed]
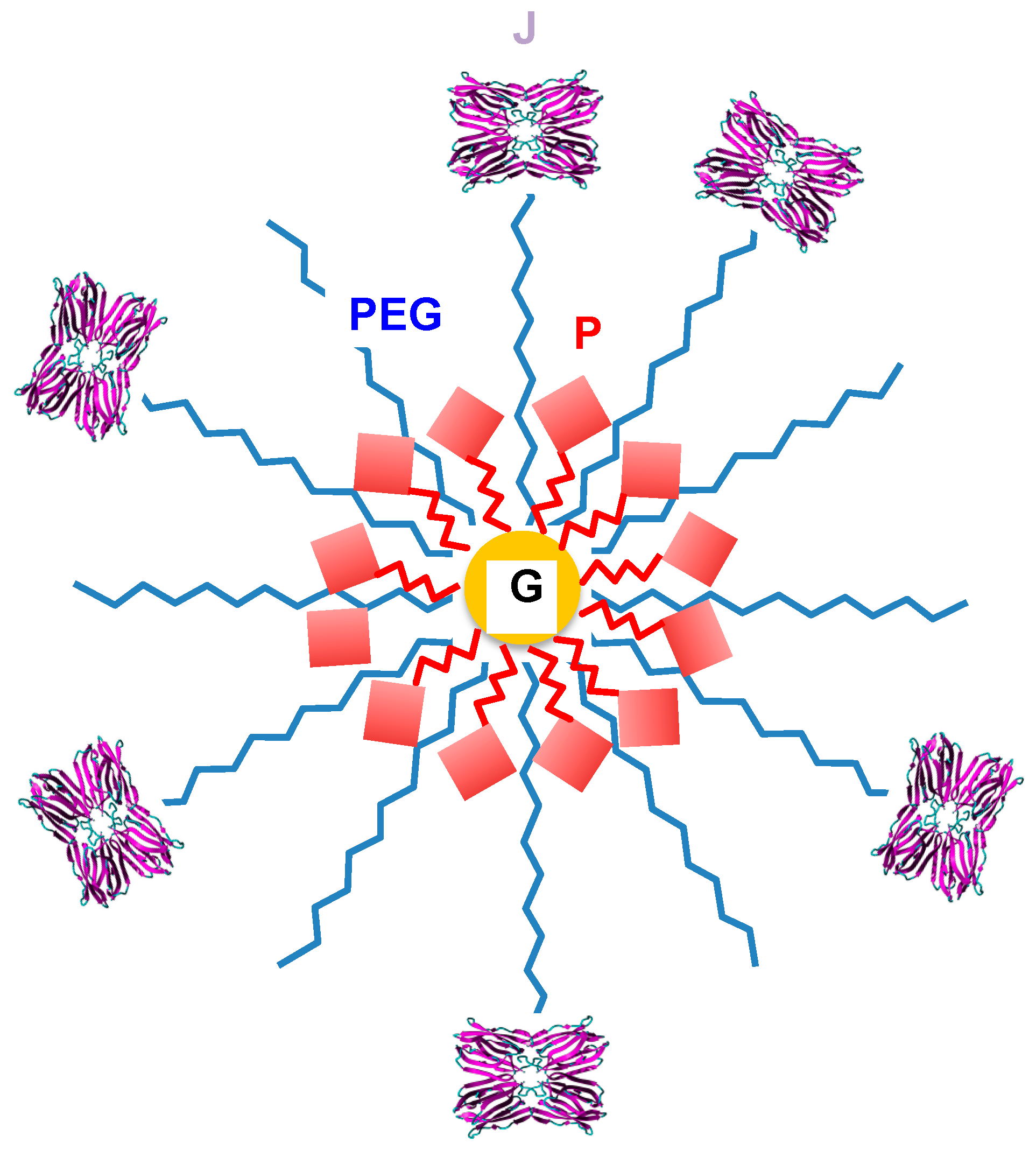
- Obaid, G.; Chambrier, I.; Cook, M.J.; Russell, D.A. Targeting the oncofetal Thomsen-Friedenreich disaccharide using jalaclin-PEG phthalocyanine gold nanoparticles for photodynamic cancer therapy. Angew. Chem. Int. Ed. 2012, 51, 6158–6162. [Google Scholar] [CrossRef] [PubMed]
- Obaid, G.; Chambrier, I.; Cook, M.J.; Russell, D.A. Cancer targeting with biomolecules: A comparative study of photodynamic therapy efficacy using antibody or lectin conjugated phthalocyanine-PEG gold nanoparticles. Photochem. Photobiol. Sci. 2015, 14, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Hockl, P.F.; Wolosiuk, A.; Pérez-Sáez, J.M.; Bordoni, A.V.; Croci, D.O.; Toum-Terrones, Y.; Soler-Illia, G.J. Glyco-nano-oncology: Novel therapeutic opportunities by combining small and sweet. Pharmacol. Res. 2016, 109, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Benoist, H.; Culerrier, R.; Poiroux, G.; Ségui, B.; Jauneau, A.; van Damme, E.J.M.; Peumans, W.J.; Barre, A.; Rougé, P. Two structurally identical mannose-specific jacalin-related lectins display different effects on human T lymphocytes activation and cell death. J. Leukoc. Biol. 2009, 86, 103–114. [Google Scholar] [CrossRef] [PubMed]
- Pereira, P.M.R.; Silva, S.; Cavaleiro, J.A.S.; Ribeiro, C.A.F.; Tomé, J.P.C.; Fernandes, R. Galactodendritic phthalocyanine targets carbohydrate-binding proteins enhancing photodynamic therapy. PLoS ONE 2014, 9, e95529. [Google Scholar] [CrossRef] [PubMed]
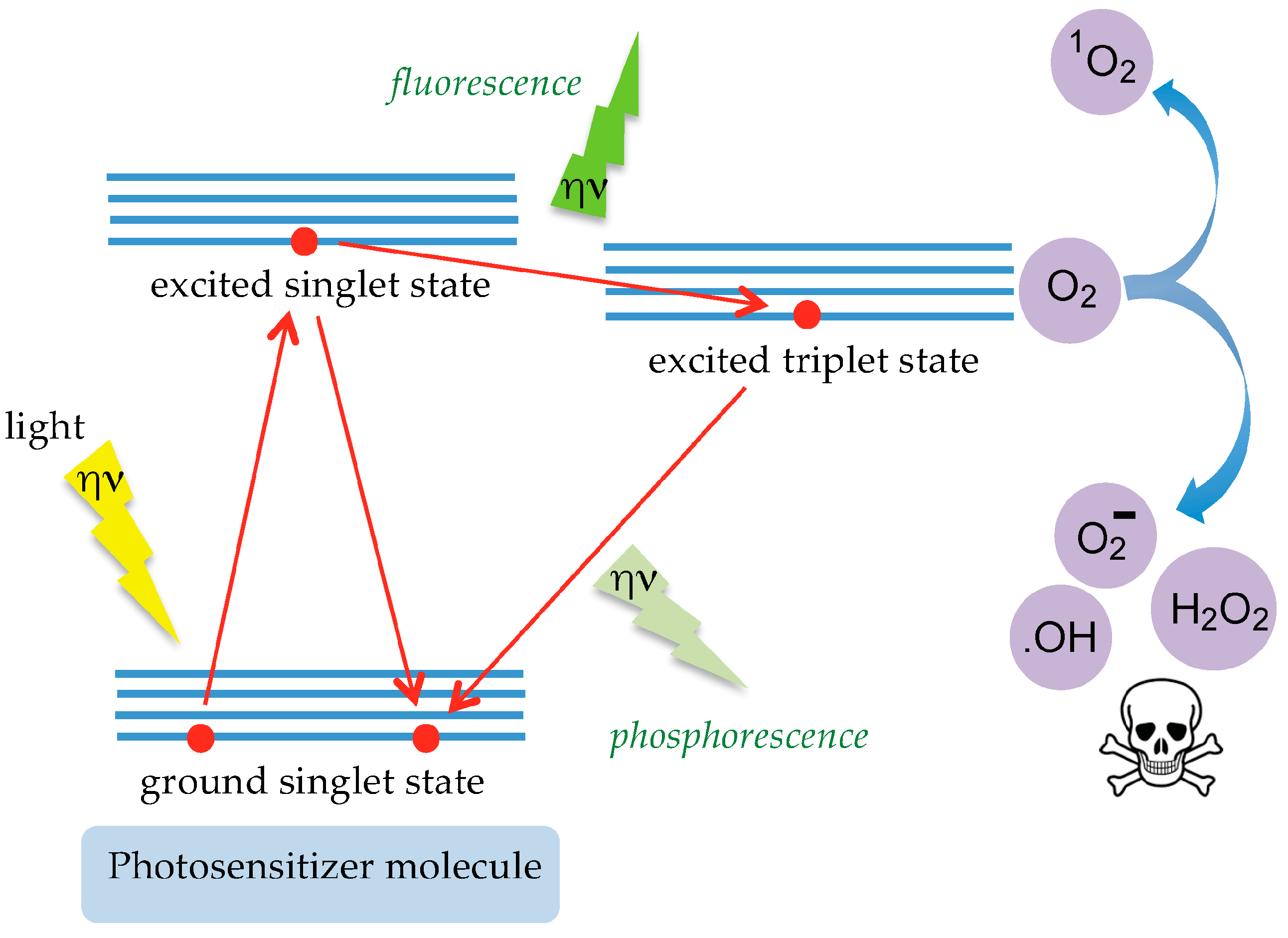
- Vrouenraets, M.B.; Visser, G.W.; Snow, G.B.; van Dongen, G.A. Basic principles, applications in oncology and improved selectivity of photodynamic therapy. Anticancer Res. 2003, 23, 505–522. [Google Scholar] [PubMed]
- Sibata, C.H.; Colussi, V.C.; Oleinick, N.L.; Kinsella, T.J. Photodynamic therapy in oncology. Expert Opin. Pharmacother. 2001, 2, 917–927. [Google Scholar] [PubMed]
- Gudgin Dickson, E.F.; Goyan, R.L.; Pottier, R.H. New directions in photodynamic therapy. Cell. Mol. Biol. 2002, 48, 939–954. [Google Scholar] [PubMed]
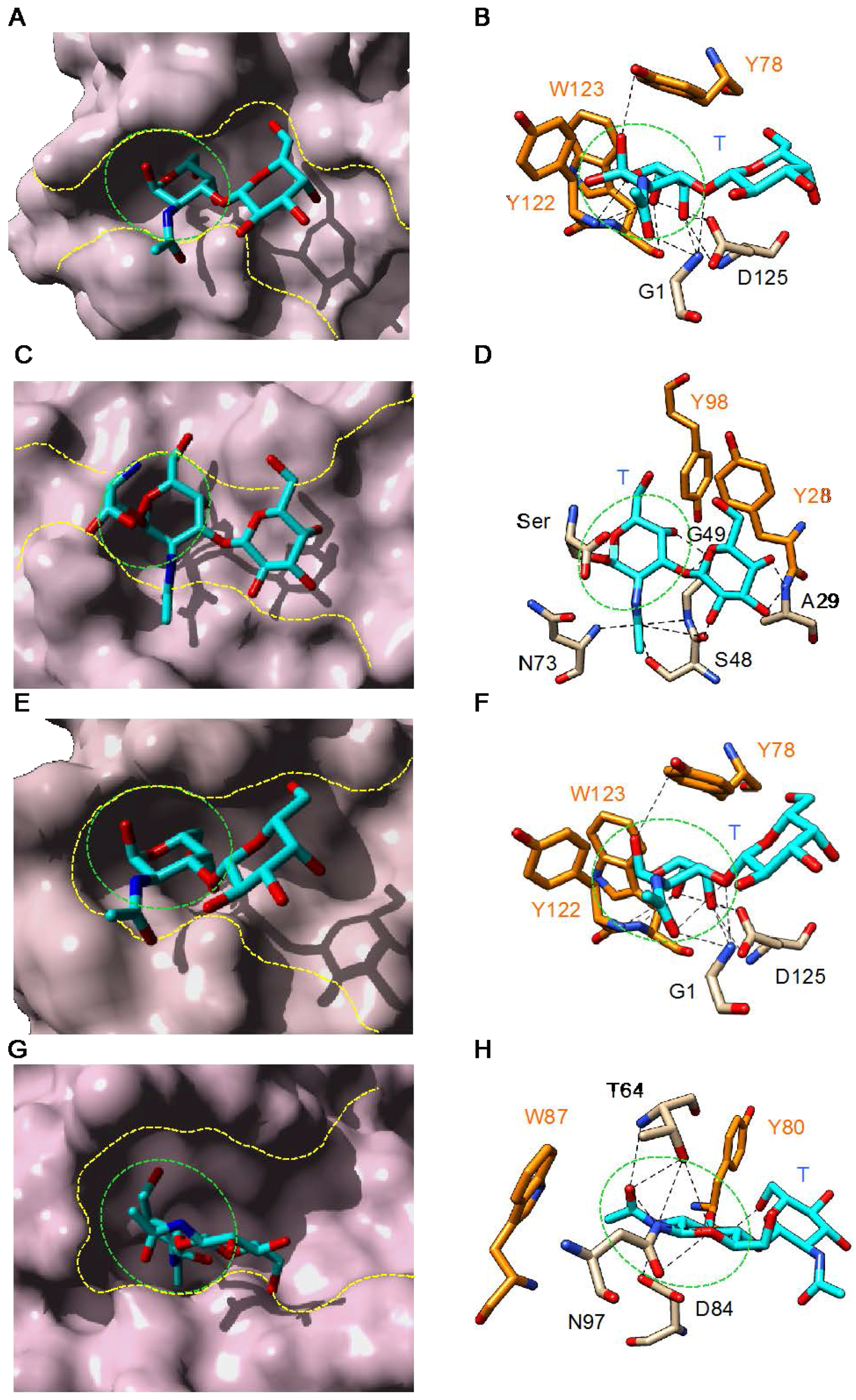

