The Value of In Vitro Tests to Diminish Drug Challenges
Abstract
:1. Introduction
2. In Vitro Tests for the Diagnosis of Drug Hypersensitivity Reactions (DHR)
3. IgE-Mediated Reactions
3.1. Immunoassays
3.2. Basophil Activation Test
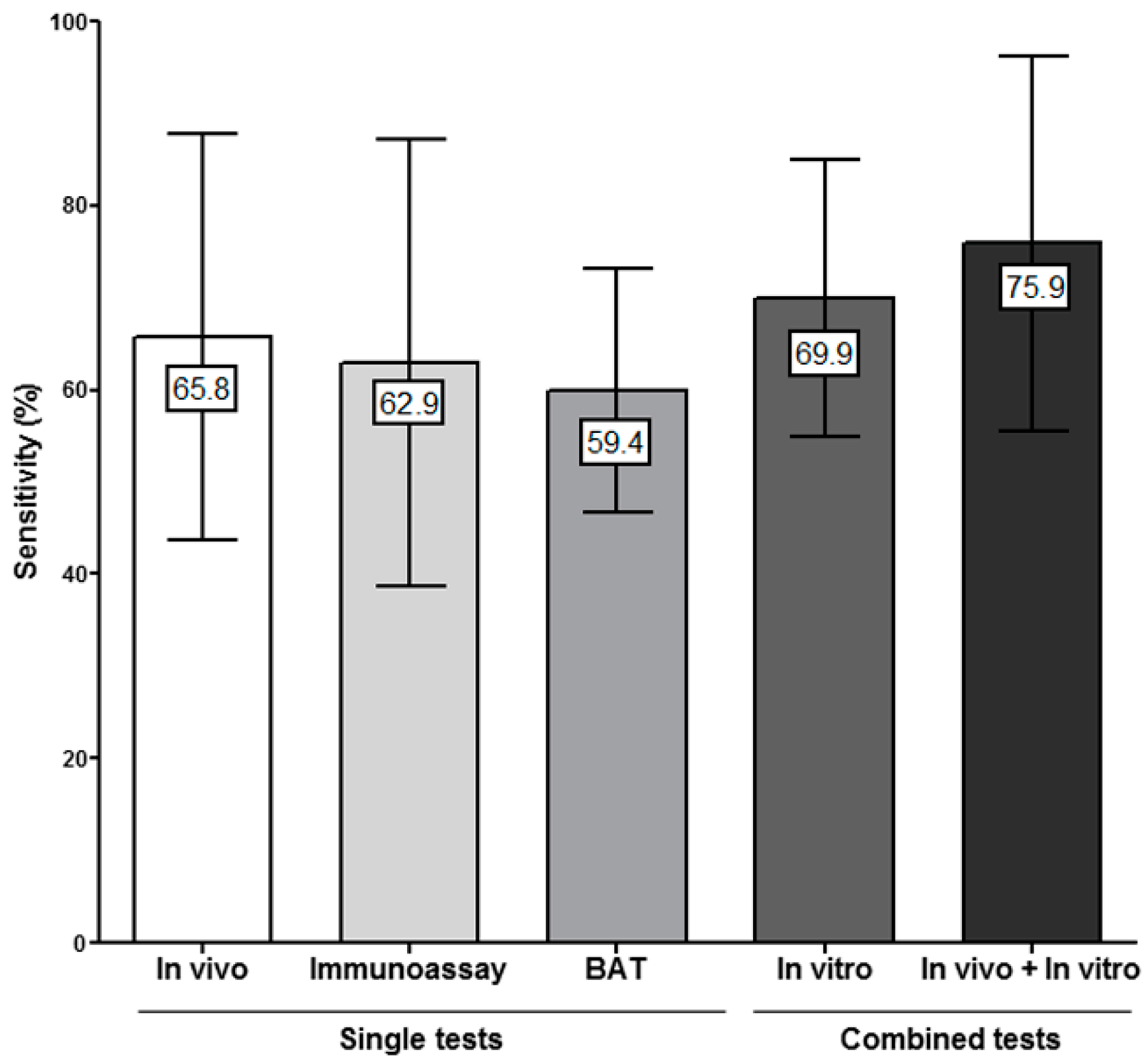
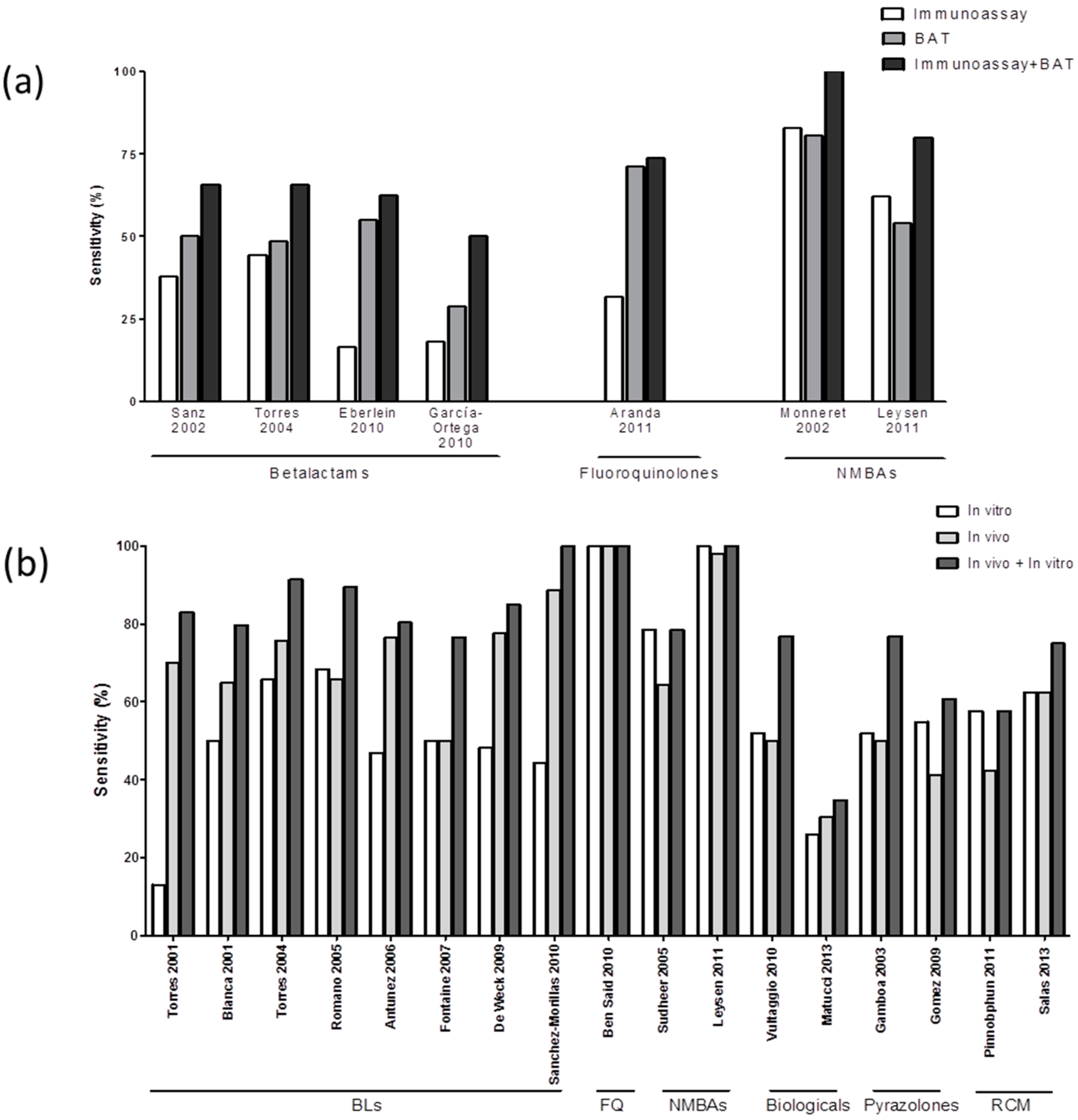
3.3. Combining In Vitro Tests for Evaluating Immediate Drug Hypersensitivity Reactions (IDHR)
3.4. Combining In Vitro and In Vivo Tests for Evaluating IDHR
4. T Cell-Mediated Reactions
4.1. Lymphocyte Transformation Test (LTT)
4.2. Enzyme-Linked Immunosorbent Spot (ELISpot)
4.3. Flow Cytometry and Enzyme-Linked Immunosorbent Assay (ELISA)
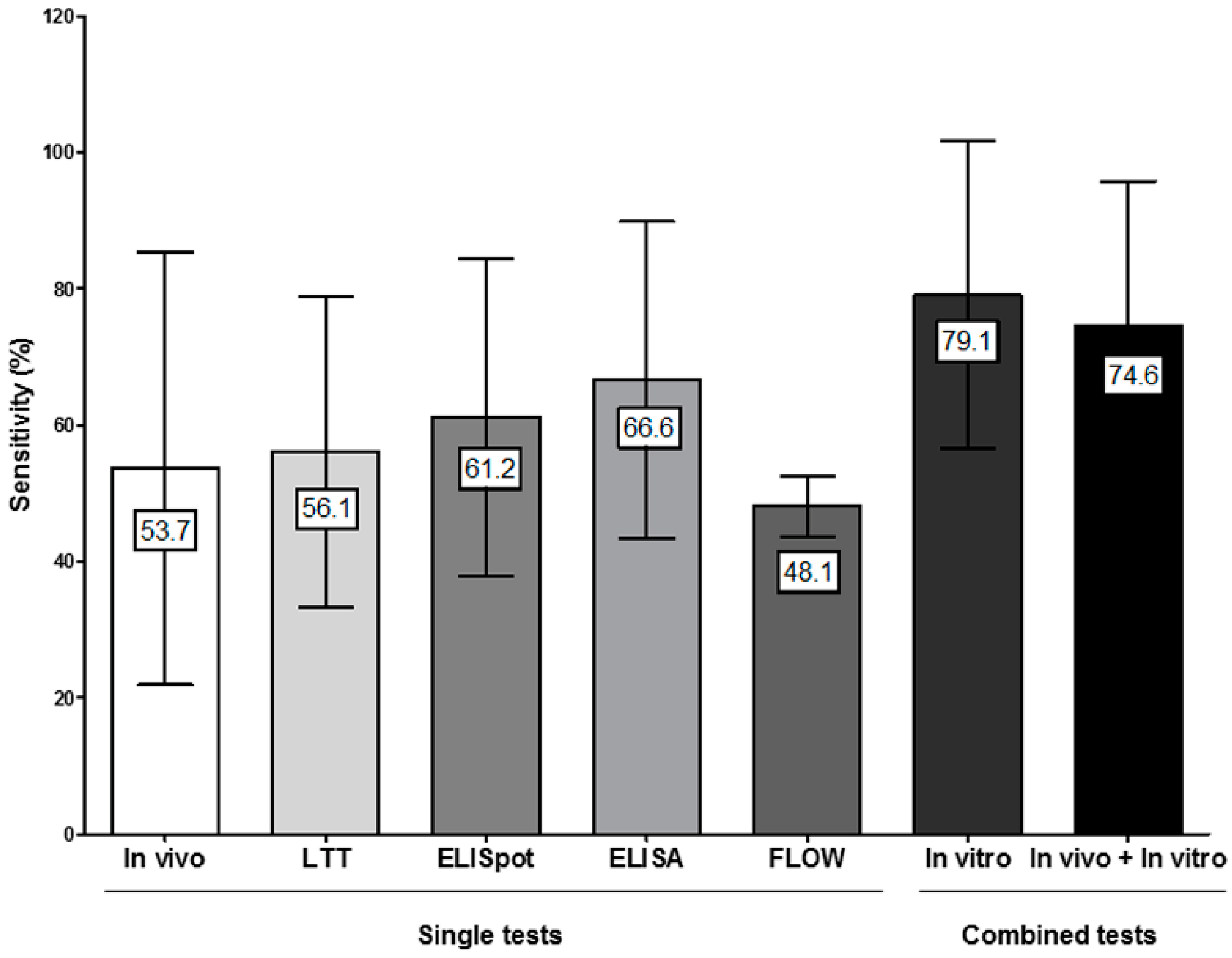
4.4. Combining In Vitro Tests for Evaluating Non-Immediate Drug Hypersensitivity Reactions (NIDHR)
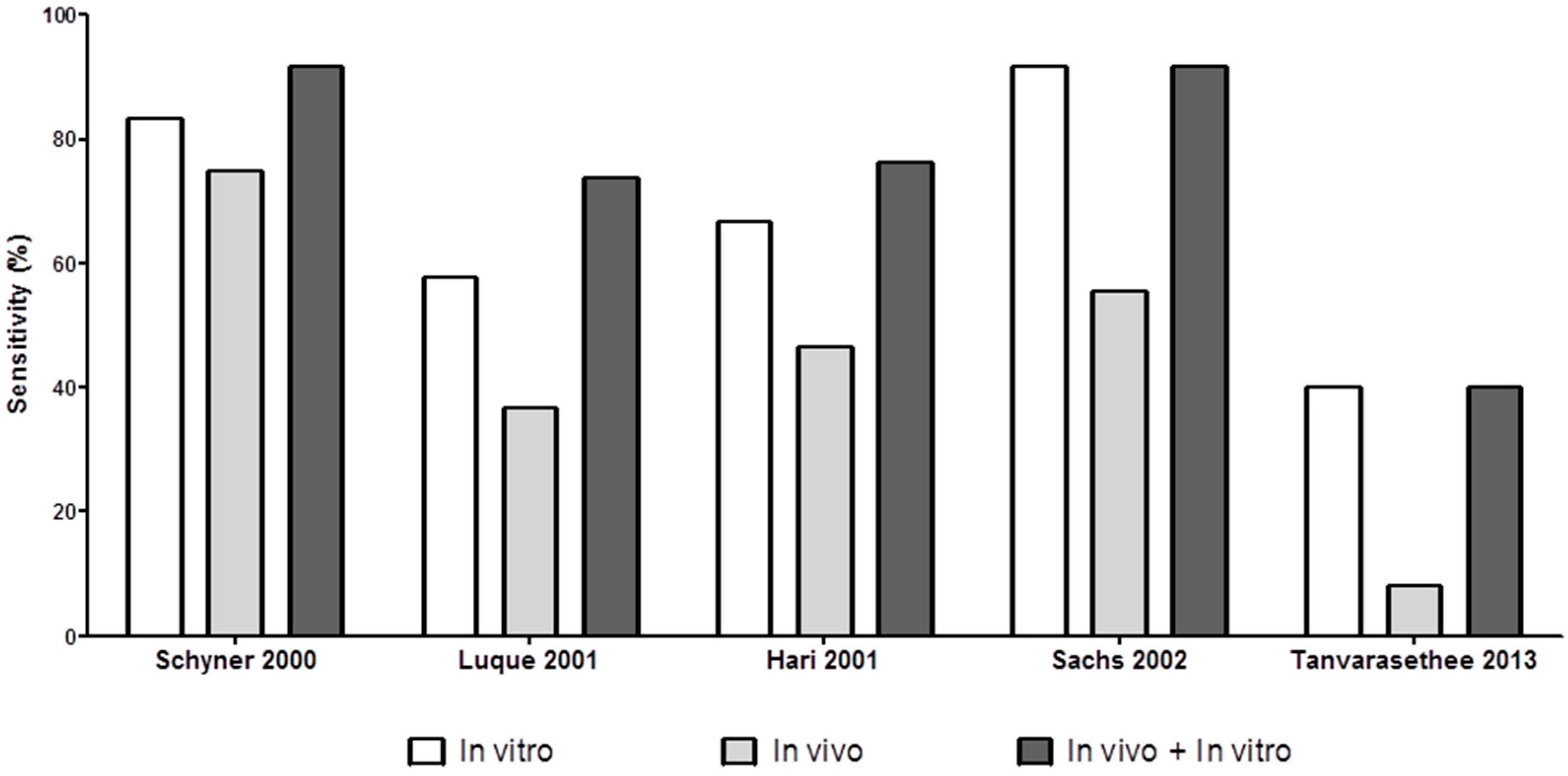
4.5. Combining In Vitro and In Vivo Tests for Evaluating NIDHR
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| DHR | Drug Hypersensitivity Reactions |
| SJS | Steven–Johnson Syndrome |
| TEN | Toxic Epidermal Necrolysis |
| IDHR | Immediate DH |
| sIgE | Specific IgE |
| NIDHR | Non-Immediate DHR |
| STs | Skin Tests |
| DPT | Drug Provocation Tests |
| BLs | β-Lactam |
| RCM | Radio Contrast Media |
| BAT | Basophil Activation Test |
| LTT | Lymphocyte Transformation Tests |
| ELISpot | Enzyme-Linked Immunosorbent Spot Assay |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| PPV | Positive Predictive Value |
| NPV | Negative Predictive Value |
| RAST | Radioallergosorbent Test |
| NMBAs | Neuromuscular Blocking Agents |
| FQs | Fluoroquinolones |
| NSAIDs | Non-Steroidal Anti-Inflammatory Drugs |
