Reclassifying Anaphylaxis to Neuromuscular Blocking Agents Based on the Presumed Patho-Mechanism: IgE-Mediated, Pharmacological Adverse Reaction or “Innate Hypersensitivity”?
Abstract
:1. Introduction
2. Type A Adverse Drug Reactions Are Often Misinterpreted as Being “Allergic”
3. Type B Adverse Drug Reaction Can Be Predictable and Dose Dependent
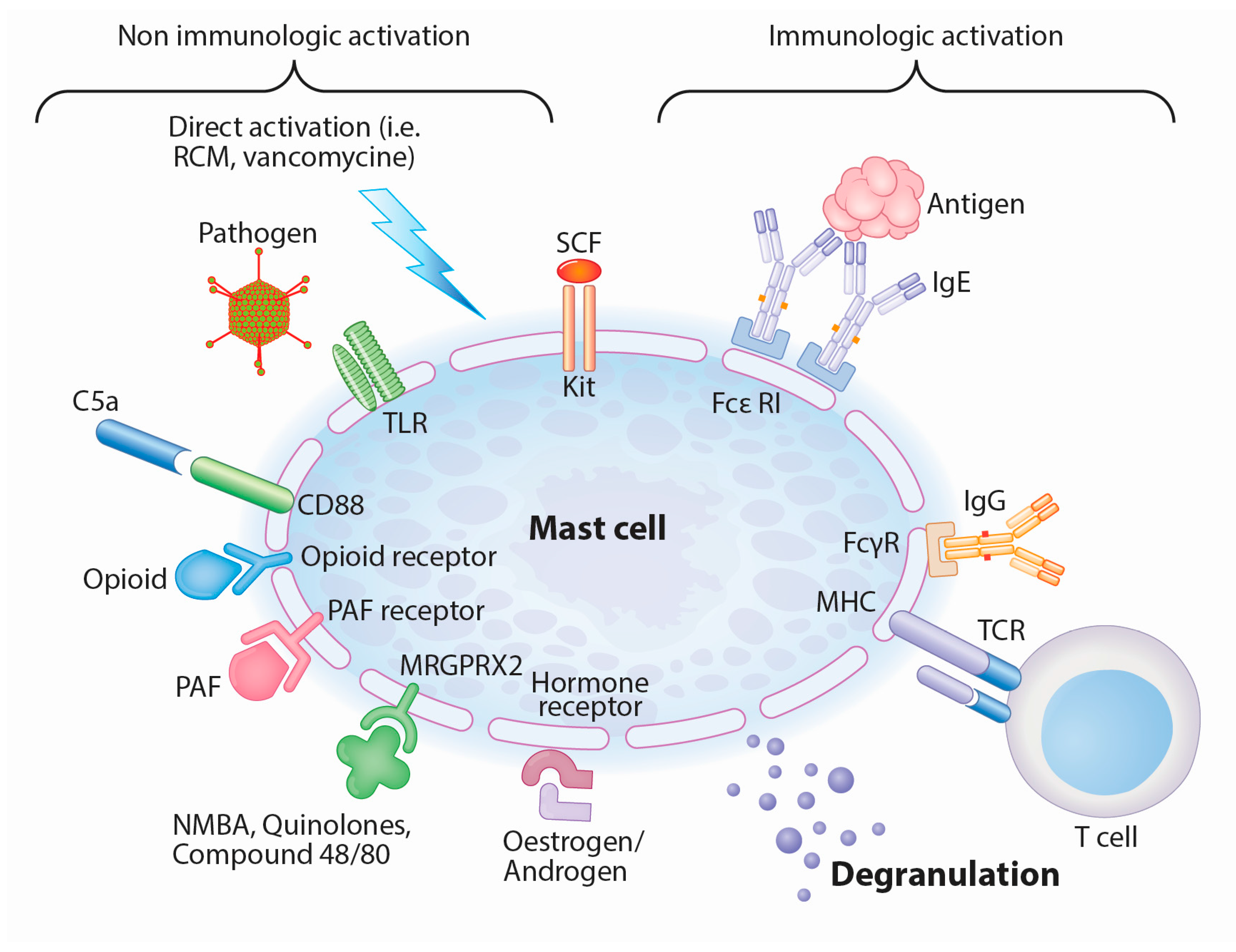
4. Mast Cells as Central Players in IgE-Mediated and Mas-Related G-Protein-Coupled Receptor Member X2-Mediated (MRGPRX2-Mediated) Anaphylactic Reactions
5. Mast Cells Can Be Stimulated by Various Co-Factors and Mas-related G-Protein-Coupled Receptor Member X2 (MRGPRX2) Receptor Activation
6. Anaphylactic Reactions upon First Exposure Might Be due to IgE Cross-Sensitization or a Pseudo-Allergic Reaction
7. The Supposed Involvement of the MRGPRX2 Receptor in Adverse Drug Reactions (ADR) to Neuromuscular Blocking Agent (NMBA) Leads to the Hypothesis of an Underlying “Innate Hypersensitivity”
8. Perioperative Anaphylaxis due to NMBA Revised
8.1. Skin Tests to NMBA Have to Be Evaluated with Caution
8.2. In Vitro Analysis of NMBA Anaphylaxis Shows Conflicting Results as to the Underlying Mechanism
9. Discussion
10. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| NMBA | Neuromuscular blocking agents |
| NSAID | Non steroidal anti-inflammatory drugs |
| EAACI | European Academy for Allergology and Clinical Immunology |
| MRGPRX2 | Mas-related G-protein coupled receptor member X2 |
| ADR | Adverse drug reactions |
| TLR(s) | Toll-like receptors |
| PAR(s) | Protease-activated receptors |
| FcεRI | High affinity IgE receptors |
| QA | Quaternary ammonium |
| BAT | Basophil activation test |
| DRESS | Drug reaction with Eosinophilia and systemic symptoms |
| RCM | Radiocontrast media |
| SCF | Stem cell factor |
| FcγR | IgG receptor |
| TCR | T-cell receptor |
| PAF | Platelet activating factor |
| MHC | Major histocompatibility complex |
References
- Ewan, P.W.; Dugué, P.; Mirakian, R.; Dixon, T.A.; Harper, J.N.; Nasser, S.M. BSACI BSACI guidelines for the investigation of suspected anaphylaxis during general anaesthesia. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2010, 40, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Mertes, P.M.; Laxenaire, M.-C.; Alla, F. Groupe d’Etudes des Réactions Anaphylactoïdes Peranesthésiques Anaphylactic and anaphylactoid reactions occurring during anesthesia in France in 1999–2000. Anesthesiology 2003, 99, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Farnam, K.; Chang, C.; Teuber, S.; Gershwin, M.E. Nonallergic drug hypersensitivity reactions. Int. Arch. Allergy Immunol. 2012, 159, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Czech, W.; Schöpf, E.; Kapp, A. Release of sulfidoleukotrienes in vitro: Its relevance in the diagnosis of pseudoallergy to acetylsalicylic acid. Inflamm. Res. Off. J. Eur. Histamine Res. Soc. 1995, 44, 291–295. [Google Scholar] [CrossRef]
- Mali, S. Anaphylaxis during the perioperative period. Anesth. Essays Res. 2012, 6, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Johansson, S.G.; Hourihane, J.O.; Bousquet, J.; Bruijnzeel-Koomen, C.; Dreborg, S.; Haahtela, T.; Kowalski, M.L.; Mygind, N.; Ring, J.; van Cauwenberge, P.; et al. EAACI (the European Academy of Allergology and Cinical Immunology) nomenclature task force A revised nomenclature for allergy. An EAACI position statement from the EAACI nomenclature task force. Allergy 2001, 56, 813–824. [Google Scholar] [CrossRef] [PubMed]
