Comparing Proteolytic Fingerprints of Antigen-Presenting Cells during Allergen Processing
Abstract
:1. Introduction
2. Results
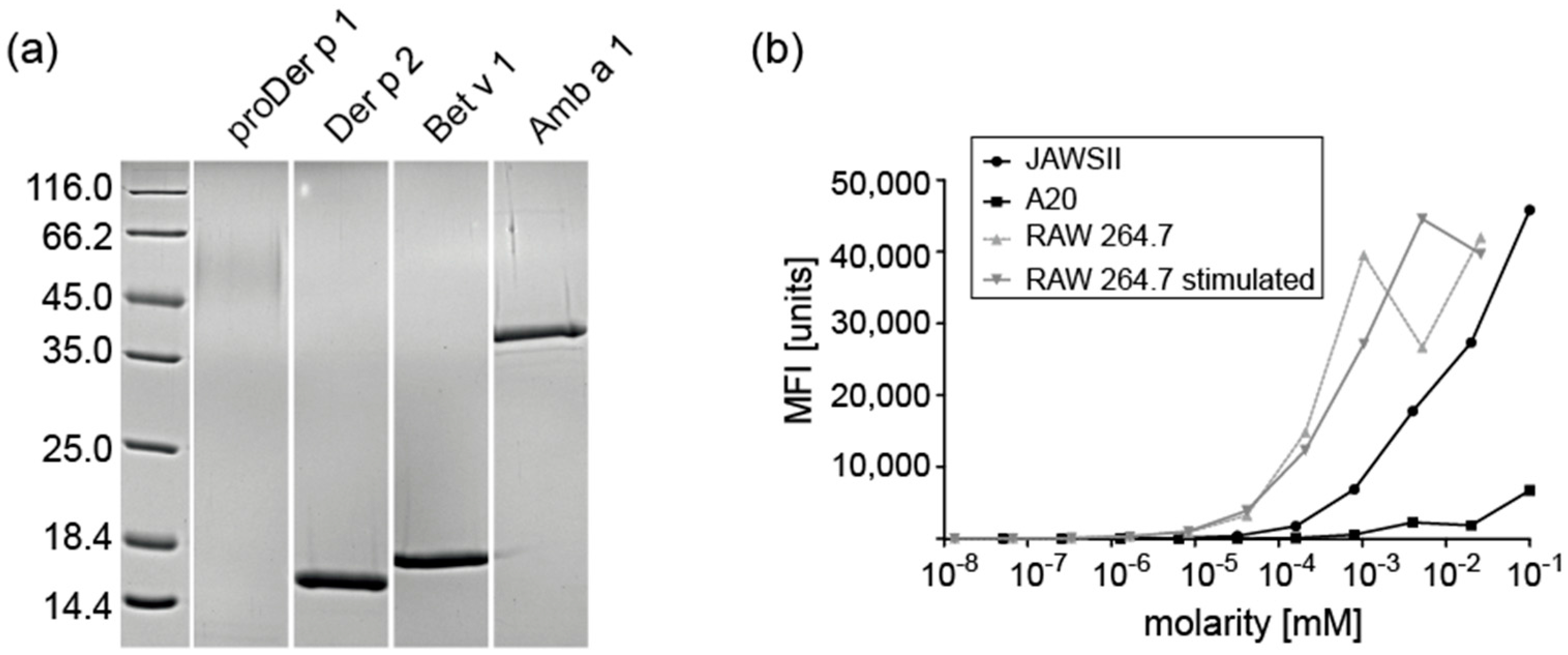
2.1. Expression and Purification of Recombinant Allergens
2.2. Subcellular Fractionation of Endolysosomes and Characterization of Proteolytic Activity
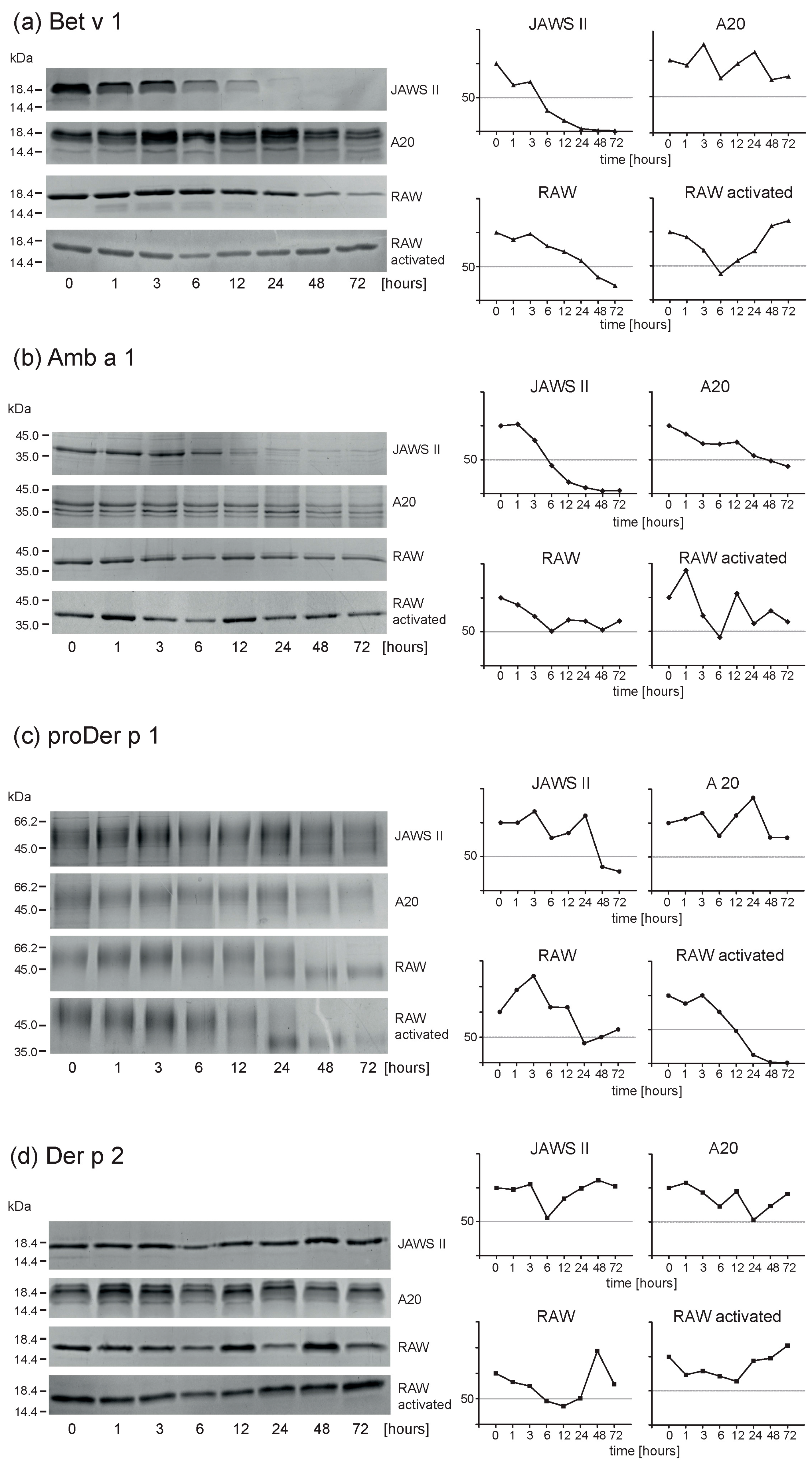
2.3. Endolysosomal Degradations of Inhalant Allergens
2.4. Activated Macrophages Display Identical Proteolysis Pattern as Resting Cells
2.5. Mass Spectrometric Analyses of Degradation Patterns Revealed No Differences Between Cell Lines
3. Discussion
4. Materials and Methods
4.1. Production of Recombinant Allergens
4.2. Cell Culture
4.3. Endolysosomal Fractionation
4.4. Determination of Proteolytic Activity
4.5. Degradation Assays
4.6. Mass Spectrometry
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| AEP | Asparaginyl endopeptidase |
| APC | Antigen-presenting cell |
| CD | Cluster of differentiation |
| CLIP | Class II-associated invariant chain peptide |
| DC | Dendritic cell |
| DTT | Dithiothreitol |
| FA | Formic acid |
| FCSi | Fetal calf serum (heat inactivated) |
| GM-CSF | Granulocyte macrophage—colony stimulating factor |
| HLA | Human leucocyte antigen |
| LPS | Lipopolysaccharide |
| MHC | Major histocompatibility complex |
| MS | Mass spectrometry |
| PAGE | Polyacrylamide gel electrophoresis |
| RFU | Relative fluorescent unit |
| SDS | Sodium dodecyl sulfate |
| TFA | Trifluoroacetic acid |
References
- Delamarre, L.; Pack, M.; Chang, H.; Mellman, I.; Trombetta, E.S. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science 2005, 307, 1630–1634. [Google Scholar] [CrossRef] [PubMed]
- Jensen, P.E. Recent advances in antigen processing and presentation. Nat. Immunol. 2007, 8, 1041–1048. [Google Scholar] [CrossRef] [PubMed]
- Roche, P.A.; Furuta, K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat. Rev. Immunol. 2015, 15, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Nitta, T.; Suzuki, H. Thymic stromal cell subsets for T cell development. Cell. Mol. Life Sci. 2016, 73, 1021–1037. [Google Scholar] [CrossRef] [PubMed]
- Valenta, R.; Ferreira, F.; Focke-Tejkl, M.; Linhart, B.; Niederberger, V.; Swoboda, I.; Vrtala, S. From allergen genes to allergy vaccines. Annu. Rev. Immunol. 2010, 28, 211–241. [Google Scholar] [CrossRef] [PubMed]