| CFSE | Carboxyfluorescein Diacetate Succinimidyl Ester |
References
- Dona, I.; Blanca-Lopez, N.; Torres, M.J.; Garcia-Campos, J.; Garcia-Nunez, I.; Gomez, F.; Salas, M.; Rondon, C.; Canto, M.G.; Blanca, M. Drug hypersensitivity reactions: Response patterns, drug involved, and temporal variations in a large series of patients. J. Investig. Allergol. Clin. Immunol. 2012, 22, 363–371. [Google Scholar] [PubMed]
- Macy, E.; Contreras, R. Health care use and serious infection prevalence associated with penicillin “allergy” in hospitalized patients: A cohort study. J. Allergy Clin. Immunol. 2014, 133, 790–796. [Google Scholar] [CrossRef] [PubMed]
- Coombs, R.R.A. Classification of allergic reactions responsible for clinical hypersensitivity and disease. In Clinical Aspects of Immunology; Gell, P.G.H., Ed.; Oxford University Press: Oxford, UK, 1968; pp. 575–596. [Google Scholar]
- Pichler, W.J. Delayed drug hypersensitivity reactions. Ann. Int. Med. 2003, 139, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Demoly, P.; Kropf, R.; Bircher, A.; Pichler, W.J. Drug hypersensitivity: Questionnaire. EAACI interest group on drug hypersensitivity. Allergy 1999, 54, 999–1003. [Google Scholar] [CrossRef] [PubMed]
- Brockow, K.; Romano, A.; Blanca, M.; Ring, J.; Pichler, W.; Demoly, P. General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy 2002, 57, 45–51. [Google Scholar] [PubMed]
- Demoly, P.; Bousquet, J. Drug allergy diagnosis work up. Allergy 2002, 57 (Suppl. S72), 37–40. [Google Scholar] [CrossRef] [PubMed]