- De Pater, G.H.; Florvaag, E.; Johansson, S.G.O.; Irgens, Å.; Petersen, M.N.H.; Guttormsen, A.B. Six years without pholcodine; Norwegians are significantly less IgE-sensitized and clinically more tolerant to neuromuscular blocking agents. Allergy 2016. [Google Scholar] [CrossRef] [PubMed]
- Brusch, A.M.; Clarke, R.C.; Platt, P.R.; Phillips, E.J. Exploring the link between pholcodine exposure and neuromuscular blocking agent anaphylaxis. Br. J. Clin. Pharmacol. 2014, 78, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Krøigaard, M.; Garvey, L.H.; Menné, T.; Husum, B. Allergic reactions in anaesthesia: Are suspected causes confirmed on subsequent testing? Br. J. Anaesth. 2005, 95, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Celik, G.; Pichler, W.; Adkinson, N.F. Drug Allergy. In Middleton’s Allergy: Principles & Practice; Adkinson, N.F., Middleton, E., Eds.; Mosby/Elsevier: Philadelphia, PA, USA, 2009. [Google Scholar]
- McNeil, B.D.; Pundir, P.; Meeker, S.; Han, L.; Undem, B.J.; Kulka, M.; Dong, X. Identification of a mast-cell-specific receptor crucial for pseudo-allergic drug reactions. Nature 2015, 519, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Ebo, D.G.; Fisher, M.M.; Hagendorens, M.M.; Bridts, C.H.; Stevens, W.J. Anaphylaxis during anaesthesia: Diagnostic approach. Allergy 2007, 62, 471–487. [Google Scholar] [CrossRef] [PubMed]
- Dewachter, P.; Mouton-Faivre, C.; Emala, C.W. Anaphylaxis and anesthesia: Controversies and new insights. Anesthesiology 2009, 111, 1141–1150. [Google Scholar] [CrossRef] [PubMed]
- Rawlins, M.; Thompson, J. Pathogenesis of adverse drug reactions. In Textbook of Adverse Drug Reactions; Davies, D.M., Ed.; Oxford University Press: Oxford, UK; New York, NY, USA, 1977. [Google Scholar]
- Waller, D.G. Allergy, pseudo-allergy and non-allergy. Br. J. Clin. Pharmacol. 2011, 71, 637–638. [Google Scholar] [CrossRef] [PubMed]
- Edwards, I.R.; Aronson, J.K. Adverse drug reactions: Definitions, diagnosis, and management. Lancet Lond. Engl. 2000, 356, 1255–1259. [Google Scholar] [CrossRef]
- Pichler, W. Drug allergy: Classification and clinical features. In UpToDate; Adkinson, N.F., Feldweg, A.M., Eds.; UpToDate: Waltham, MA, USA, 2016. [Google Scholar]
- Yun, J.; Mattsson, J.; Schnyder, K.; Fontana, S.; Largiadèr, C.R.; Pichler, W.J.; Yerly, D. Allopurinol hypersensitivity is primarily mediated by dose-dependent oxypurinol-specific T cell response. Clin. Exp. Allergy 2013, 43, 1246–1255. [Google Scholar] [CrossRef] [PubMed]
- Stamp, L.K.; Taylor, W.J.; Jones, P.B.; Dockerty, J.L.; Drake, J.; Frampton, C.; Dalbeth, N. Starting dose is a risk factor for allopurinol hypersensitivity syndrome: A proposed safe starting dose of allopurinol. Arthritis Rheum. 2012, 64, 2529–2536. [Google Scholar] [CrossRef] [PubMed]
- Hennino, A.; Bérard, F.; Guillot, I.; Saad, N.; Rozières, A.; Nicolas, J.-F. Pathophysiology of urticaria. Clin. Rev. Allergy Immunol. 2006, 30, 3–11. [Google Scholar] [CrossRef]
- D’Andrea, M.R.; Rogahn, C.J.; Andrade-Gordon, P. Localization of protease-activated receptors-1 and -2 in human mast cells: Indications for an amplified mast cell degranulation cascade. Biotech. Histochem. 2000, 75, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, H.; Gupta, K.; Ali, H. Roles of Mas-related G protein-coupled receptor X2 on mast cell-mediated host defense, pseudoallergic drug reactions, and chronic inflammatory diseases. J. Allergy Clin. Immunol. 2016, 138, 700–710. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.S. Mast-cell responses to pathogens. Nat. Rev. Immunol. 