- Woodfolk, J.A. T cell responses to allergens. J. Allergy Clin. Immunol. 2007, 119, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Egger, M.; Jurets, A.; Wallner, M.; Briza, P.; Ruzek, S.; Hainzl, S.; Pichler, U.; Kitzmuller, C.; Bohle, B.; Huber, C.G.; et al. Assessing protein immunogenicity with a dendritic cell line-derived endolysosomal degradome. PLoS ONE 2011, 6, e17278. [Google Scholar] [CrossRef] [PubMed]
- Mutschlechner, S.; Egger, M.; Briza, P.; Wallner, M.; Lackner, P.; Karle, A.; Vogt, A.B.; Fischer, G.F.; Bohle, B.; Ferreira, F. Naturally processed T cell-activating peptides of the major birch pollen allergen. J. Allergy Clin. Immunol. 2010, 125, 711–718.e2. [Google Scholar] [CrossRef] [PubMed]
- Wallner, M.; Hauser, M.; Himly, M.; Zaborsky, N.; Mutschlechner, S.; Harrer, A.; Asam, C.; Pichler, U.; van Ree, R.; Briza, P.; et al. Reshaping the Bet v 1 fold modulates TH polarization. J. Allergy Clin. Immunol. 2011, 127, 1571–1578.e9. [Google Scholar] [CrossRef] [PubMed]
- Rozman-Pungercar, J.; Kopitar-Jerala, N.; Bogyo, M.; Turk, D.; Vasiljeva, O.; Stefe, I.; Vandenabeele, P.; Bromme, D.; Puizdar, V.; Fonovic, M.; et al. Inhibition of papain-like cysteine proteases and legumain by caspase-specific inhibitors: When reaction mechanism is more important than specificity. Cell Death Differ. 2003, 10, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Ghoneim, H.; Klinkert, M.Q. Biochemical properties of purified cathepsin B from Schistosoma mansoni. Int. J. Parasitol. 1995, 25, 1515–1519. [Google Scholar] [CrossRef]
- Huotari, J.; Helenius, A. Endosome maturation. EMBO J. 2011, 30, 3481–3500. [Google Scholar] [CrossRef] [PubMed]
- Jacquet, A.; Magi, M.; Petry, H.; Bollen, A. High-level expression of recombinant house dust mite allergen Der p 1 in Pichia pastoris. Clin. Exp. Allergy 2002, 32, 1048–1053. [Google Scholar] [CrossRef] [PubMed]
- Derewenda, U.; Li, J.; Derewenda, Z.; Dauter, Z.; Mueller, G.A.; Rule, G.S.; Benjamin, D.C. The crystal structure of a major dust mite allergen Der p 2, and its biological implications. J. Mol. Biol. 2002, 318, 189–197. [Google Scholar] [CrossRef]
- De Halleux, S.; Stura, E.; VanderElst, L.; Carlier, V.; Jacquemin, M.; Saint-Remy, J.M. Three-dimensional structure and IgE-binding properties of mature fully active Der p 1, a clinically relevant major allergen. J. Allergy Clin. Immunol. 2006, 117, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Chevigne, A.; Barumandzadeh, R.; Groslambert, S.; Cloes, B.; Dehareng, D.; Filee, P.; Marx, J.C.; Frere, J.M.; Matagne, A.; Jacquet, A.; et al. Relationship between propeptide pH unfolding and inhibitory ability during ProDer p 1 activation mechanism. J. Mol. Biol. 2007, 374, 170–185. [Google Scholar] [CrossRef] [PubMed]