- Benahmed, S.; Picot, M.C.; Dumas, F.; Demoly, P. Accuracy of a pharmacovigilance algorithm in diagnosing drug hypersensitivity reactions. Arch. Int. Med. 2005, 165, 1500–1505. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.J.; Romano, A.; Celik, G.; Demoly, P.; Khan, D.A.; Macy, E.; Park, M.; Blumenthal, K.; Aberer, W.; Castells, M.; et al. Approach to the diagnosis of drug hypersensitivity reactions: Similarities and differences between Europe and North America. Clin. Transl. Allergy 2017, 7, 7. [Google Scholar] [CrossRef] [PubMed]
- Aberer, W.; Bircher, A.; Romano, A.; Blanca, M.; Campi, P.; Fernandez, J.; Brockow, K.; Pichler, W.J.; Demoly, P.; European Network for Drug Allergy. Drug provocation testing in the diagnosis of drug hypersensitivity reactions: General considerations. Allergy 2003, 58, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, C.; Celik, G.; Rouzaire, P.; Whitaker, P.; Bonadonna, P.; Cernadas, J.R.; Vultaggio, A.; Brockow, K.; Caubet, J.C.; Makowska, J.; et al. In vitro tests for drug hypersensitivity reactions: An ENDA/EAACI drug allergy interest group position paper. Allergy 2016, 71, 1103–1134. [Google Scholar] [CrossRef] [PubMed]
- Ebo, D.G.; Leysen, J.; Mayorga, C.; Rozieres, A.; Knol, E.F.; Terreehorst, I. The in vitro diagnosis of drug allergy: Status and perspectives. Allergy 2011, 66, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Vultaggio, A.; Matucci, A.; Virgili, G.; Rossi, O.; Fili, L.; Parronchi, P.; Romagnani, S.; Maggi, E. Influence of total serum IgE levels on the in vitro detection of β-lactams-specific IgE antibodies. Clin. Exp. Allergy 2009, 39, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Ariza, A.; Mayorga, C.; Salas, M.; Dona, I.; Martin-Serrano, A.; Perez-Inestrosa, E.; Perez-Sala, D.; Guzman, A.E.; Montanez, M.I.; Torres, M.J. The influence of the carrier molecule on amoxicillin recognition by specific IgE in patients with immediate hypersensitivity reactions to betalactams. Sci. Rep. 2016, 6, 35113. [Google Scholar] [CrossRef] [PubMed]