2004, 4, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Zuberbier, T.; Pfrommer, C.; Specht, K.; Vieths, S.; Bastl-Borrmann, R.; Worm, M.; Henz, B.M. Aromatic components of food as novel eliciting factors of pseudoallergic reactions in chronic urticaria. J. Allergy Clin. Immunol. 2002, 109, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Bonadonna, P.; Pagani, M.; Aberer, W.; Bilò, M.B.; Brockow, K.; Oude Elberink, H.; Garvey, L.; Mosbech, H.; Romano, A.; Zanotti, R.; et al. Drug hypersensitivity in clonal mast cell disorders: ENDA/EAACI position paper. Allergy 2015, 70, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Zuberbier, T.; Asero, R.; Bindslev-Jensen, C.; Walter Canonica, G.; Church, M.K.; Giménez-Arnau, A.M.; Grattan, C.E.H.; Kapp, A.; Maurer, M.; Merk, H.F.; et al. Dermatology Section of the European Academy of Allergology and Clinical Immunology; Global Allergy and Asthma European Network; European Dermatology Forum; World Allergy Organization EAACI/GA (2) LEN/EDF/WAO guideline: Management of urticaria. Allergy 2009, 64, 1427–1443. [Google Scholar] [CrossRef] [PubMed]
- Zuberbier, T.; Chantraine-Hess, S.; Hartmann, K.; Czarnetzki, B.M. Pseudoallergen-free diet in the treatment of chronic urticaria. A prospective study. Acta Derm. Venereol. 1995, 75, 484–487. [Google Scholar] [PubMed]
- Dewachter, P.; Castells, M.C.; Hepner, D.L.; Mouton-Faivre, C. Perioperative management of patients with mastocytosis. Anesthesiology 2014, 120, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Baldo, B.A.; Fisher, M.M.; Pham, N.H. On the origin and specificity of antibodies to neuromuscular blocking (muscle relaxant) drugs: An immunochemical perspective. Clin. Exp. Allergy 2009, 39, 325–344. [Google Scholar] [CrossRef] [PubMed]
- Moss, J. Muscle relaxants and histamine release. Acta Anaesthesiol. Scand. Suppl. 1995, 106, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Claudius, C.; Garvey, L.H.; Viby-Mogensen, J. The undesirable effects of neuromuscular blocking drugs. Anaesthesia 2009, 64 (Suppl. S1), 10–21. [Google Scholar] [CrossRef] [PubMed]
- Florvaag, E.; Johansson, S.G.O.; Irgens, Å.; de Pater, G.H. IgE-sensitization to the cough suppressant pholcodine and the effects of its withdrawal from the Norwegian market. Allergy 2011, 66, 955–960. [Google Scholar] [CrossRef] [PubMed]
- Florvaag, E.; Johansson, S.G.O.; Oman, H.; Harboe, T.; Nopp, A. Pholcodine stimulates a dramatic increase of IgE in IgE-sensitized individuals. A pilot study. Allergy 2006, 61, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Kaminsky, E.B.; Kaul, V.; Paschall, J.; Church, D.M.; Bunke, B.; Kunig, D.; Moreno-De-Luca, D.; Moreno-De-Luca, A.; Mulle, J.G.; Warren, S.T.; et al. An evidence-based approach to establish the functional and clinical significance of copy number variants in intellectual and developmental disabilities. Genet. Med. 2011, 13, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.T.; Adam, M.P.; Aradhya, S.; Biesecker, L.G.; Brothman, A.R.; Carter, N.P.; Church, D.M.; Crolla, J.A.; Eichler, E.E.; Epstein, C.J.; et al. Consensus statement: Chromosomal microarray is a first-tier clinical diagnostic test for individuals with developmental disabilities or congenital anomalies. Am. J. Hum. Genet. 2010, 86, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, Y.; Lin, A.A.; Cavalli-Sforza, L.L.; Zhao, Z.; Su, B. Adaptive evolution of MRGX2, a human sensory neuron specific gene involved in nociception. Gene 2005, 352, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Nel, L.; Eren, E. Peri-operative anaphylaxis. Br. J. Clin. Pharmacol. 2011, 71, 647–658. [Google Scholar] [CrossRef] [PubMed]