- Chevigne, A.; Campizi, V.; Szpakowska, M.; Bourry, D.; Dumez, M.E.; Martins, J.C.; Matagne, A.; Galleni, M.; Jacquet, A. The Lys-Asp-Tyr triad within the mite allergen Der p 1 propeptide is a critical structural element for the pH-dependent initiation of the protease maturation. Int. J. Mol. Sci. 2017, 18, 1087. [Google Scholar] [CrossRef] [PubMed]
- Takai, T.; Mizuuchi, E.; Kikuchi, Y.; Nagamune, T.; Okumura, K.; Ogawa, H. Glycosylation of recombinant proforms of major house dust mite allergens Der p 1 and Der f 1 decelerates the speed of maturation. Int. Arch. Allergy Immunol. 2006, 139, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Bird, P.I.; Trapani, J.A.; Villadangos, J.A. Endolysosomal proteases and their inhibitors in immunity. Nat. Rev. Immunol. 2009, 9, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Watts, C.; Matthews, S.P.; Mazzeo, D.; Manoury, B.; Moss, C.X. Asparaginyl endopeptidase: Case history of a class II MHC compartment protease. Immunol. Rev. 2005, 207, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Manoury, B.; Mazzeo, D.; Fugger, L.; Viner, N.; Ponsford, M.; Streeter, H.; Mazza, G.; Wraith, D.C.; Watts, C. Destructive processing by asparagine endopeptidase limits presentation of a dominant T cell epitope in MBP. Nat. Immunol. 2002, 3, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Vaithilingam, A.; Lai, N.Y.; Duong, E.; Boucau, J.; Xu, Y.; Shimada, M.; Gandhi, M.; Le Gall, S. A simple methodology to assess endolysosomal protease activity involved in antigen processing in human primary cells. BMC Cell. Biol. 2013, 14, 35. [Google Scholar] [CrossRef] [PubMed]
- Hofer, H.; Asam, C.; Hauser, M.; Nagl, B.; Laimer, J.; Himly, M.; Briza, P.; Ebner, C.; Lang, R.; Hawranek, T.; et al. Tackling Bet v 1 and associated food allergies with a single hybrid protein. J. Allergy Clin. Immunol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Jahn-Schmid, B.; Wopfner, N.; Hubinger, G.; Asero, R.; Ebner, C.; Ferreira, F.; Bohle, B. The T-cell response to Amb a 1 is characterized by 3 dominant epitopes and multiple MHC restriction elements. J. Allergy Clin. Immunol. 2010, 126, 1068–1071. [Google Scholar] [CrossRef] [PubMed]
- Hoyne, G.; Bourne, T.; Kristensen, N.; Hetzel, C.; Lamb, J. From epitopes to peptides to immunotherapy. Clin. Immunol. Immunopathol. 1996, 80, S23–S30. [Google Scholar] [CrossRef] [PubMed]