- Garcia, J.J.; Blanca, M.; Moreno, F.; Vega, J.M.; Mayorga, C.; Fernandez, J.; Juarez, C.; Romano, A.; de Ramon, E. Determination of IgE antibodies to the benzylpenicilloyl determinant: A comparison of the sensitivity and specificity of three radio allergo sorbent test methods. J. Clin. Lab. Anal. 1997, 11, 251–257. [Google Scholar] [CrossRef]
- Blanca, M.; Mayorga, C.; Torres, M.J.; Reche, M.; Moya, M.C.; Rodriguez, J.L.; Romano, A.; Juarez, C. Clinical evaluation of Pharmacia CAP System™ RAST FEIA amoxicilloyl and benzylpenicilloyl in patients with penicillin allergy. Allergy 2001, 56, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Doña, I.; Torres, M.J.; Montañez, M.I.; Fernandez, T.D. In vitro diagnostic testing for antibiotic allergy. Allergy Asthma Immunol. Res. 2017, 9, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.L.; Gamboa, P.M.; Antepara, I.; Uasuf, C.; Vila, L.; Garcia-Aviles, C.; Chazot, M.; de Weck, A.L. Flow cytometric basophil activation test by detection of CD63 expression in patients with immediate-type reactions to betalactam antibiotics. Clin. Exp. Allergy 2002, 32, 277–286. [Google Scholar] [CrossRef] [PubMed]
- De Weck, A.L.; Sanz, M.L.; Gamboa, P.M.; Aberer, W.; Sturm, G.; Bilo, M.B.; Montroni, M.; Blanca, M.; Torres, M.J.; Mayorga, L.; et al. Diagnosis of immediate-type β-lactam allergy in vitro by flow-cytometric basophil activation test and sulfidoleukotriene production: A multicenter study. J. Investig. Allergol. Clin. Immunol. 2009, 19, 91–109. [Google Scholar]