- Galvão, V.R.; Giavina-Bianchi, P.; Castells, M. Perioperative anaphylaxis. Curr. Allergy Asthma Rep. 2014, 14, 452. [Google Scholar] [CrossRef] [PubMed]
- Laxenaire, M.C.; Mertes, P.M. Groupe d’Etudes des Réactions Anaphylactoïdes Peranesthésiques Anaphylaxis during anaesthesia. Results of a two-year survey in France. Br. J. Anaesth. 2001, 87, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.M.; Munro, I. Life-threatening anaphylactoid reactions to muscle relaxants. Anesth. Anal. 1983, 62, 559–564. [Google Scholar] [CrossRef]
- Spoerl, D.; D’Incau, S.; Roux-Lombard, P.; Harr, T.; Czarnetzki, C. Non-IgE-Dependent Hypersensitivity to Rocuronium Reversed by Sugammadex: Report of Three Cases and Hypothesis on the Underlying Mechanism. Int. Arch. Allergy Immunol. 2016, 169, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.M.; Merefield, D.; Baldo, B. Failure to prevent an anaphylactic reaction to a second neuromuscular blocking drug during anaesthesia. Br. J. Anaesth. 1999, 82, 770–773. [Google Scholar] [CrossRef] [PubMed]
- Leysen, J.; Uyttebroek, A.; Sabato, V.; Bridts, C.H.; de Clerck, L.S.; Ebo, D.G. Predictive value of allergy tests for neuromuscular blocking agents: Tackling an unmet need. Clin. Exp. Allergy 2014, 44, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Hagau, N.; Gherman-Ionica, N.; Sfichi, M.; Petrisor, C. Threshold for basophil activation test positivity in neuromuscular blocking agents hypersensitivity reactions. Allergy Asthma Clin. Immunol. 2013, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.W.; Mertes, P.M.; Petitpain, N.; Hasdenteufel, F.; Malinovsky, J.M. GERAP Hypersensitivity reactions during anesthesia. Results from the ninth French survey (2005–2007). Minerva Anestesiol. 2012, 78, 868–878. [Google Scholar] [PubMed]
- Mertes, P.-M.; Laxenaire, M.-C. GERAP (Anaphylactic and anaphylactoid reactions occurring during anaesthesia in France. Seventh epidemiologic survey (January 2001–December 2002)). Ann. Fr. Anesth. Reanim. 2004, 23, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Harboe, T.; Guttormsen, A.B.; Irgens, A.; Dybendal, T.; Florvaag, E. Anaphylaxis during anesthesia in Norway: A 6-year single-center follow-up study. Anesthesiology 2005, 102, 897–903. [Google Scholar] [CrossRef] [PubMed]
- Karila, C.; Brunet-Langot, D.; Labbez, F.; Jacqmarcq, O.; Ponvert, C.; Paupe, J.; Scheinmann, P.; de Blic, J. Anaphylaxis during anesthesia: Results of a 12-year survey at a French pediatric center. Allergy 2005, 60, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Kvedariene, V.; Kamey, S.; Ryckwaert, Y.; Rongier, M.; Bousquet, J.; Demoly, P.; Arnoux, B. Diagnosis of neuromuscular blocking agent hypersensitivity reactions using cytofluorimetric analysis of basophils. Allergy 2006, 61, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.Y.; Caballero, M.R.; Lukawska, J.; Dugué, P. Anaphylaxis during general anaesthesia: One-year survey from a British allergy clinic. Singap. Med. J. 2008, 49, 483–487. [Google Scholar]
- Laroche, D.; Chollet-Martin, S.; Léturgie, P.; Malzac, L.; Vergnaud, M.-C.; Neukirch, C.; Venemalm, L.; Guéant, J.-L.; Roland, P.N. Evaluation of a new routine diagnostic test for immunoglobulin E sensitization to neuromuscular blocking agents. Anesthesiology 2011, 114, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Aalberse, R.C.; Kleine Budde, I.; Mulder, M.; Stapel, S.O.; Paulij, W.; Leynadier, F.; Hollmann, M.W. Differentiating the cellular and humoral components of neuromuscular blocking agent-induced anaphylactic reactions in patients undergoing anaesthesia. Br. J. Anaesth. 2011, 106, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, L.F.; Pereira, A.; Chiriac, A.M.; Bonnet-Boyer, M.-C.; Demoly, P. Negative predictive value of skin tests to neuromuscular blocking agents. Allergy 2012, 67, 439–441. [Google Scholar] [CrossRef] [PubMed]