| Plant/Fungus | Lectin | Specificity | References |
|---|---|---|---|
| Abrus precatorius (P) | APA | T | [32] |
| Agaricus bisporus (F) | ABL | T | [33,34] |
| Agrocybe aegerita (F) | AAL | ST/T | [35] |
| Agropyrum repens (P) | ARL | T | [36] |
| Amaranthus caudatus (P) | Amaranthin | T/Tn | [37,38] |
| Amaranthus leucocarpus (P) | ALL | T/Tn | [39] |
| Arachis hypogaea (P) | PNA | ST > T > Tn | [38,40,41] |
| Artocarpus incisa (P) | Frutalin | T | [42] |
| Artocarpus integrifolia (P) | Jacalin | ST/T/Tn | [43] |
| Champedak GBL | Tn of O-mucin | [44] | |
| Artocarpus lakoocha (P) | ALL | T/Tn cluster | [45] |
| Bauhinia forficata (P) | BfL | Tn | [46] |
| Bauhinia purpurea (P) | BPA | T/Tn cluster | [47] |
| Caragana arborescens (P) | CAA | ST, Forssman | [48] |
| Codium fragile (alga) | CFA | T/Tn, Forssman | [49] |
| Dolichos biflorus (P) | Tn | Tn | [50] |
| Glechoma hederacea (P) | Gleheda | T/Tn | [51] |
| Glycine max (P) | SBA | Tn, mucin | [52] |
| Griffonia (Bandeirea) simplicifolia (P) | Gs I-A4 | Tn | [53,54] |
| Lactarius deliciosus (F) | LDL | T | [55] |
| Lactarius deterrimus (F) | LDetL | T | [56] |
| Laelia autumnalis (P) | LAL | T/Tn | [57] |
| Maclura pomifera (P) | MPA | T/Tn | [58,59] |
| Moluccella laevis (P) | MLL | Tn, Forssman | [60,61] |
| Momordica charantia (P) | BGSL | T | [62] |
| Morus nigra (P) | Morniga-G | Tn/T cluster | [63] |
| Myrsine coriacea (P) | McL | Tn | [64] |
| Psophocarpus tetragonolobus (P) | WBL | Tn | [65] |
| Ricinus communis (P) | Ricin | T/Tn | [66] |
| RCA-I | T | [67] | |
| Salvia bogotensis (P) | SBL | Tn | [68] |
| Salvia sclarea (P) | SSL | Tn | [69,70,71] |
| Salvia hominum (P) | SHL | Tn | [72] |
| Sambucus nigra (P) | SNA | Tn cluster | [73] |
| SNA-II | Tn | [74] | |
| SNA-IV | Tn | Unpublished | |
| Sclerotium rolfsii (F) | SRL | Tn cluster | [75] |
| Sophora japonica (P) | SJL | T | [76] |
| Triticum vulgare (P) | WGA | Tn cluster | [77,78] |
| Vateirea macrocarpa (P) | VML | T/Tn | [79] |
| Vicia graminea (P) | VguL | T | [80] |
| Vicia villosa (P) | VVA B4 | Tn | [81] |
| Viscum album (P) | ML-I | T | [82] |