- Fontaine, C.; Mayorga, C.; Bousquet, P.J.; Arnoux, B.; Torres, M.J.; Blanca, M.; Demoly, P. Relevance of the determination of serum-specific IgE antibodies in the diagnosis of immediate β-lactam allergy. Allergy 2007, 62, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Aviles, C.; Sanz, M.L.; Gamboa, P.M.; Urrutia, I.; Antepara, I.; Jauregui, I.; de Weck, A.L. Antigen specific quantification of sulfidoleukotrienes in patients allergic to betalactam antibiotics. J. Investig. Allergol. Clin. Immunol. 2005, 15, 37–45. [Google Scholar] [PubMed]
- Vultaggio, A.; Virgili, G.; Gaeta, F.; Romano, A.; Maggi, E.; Matucci, A. High serum β-lactams specific/total IgE ratio is associated with immediate reactions to β-lactams antibiotics. PLoS ONE 2015, 10, e0121857. [Google Scholar] [CrossRef] [PubMed]
- Ebo, D.G.; Venemalm, L.; Bridts, C.H.; Degerbeck, F.; Hagberg, H.; de Clerck, L.S.; Stevens, W.J. Immunoglobulin E antibodies to rocuronium: A new diagnostic tool. Anesthesiology 2007, 107, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Leysen, J.; Bridts, C.H.; de Clerck, L.S.; Vercauteren, M.; Lambert, J.; Weyler, J.J.; Stevens, W.J.; Ebo, D.G. Allergy to rocuronium: From clinical suspicion to correct diagnosis. Allergy 2011, 66, 1014–1019. [Google Scholar] [CrossRef] [PubMed]
- Monneret, G.; Benoit, Y.; Debard, A.L.; Gutowski, M.C.; Topenot, I.; Bienvenu, J. Monitoring of basophil activation using CD63 and CCR3 in allergy to muscle relaxant drugs. Clin. Immunol. 2002, 102, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Guilloux, L.; Ricard-Blum, S.; Ville, G.; Motin, J. A new radioimmunoassay using a commercially available solid support for the detection of IgE antibodies against muscle relaxants. J. Allergy Clin. Immunol. 1992, 90, 153–159. [Google Scholar] [CrossRef]