- Antunes, J.; Kochuyt, A.-M.; Ceuppens, J.L. Perioperative allergic reactions: Experience in a Flemish referral centre. Allergol. Immunopathol. Madr. 2014, 42, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Krishna, M.T.; York, M.; Chin, T.; Gnanakumaran, G.; Heslegrave, J.; Derbridge, C.; Huissoon, A.; Diwakar, L.; Eren, E.; Crossman, R.J.; et al. Multi-centre retrospective analysis of anaphylaxis during general anaesthesia in the United Kingdom: Aetiology and diagnostic performance of acute serum tryptase. Clin. Exp. Immunol. 2014, 178, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Soetens, F.M.; Smolders, F.J.; Meeuwis, H.C.; Van der Donck, A.G.; van der Aa, P.H.; De Vel, M.A.; Vanhoof, M.J.; Soetens, M.A. Intradermal skin testing in the investigation of suspected anaphylactic reactions during anaesthesia—A retrospective survey. Acta Anaesthesiol. Belg. 2003, 54, 59–63. [Google Scholar] [PubMed]
- Hagau, N.; Gherman, N.; Cocis, M.; Petrisor, C. Antibiotic-induced immediate type hypersensitivity is a risk factor for positive allergy skin tests for neuromuscular blocking agents. Allergol. Int. 2016, 65, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Berg, C.M.; Heier, T.; Wilhelmsen, V.; Florvaag, E. Rocuronium and cisatracurium-positive skin tests in non-allergic volunteers: Determination of drug concentration thresholds using a dilution titration technique. Acta Anaesthesiol. Scand. 2003, 47, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Reddy, J.I.; Cooke, P.J.; van Schalkwyk, J.M.; Hannam, J.A.; Fitzharris, P.; Mitchell, S.J. Anaphylaxis is more common with rocuronium and succinylcholine than with atracurium. Anesthesiology 2015, 122, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Veien, M.; Szlam, F.; Holden, J.T.; Yamaguchi, K.; Denson, D.D.; Levy, J.H. Mechanisms of nonimmunological histamine and tryptase release from human cutaneous mast cells. Anesthesiology 2000, 92, 1074–1081. [Google Scholar] [CrossRef] [PubMed]
- Marone, G.; Stellato, C.; Mastronardi, P.; Mazzarella, B. Mechanisms of activation of human mast cells and basophils by general anesthetic drugs. Ann. Fr. Anesth. Reanim. 1993, 12, 116–125. [Google Scholar] [CrossRef]
- Uyttebroek, A.P.; Sabato, V.; Leysen, J.; Bridts, C.H.; De Clerck, L.S.; Ebo, D.G. Flowcytometric diagnosis of atracurium-induced anaphylaxis. Allergy 2014, 69, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Leysen, J.; de Witte, L.; Sabato, V.; Faber, M.; Hagendorens, M.; Bridts, C.; de Clerck, L.; Ebo, D. IgE-mediated allergy to pholcodine and cross-reactivity to neuromuscular blocking agents: Lessons from flow cytometry. Cytom. B Clin. Cytom. 2013, 84, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Fisher, M.M.; Baldo, B.A. Immunoassays in the diagnosis of anaphylaxis to neuromuscular blocking drugs: The value of morphine for the detection of IgE antibodies in allergic subjects. Anaesth. Intensive Care 2000, 28, 167–170. [Google Scholar] [PubMed]
- Decuyper, I.I.; Ebo, D.G.; Uyttebroek, A.P.; Hagendorens, M.M.; Faber, M.A.; Bridts, C.H.; de Clerck, L.S.; Sabato, V. Quantification of specific IgE antibodies in immediate drug hypersensitivity: More shortcomings than potentials? Clin. Chim. Acta 2016, 460, 184–189. [Google Scholar] [CrossRef] [PubMed]