| Wisteria floribunda (P) | WFA | Tn | [83] |
| Xerocomus chrysenteron (F) | XCL | Tn | [84] |
| Ximenia americana (P) | Riproximin | Tn cluster | [85] |
| Cancer Cell Line (H: Human, M: Mouse, R: Rat, Hamster: h) | Lectin | Toxicity | Proliferation Inhibition | Ref. |
|---|---|---|---|---|
| HT29 colon (H), MCF-7 breast (H) | ABL | - | + | [153] |
| HeLa (H), SW480 lymph node metastasis (H); SGC-7901, BGC-823 | AAL | + (M) | + | [35] |
| gastric cancer (H); MGC80-3 gastric adeno-carcinoma (H); HL-60 | ||||
| leukemia (H); S-180 sarcoma (M) | ||||
| NCI-60 tumor cell line panel (H), LOX IMVI melanoma (H) | BfL | - | + | [46] |
| SK-MEL-28 melanoma (H), HT-144 melanoma (H), C32 melanoma | GSA-IA4 | + | + | [54,154] |
| (H), LS174t, SW1116 colon cancer (H) | ||||
| A431 epidermoid carcinoma (H); HT29 colorectal carcinoma (H) | Jacalin, PNA | + | + | [155,156] |
| JAr choriocarcinoma (H); H3B hepato-carcinoma (H); B16 | ||||
| melanoma (M) | ||||
| EAC Ehrlich ascites carcinoma; A549 lung carcinoma (H); CNE-1 | MCL | + | + | [157,158,159] |
| CNE-2 nasopharyngeal carcinoma (H) | ||||
| BT20, BT549, MCF7, HS578T, HBL100, T47D breast cancer (H) | ML-I, -II, -III | + | [141,160] | |
| SK-Hep-1, SK-Hep-3B hepatocarcinoma (H) | ||||
| HT-29 colon (H) | McL | + | + | [64] |
| G-361 melanoma (H); HepG2 hepatoma (H); SKGIIIa cervical | Ricin | + | + | [161,162] |
| carcinoma (H) | ||||
| Raji, Daudi lymphoma cell lines (H); JAr choriocarcinoma (H); | SBA | + | - | [155,163] |
| H3B hepato-carcinoma (H); B16 melanoma (M) | ||||
| HT-29 colon (H) | SRL | + | + | [164] |
| MCF7, MDA-MB231 breast carcinoma (H); U87-MG brain tumor (H) | Riproximin | + | - | [85] |
| HEp2 larynx (H); NCI-H460 lung (H); HT29 colon (H); PC3 | ||||
| prostate (H); SKW3, K562, BV173 leukemia (H) |
| Lectin | Mechanism | Ref. |
|---|---|---|
| (Abrus precatorius) Abrin | Abrin (type II RIP) induced the caspase 3-dependent but caspase 8-independent apoptotic pathway, mitochondrial membrane potential damage and production of ROS in Jurkat cells. | [165] |