- Rouzaire, P.; Proton, G.; Bienvenu, F.; Guilloux, L.; Benoit, Y.; Piriou, V.; Bienvenu, J.; Mertes, P. IgE antibody detection in the diagnosis of hypersensitivity to neuromuscular blocking agents. Acta Anaesthesiol. Scand. 2012, 56, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Mata, E.; Gueant, J.L.; Moneret-Vautrin, D.A.; Bermejo, N.; Gerard, P.; Nicolas, J.P.; Laxenaire, M.C. Clinical evaluation of in vitro leukocyte histamine release in allergy to muscle relaxant drugs. Allergy 1992, 47, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Laroche, D.; Chollet-Martin, S.; Leturgie, P.; Malzac, L.; Vergnaud, M.C.; Neukirch, C.; Venemalm, L.; Gueant, J.L.; Nicaise Roland, P. Evaluation of a new routine diagnostic test for immunoglobulin E sensitization to neuromuscular blocking agents. Anesthesiology 2011, 114, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Garvey, L.H.; Kroigaard, M.; Poulsen, L.K.; Skov, P.S.; Mosbech, H.; Venemalm, L.; Degerbeck, F.; Husum, B. IgE-mediated allergy to chlorhexidine. J. Allergy Clin. Immunol. 2007, 120, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Aranda, A.; Mayorga, C.; Ariza, A.; Dona, I.; Rosado, A.; Blanca-Lopez, N.; Andreu, I.; Torres, M.J. In vitro evaluation of IgE-mediated hypersensitivity reactions to quinolones. Allergy 2011, 66, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, M.; Severino, M.; Testi, S.; Macchia, D.; Ermini, G.; Pichler, W.J.; Campi, P. Detection of specific IgE to quinolones. J. Allergy Clin. Immunol. 