- Baldo, B.A.; Fisher, M.M. Substituted ammonium ions as allergenic determinants in drug allergy. Nature 1983, 306, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Ebo, D.G.; Bridts, C.H.; Hagendorens, M.M.; Mertens, C.H.; de Clerck, L.S.; Stevens, W.J. Flow-assisted diagnostic management of anaphylaxis from rocuronium bromide. Allergy 2006, 61, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Sabato, V.; van Gasse, A.; Cop, N.; Claesen, K.; Decuyper, I.I.; Faber, M.A.; Bridts, C.; Mertens, C.; Hagendorens, M.; de Clerck, L.; et al. The Mas-Related G Protein-Coupled Receptor MRGPRX2 Is Expressed on Human Basophils and up-Regulated upon Activation. In Proceedings of the AAAAI Conference, Atlanta, GA, USA, 3–6 March 2017. Abstract 536. [Google Scholar]
- Johansson, S.G.O.; Oman, H.; Degerbeck, F.; Tunelli, J.; Florvaag, E.; Nopp, A. Anaphylaxis to atracurium—A non-QAI-dependent reaction? Acta Anaesthesiol. Scand. 2012, 56, 262–263. [Google Scholar] [CrossRef] [PubMed]
- Uyttebroek, A.P.; Sabato, V.; Bridts, C.H.; De Clerck, L.S.; Ebo, D.G. Immunoglobulin E antibodies to atracurium: A new diagnostic tool? Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2015, 45, 485–487. [Google Scholar] [CrossRef] [PubMed]
- Naguib, M.; Samarkandi, A.H.; Bakhamees, H.S.; Magboul, M.A.; El-Bakry, A.K. Histamine-release haemodynamic changes produced by rocuronium, vecuronium, mivacurium, atracurium and tubocurarine. Br. J. Anaesth. 1995, 75, 588–592. [Google Scholar] [CrossRef] [PubMed]
| Pseudo-Allergic (Mas-Related G-Protein-Coupled Receptor Member X2 (MRGPRX2) Activation) | Non-IgE Mediated, Immunologic Activation (IgG, rarely Described to be Involved in Immediate Type Reaction) | Non-IgE Mediated, Non Immunologic Activation (i.e., Opioid, Complement) | IgE Mediated | Non-Allergic (Immune System Not Primary Involved) | |
|---|---|---|---|---|---|
| Mast cell involvement | Yes | Yes | Yes | Yes | No |
| Skin test (immediate reading) | Positive | Negative | Positive | Positive | Negative |
| Specific IgE | Can be positive without clinical relevance | Can be positive without clinical relevance | Can be positive without clinical relevance | Presumably positive | Can be positive without clinical relevance |
| Basophil activation test (BAT) | Can be positive without clinical relevance | Presumably negative | For most negative | Presumably positive | Negative |
| Could explain reaction after first time exposure | Yes | No, except if previous sensitization by cross-reactivity | Yes | No, except if previous sensitization by cross-reactivity | Yes |
| Dose dependency | Yes | Probably | Yes | Classically no, marginally significant | Yes |
| Adverse drug reactions (ADR) Classification | New proposal: type A | Type B | Type B | Type B | Type A |
| Re-administration possible | Theoretically possible, with reduced speed or lower doses. No data available yet | Theoretically not recommended | Theoretically not recommended | Not recommended (consider desensitization protocol) | Yes, with reduced speed or lower doses if not pharmacologically contraindicated |
| Title | Relevant Data and Remarks |
|---|---|
| Anaphylactic and anaphylactoid reactions occurring during anesthesia in France in 1999–2000 [2]. | Anaphylactic and anaphylactoid reactions were diagnosed in 518 cases (66%) and 271 cases (34%), respectively. The most common causes of anaphylaxis were NMBA (n = 306, 58.2%). Anaphylaxis was diagnosed on the basis of clinical history, skin tests, and/or specific immunoglobulin E assay. In case of negative tests, an anaphylactoid reaction was diagnosed. |