| (Abrus precatorius) A. p. lectin | Peptides from A. p. lectin induced drastic loss of mitochondrial membrane potential and increase in ROS, leading to symptoms of early apoptosis through a deregulation of Akt and activation of both JNK, MAPK, p53 and autophagy in HeLa cells. | [166] |
| (Abrus precatorius) Abrin P2 | Abrin P2 suppressed the proliferation of colon HCT-8 cell line and provoked a cell cycle arrest at the S and G2/M phases. Abrin P2 inhibited cell proliferation via the down-regulation of cyclin B1 and the nuclear antigen Ki67, and the up-regulation of P21. The abrin P2-induced apoptosis was dose- and time-dependent. | [167] |
| (Abrus precatorius) agglutinin AGG | AGG administered to human breast xenografted athymic nude mice mediated anti-tumorigenic effects through induction of extrinsic apoptosis via Akt-dependent ROS generation, and inhibition of angiogenesis via inhibition of expression of the pro-angiogenic factor IGFBP2 in an AKT-dependent manner. | [168] |
| (Agrocybe aegerita) lectin AAL | AAL inhibited the growth of different tumor cell lines HeLa, SW480, SGC-7901, MGC80-3, BGC-823 and HL-60 and induced apoptosis in HeLa cells. It also displayed DNAse activity. | [35] |
| (Arachis hypogaea) peanut agglutinin PNA | PNA induced autophagy and apoptotic cell death in HeLa cells, associated to a concomitant increase in ROS. | [169] |
| (Artocarpus integrifolia) jacalin | Rounding of A431 (epidermoid carcinoma) and HT29 (colorectal carcinoma) cells due to the stress-induced phosphorylation of caveolin-1 and p38 and down-regulation of EGFr. | [155] |
| (Bauhinia forficata) lectin BfL | BfL inhibited the adhesion of breast cancer MCF7 cells on laminin, collagen I and fibronectin, decreased the α1, α6 and β1 integrin subunit expression and increased the α5 subunit expression. BfL caused necrosis of MCF7 cells with caspase-9 inhibition, DNA fragmentation and cell cycle arrest in the G2/M phase. | [170] |
| (Glycine max) soybean agglutinin SBL | SBL-mediated autophagy, apoptosis and DNA damage in HeLa cells depend on the generation of ROS. Pre-treatment of HeLa cells by the ROS scavenger N-acetylcysteine reduced both SBL-induced autophagy, apoptosis and DNA damage. | [171] |