- Crack, L.R.; Chan, H.W.; McPherson, T.; Ogg, G.S. Identification of an immunodominant region of the major house dust mite allergen Der p 2 presented by common human leucocyte antigen alleles. Clin. Exp. Dermatol. 2012, 37, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Neeno, T.; Krco, C.J.; Harders, J.; Baisch, J.; Cheng, S.; David, C.S. HLA-DQ8 transgenic mice lacking endogenous class II molecules respond to house dust allergens: Identification of antigenic epitopes. J. Immunol. 1996, 156, 3191–3195. [Google Scholar] [PubMed]
- O’Brien, R.M.; Thomas, W.R.; Nicholson, I.; Lamb, J.R.; Tait, B.D. An immunogenetic analysis of the T-cell recognition of the major house dust mite allergen Der p 2: Identification of high- and low-responder HLA-DQ alleles and localization of T-cell epitopes. Immunology 1995, 86, 176–182. [Google Scholar] [PubMed]
- Verhoef, A.; Higgins, J.A.; Thorpe, C.J.; Marsh, S.G.; Hayball, J.D.; Lamb, J.R.; O’Hehir, R.E. Clonal analysis of the atopic immune response to the group 2 allergen of Dermatophagoides spp.: Identification of HLA-DR and -DQ restricted T cell epitopes. Int. Immunol. 1993, 5, 1589–1597. [Google Scholar] [PubMed]
- O’Hehir, R.E.; Verhoef, A.; Panagiotopoulou, E.; Keswani, S.; Hayball, J.D.; Thomas, W.R.; Lamb, J.R. Analysis of human T cell responses to the group II allergen of Dermatophagoides species: Localization of major antigenic sites. J. Allergy Clin. Immunol. 1993, 92, 105–113. [Google Scholar] [CrossRef]
- Wu, B.; Elst, L.V.; Carlier, V.; Jacquemin, M.G.; Saint-Remy, J.M. The Dermatophagoides pteronyssinus group 2 allergen contains a universally immunogenic T cell epitope. J. Immunol. 2002, 169, 2430–2435. [Google Scholar] [CrossRef] [PubMed]
- Wambre, E.; Bonvalet, M.; Bodo, V.B.; Maillere, B.; Leclert, G.; Moussu, H.; von Hofe, E.; Louise, A.; Balazuc, A.M.; Ebo, D.; et al. Distinct characteristics of seasonal (Bet v 1) vs. Perennial (Der p 1/Der p 2) allergen-specific CD4+ T cell responses. Clin. Exp. Allergy 2011, 41, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Swoboda, I.; Jilek, A.; Ferreira, F.; Engel, E.; Hoffmann-Sommergruber, K.; Scheiner, O.; Kraft, D.; Breiteneder, H.; Pittenauer, E.; Schmid, E.; et al. Isoforms of Bet v 1, the major birch pollen allergen, analyzed by liquid chromatography, mass spectrometry, and cDNA cloning. J. Biol. Chem. 1995, 270, 2607–2613. [Google Scholar] [CrossRef] [PubMed]