| Anaphylactic and anaphylactoid reactions occurring during anaesthesia in France. Seventh epidemiologic survey (January 2001–December 2002) [46]. | Anaphylactic and anaphylactoid reactions were diagnosed in 491 cases (69%) and 221 cases (31%), respectively. The most common causes of anaphylaxis were NMBA (n = 271, 55%). Anaphylaxis was diagnosed on the basis of clinical history if skin tests were positive or in case of elevated tryptase values and the presence of specific IgE. In case of negative tests, an anaphylactoid reaction was diagnosed. |
| Anaphylaxis during Anesthesia in Norway [47]. | Eighty-three cases were examined: IgE–mediated anaphylaxis was established in 71.1% of the cases, and NMBA were by far the most frequent culprit drug (93.2%). IgE-mediated anaphylaxis was identified based on a modified categorization grading of causality of the IgE-mediated reactions (investigated by skin prick test, intradermal test, histamine releasing test, specific IgE against morphine and P-aminophenyl phosphoryl choline) |
| Anaphylaxis during anesthesia: results of a 12-year survey at a French pediatric center [48]. | Out of 68 adverse reactions, IgE-mediated anaphylaxis was diagnosed in 51 children: 31 (60.8%) for NMBA, 14 (27%) for latex, seven (14%) for colloids, five (9%) for opioids and six (12%) for hypnotics. IgE-mediated anaphylaxis was diagnosed on the basis of the skin tests results concordant with the patients’ clinical history of adverse reactions and the anesthetic protocol. |
| Diagnosis of NMBA hypersensitivity reactions using cytofluorimetric analysis of basophils [49]. | In 47 NMBA allergic patients, cytofluorimetric analysis of basophils was positive in 17 subjects. The diagnosis of allergy to NMBA was established from a characteristic clinical history (urticaria, bronchospasm and/or anaphylactic shock a few minutes after the start of anesthesia) and the positivity of NMBA skin tests. |
| Anaphylaxis during general anaesthesia: one-year survey from a British allergy clinic [50]. | Out of the 23 patients who presented with anaphylaxis during anesthesia, 15 patients were found to have a positive skin test to at least one NMBA. |
| Evaluation of a new routine diagnostic test for IgE sensitization to NMBA [51]. | In 168 patients exposed to NMBA, quaternary ammonium (QA)-specific IgE was found in 84.2% of skin test-positive reactors. The frequency of QA-specific IgE positivity was significantly higher in skin test-negative reactors (24.6%) than in controls (9.3%), suggesting NMBA sensitivity. |
| Differentiating the cellular and humoral components of neuromuscular blocking agent-induced anaphylactic reactions in patients undergoing anaesthesia [52]. | On the basis of intradermal skin testing and clinical evaluation, allergy to NMBA was considered likely in 48 of 61 patients (79%). Correlation between skin test reactivity to rocuronium and IgE to rocuronium was low. In contrast, striking correlation between IgE to rocuronium and skin test reactivity to succinylcholine was found (p < 0.001). |
| IgE-sensitization to the cough suppressant pholcodine and the effects of its withdrawal from the Norwegian market [32]. | Methods used to identify NMBA induced anaphylaxis are not reported. Decrease of perioperative anaphylaxis after pholcodine withdrawal was noted. However, the total amount of NMBA usage was not reported. |