| (Momordica charantia) lectin MCL | MCL induced apoptosis, DNA fragmentation, G1 phase arrest and mitochondrial injury in nasopharyngeal carcinoma NPC cells in vitro and in vivo, associated with regulation of p38 MAPK, NK and ERK phosphorylation and NO production. MCL increased cytochrome c release in the cytosol, activated caspase-3, -8 and -9 and enhanced production of PARP. | [157] |
| (Momordica charantia) lectin MCL | MCL treatment induced G2/M phase arrest, autophagy, DNA fragmentation, mitochondrial injury and apoptosis in HCC cells. Activation of caspase and MAPK pathway was involved in the MCL-induced apoptosis. Up-regulation of truncated Bid (tBid) was shown to occur during the MCL treatment. | [172] |
| (Momordica charantia) RIP MAP30 | MAP30 from Momordica charantia promotes apoptosis in both Hep G2 cells (hepatocellular carcinoma) and Hep G2-bearing mice. The contribution of both caspase-8 regulated extrinsic and caspase-9 intrinsic caspase cascades was evidenced. | [173] |
| (Momordica charantia) α-momorcharin and MAP30 | Both RIPs induced cell cycle arrest in S-phase, DNA fragmentation and apoptosis in A549 lung carcinoma cells. Inhibition of cell proliferation was dose- and time-dependent. | [158] |
| (Sambucus nigra) agglutinin SNA | SNA activates the signaling pathways of AKT and ERK1/2 in ovarian carcinoma cells. The mitochondrial outer membrane permeabilization resulted in ROS generation and cytochrome c release in the cytosol. The perturbed mitochondrial respiration resulted in the G2/M phase cell cycle arrest. | [174] |
| (Sclerotium rolfsii) lectin SRL | SRL caused dose-dependent inhibition of proliferation of MCF-7 and ZR-75 breast cancer cells via induction of cellular apoptosis. Inhibitors of caspase-3, -8 and -9 prevented the apoptosis to occur. | [164] |
| (Viscum album) Korean mistletoe lectin VCA | VCA elicited apoptosis in SK-Hep-1 p53-positive and Hep 3B p53-negative hepatocarcinoma cell lines by down-regulation of Bcl-2 and up-regulation of Bax functioning upstream of caspase-3. Down-regulation of telomerase activity occurred in both VCA-treated cells. | [141] |