- Chua, K.Y.; Huang, C.H.; Shen, H.D.; Thomas, W.R. Analysis of sequence polymorphism of a major mite allergen, Der p 2. Clin. Exp. Allergy 1996, 26, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Bond, J.F.; Garman, R.D.; Keating, K.M.; Briner, T.J.; Rafnar, T.; Klapper, D.G.; Rogers, B.L. Multiple Amb a I allergens demonstrate specific reactivity with IgE and T cells from ragweed-allergic patients. J. Immunol. 1991, 146, 3380–3385. [Google Scholar] [PubMed]
- Thomas, W.R. Hierarchy and molecular properties of house dust mite allergens. Allergol. Int. 2015, 64, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Kanellopoulos-Langevin, C.; Merwin, R.M.; Sachs, D.H.; Asofsky, R. Establishment and characterization of BALB/c lymphoma lines with B cell properties. J. Immunol. 1979, 122, 549–554. [Google Scholar] [PubMed]
- Raschke, W.C.; Baird, S.; Ralph, P.; Nakoinz, I. Functional macrophage cell lines transformed by abelson leukemia virus. Cell 1978, 15, 261–267. [Google Scholar] [CrossRef]
- Jiang, X.; Shen, C.; Rey-Ladino, J.; Yu, H.; Brunham, R.C. Characterization of murine dendritic cell line JAWS II and primary bone marrow-derived dendritic cells in Chlamydia muridarum antigen presentation and induction of protective immunity. Infect. Immun. 2008, 76, 2392–2401. [Google Scholar] [CrossRef] [PubMed]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]

© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofer, H.; Weidinger, T.; Briza, P.; Asam, C.; Wolf, M.; Twaroch, T.E.; Stolz, F.; Neubauer, A.; Dall, E.; Hammerl, P.; et al. Comparing Proteolytic Fingerprints of Antigen-Presenting Cells during Allergen Processing. Int. J. Mol. Sci. 2017, 18, 1225. https://doi.org/10.3390/ijms18061225
Hofer H, Weidinger T, Briza P, Asam C, Wolf M, Twaroch TE, Stolz F, Neubauer A, Dall E, Hammerl P, et al. Comparing Proteolytic Fingerprints of Antigen-Presenting Cells during Allergen Processing. International Journal of Molecular Sciences. 2017; 18(6):1225. https://doi.org/10.3390/ijms18061225
Chicago/Turabian StyleHofer, Heidi, Tamara Weidinger, Peter Briza, Claudia Asam, Martin Wolf, Teresa E. Twaroch, Frank Stolz, Angela Neubauer, Elfriede Dall, Peter Hammerl, and et al. 2017. "Comparing Proteolytic Fingerprints of Antigen-Presenting Cells during Allergen Processing" International Journal of Molecular Sciences 18, no. 6: 1225. https://doi.org/10.3390/ijms18061225
APA StyleHofer, H., Weidinger, T., Briza, P., Asam, C., Wolf, M., Twaroch, T. E., Stolz, F., Neubauer, A., Dall, E., Hammerl, P., Jacquet, A., & Wallner, M. (2017). Comparing Proteolytic Fingerprints of Antigen-Presenting Cells during Allergen Processing. International Journal of Molecular Sciences, 18(6), 1225. https://doi.org/10.3390/ijms18061225