| Negative predictive value of skin tests to NMBA [53]. | 55 patients were diagnosed with an allergy to NMBA, confirmed by clinical history, presence of specific IgE and/or positive skin test. 19 of these 55 patients had a second general anesthesia, 13 without NMBA and 6 using an NMBA for which skin tests were negative. None had had a new reaction to the injected NMBA. |
| Hypersensitivity reactions during anesthesia. Results from the ninth French survey (2005–2007) [45]. | An IgE-mediated or non-IgE-mediated reaction was diagnosed in 786 cases (63%) and 467 cases (37%), respectively. The most common causes of anaphylaxis were NMBA (N = 373, 47.4%). Allergic or IgE-mediated anaphylaxis was diagnosed on the basis of skin test and/or IgE assay results consistent with the clinical history and the anesthetic protocol. |
| Perioperative allergic reactions: experience in a Flemish referral centre [54]. | Out of 119 patients, a diagnosis of IgE-mediated reaction was established by skin tests and/or specific IgE in 76 cases (63.9%). The most common agents were NMBA (61.8%). The remaining 43 cases (36.1%) were considered as non-IgE-mediated reactions. |
| Predictive value of allergy tests for NMBA: tackling an unmet need [43]. | 272 patients with a history of perioperative allergy who had received a NMBA were reported. From the 47 patients who were re-exposed to a NMBA, 19 were initially diagnosed with suspected NMBA allergy, 13 had another IgE-mediated allergy suspected, and in the remainder 15, no IgE-mediated allergy was identified (skin test, specific IgE and BAT were used). Negative skin test and negative BAT assisted the selection of alternative NMBA, which were well tolerated in all cases. |
| Multi-centre retrospective analysis of anaphylaxis during general anaesthesia in the United Kingdom: aetiology and diagnostic performance of acute serum tryptase [55]. | In 161 patients, an IgE-mediated cause was identified in 103 patients (64%); NMBA constituted the leading cause (38%). IgE-mediated reactions were diagnosed based on skin prick test [n = 25 (24%)], intradermal test (n = 68 (66%)), serum-specific IgE (n = 9 (9%)) and challenge tests (n = 3 (1%)). |
| Six years without pholcodine; Norwegians are significantly less IgE-sensitized and clinically more tolerant to NMBA [7]. | Five to 10 years after pholcodine withdrawal, very few, if any, individuals were IgE-sensitized to QA ion, and only one case of NMBA-related anaphylaxis per 1–2 years was reported. However, exposure decreased during time of observation and less suxamethonium and more rocuronium were used. |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spoerl, D.; Nigolian, H.; Czarnetzki, C.; Harr, T. Reclassifying Anaphylaxis to Neuromuscular Blocking Agents Based on the Presumed Patho-Mechanism: IgE-Mediated, Pharmacological Adverse Reaction or “Innate Hypersensitivity”? Int. J. Mol. Sci. 2017, 18, 1223. https://doi.org/10.3390/ijms18061223
Spoerl D, Nigolian H, Czarnetzki C, Harr T. Reclassifying Anaphylaxis to Neuromuscular Blocking Agents Based on the Presumed Patho-Mechanism: IgE-Mediated, Pharmacological Adverse Reaction or “Innate Hypersensitivity”? International Journal of Molecular Sciences. 2017; 18(6):1223. https://doi.org/10.3390/ijms18061223
Chicago/Turabian StyleSpoerl, David, Haig Nigolian, Christoph Czarnetzki, and Thomas Harr. 2017. "Reclassifying Anaphylaxis to Neuromuscular Blocking Agents Based on the Presumed Patho-Mechanism: IgE-Mediated, Pharmacological Adverse Reaction or “Innate Hypersensitivity”?" International Journal of Molecular Sciences 18, no. 6: 1223. https://doi.org/10.3390/ijms18061223