| (Viscum album) Mistletoe lectin-1 ML-1 | CM-1 induced apoptosis in colorectal cancer cells by down-regulating the miR-135a&b miRNAs expression. The expression of β-catenin was up-regulated. | [149] |
| (Viscum album) Recombinant aviscumine | The mechanism of aviscumin-mediated cell death on multiple cell types was solely induced by the toxic A-chain. The mechanism is independent from the death receptor Fas and independent of the activity of the anti-apoptotic transcription factor NFκB. Treatment with aviscumine inhibited growth in various metastases mouse models including C8 colon carcinoma, Lewis lung sarcoma, Renca renal sarcoma, etc. | [175] |
| (Viscum album) Korean mistletoe lectin VCA | Treatment of B16BL6 and B16F10 melanoma cells with VCA resulted in G0/G1 phase arrest and induced an increase in both early and late apoptosis. Both VCA and mistletoe extracts increased activated multiple caspases (caspase-1, 3, 4, 5, 6, 7, 8 and 9) and a decrease of procaspase 3 and 8. | [176] |
| (Ricinus communis) agglutinin RCA and ricin A-chain | Treatment of cancer cells in vitro by ricin and ricin A-chain activates caspase 3 and caspase 8, but not caspase 9. In vivo, cell death depends on the necrotic effect of the RIP. | [177] |
| (Ricinus communis) ricin | Ricin inhibited the proliferation of HeLa cells by inducing apoptosis, chromatin condensation and nuclear fragmentation. | [178] |
| (Ricinus communis) ricin and riproximin | Unfolding protein response UPR to endoplasmic reticulum stress was induced in both HCT116 and MDA-MB-231 cells. Apoptosis was induced by concentrations of RIPs-II at which the UPR-related genes are still translated. | [179] |
| (Viscum album) Korean mistletoe lectin-II | Lectin-II induced the activation of caspase-3, -8 and -9 of myeloleukemic U937 cells in a time- and dose-dependent manner. | [180] |
| (Viscum album) mistletoe lectin-II | Apoptotic cell death of U937 cells was induced by the generation of pro-oxidants mediating the JNK/SAPK activation, cytochrome c release, activation of caspase-9- and -3-like proteases, and PARP cleavage. | [181] |
| (Viscum album) Korean mistletoe lectin VCA | Induction of apoptosis in A253 cells through activation of caspase-3 and inhibition of telomerase activity through transcriptional down-regulation of hTERT. Inhibition of telomerase activity resulted from dephosphorylation of Akt. | [182] |
| (Viscum album) European mistletoe lectin-containing extracts | In vitro and ex vivo treatment of Ewing sarcoma cells by mistletoe extracts inhibited proliferation and induced a dose-dependent apoptosis via intrinsic and extrinsic apoptotic pathways, as evidenced by activation of both caspase-8 and caspase-9. | [183] |
| (Viscum album) European mistletoe lectin-containing extracts | Treatment of Ewing sarcoma cells by mistletoe extracts impacted both gene and protein expression. Cell response to oxidative stress induced the activation of the MAPK signaling pathway. | [184] |
| (Ximenia americana) riproximin | Riproximin induced cytotoxic effects on breast cancer cell lines MDA-MB-231 and MCF-7. Riproximin treatment caused arrest in S phase and nuclear fragmentation of the cell, induced cytokine IL24/MDA-7 and ER-stress-related GADD genes. An inhibition of the genes involved in migration of colony was observed. | [185] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poiroux, G.; Barre, A.; Van Damme, E.J.M.; Benoist, H.; Rougé, P. Plant Lectins Targeting O-Glycans at the Cell Surface as Tools for Cancer Diagnosis, Prognosis and Therapy. Int. J. Mol. Sci. 2017, 18, 1232. https://doi.org/10.3390/ijms18061232
Poiroux G, Barre A, Van Damme EJM, Benoist H, Rougé P. Plant Lectins Targeting O-Glycans at the Cell Surface as Tools for Cancer Diagnosis, Prognosis and Therapy. International Journal of Molecular Sciences. 2017; 18(6):1232. https://doi.org/10.3390/ijms18061232
Chicago/Turabian StylePoiroux, Guillaume, Annick Barre, Els J. M. Van Damme, Hervé Benoist, and Pierre Rougé. 2017. "Plant Lectins Targeting O-Glycans at the Cell Surface as Tools for Cancer Diagnosis, Prognosis and Therapy" International Journal of Molecular Sciences 18, no. 6: 1232. https://doi.org/10.3390/ijms18061232