2004, 113, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.H.; Mirakhur, B.; Chan, E.; Le, Q.T.; Berlin, J.; Morse, M.; Murphy, B.A.; Satinover, S.M.; Hosen, J.; Mauro, D.; et al. Cetuximab-induced anaphylaxis and IgE specific for galactose-α-1,3-galactose. N. Engl. J. Med. 2008, 358, 1109–1117. [Google Scholar] [CrossRef] [PubMed]
- Vultaggio, A.; Matucci, A.; Nencini, F.; Pratesi, S.; Parronchi, P.; Rossi, O.; Romagnani, S.; Maggi, E. Anti-infliximab IgE and non-IgE antibodies and induction of infusion-related severe anaphylactic reactions. Allergy 2010, 65, 657–661. [Google Scholar] [CrossRef] [PubMed]
- Mariotte, D.; Dupont, B.; Gervais, R.; Galais, M.-P.; Laroche, D.; Tranchant, A.; Comby, E.; Bouhier-Leporrier, K.; Reimund, J.-M.; Le Mauff, B. Anti-cetuximab IgE ELISA for identification of patients at a high risk of cetuximab-induced anaphylaxis. mAbs 2011, 3, 396–401. [Google Scholar] [CrossRef] [PubMed]
- Matucci, A.; Pratesi, S.; Petroni, G.; Nencini, F.; Virgili, G.; Milla, M.; Maggi, E.; Vultaggio, A. Allergological in vitro and in vivo evaluation of patients with hypersensitivity reactions to infliximab. Clin. Exp. Allergy 2013, 43, 659–664. [Google Scholar] [PubMed]
- Hoffmann, H.J.; Santos, A.F.; Mayorga, C.; Nopp, A.; Eberlein, B.; Ferrer, M.; Rouzaire, P.; Ebo, D.G.; Sabato, V.; Sanz, M.L.; et al. The clinical utility of basophil activation testing in diagnosis and monitoring of allergic disease. Allergy 2015, 70, 1393–1405. [Google Scholar] [CrossRef] [PubMed]
- Ariza, A.; Montanez, M.I.; Fernandez, T.D.; Perkins, J.R.; Mayorga, C. Cellular tests for the evaluation of drug hypersensitivity. Curr. Pharm. Des. 2016, 22, 6773–6783. [Google Scholar] [CrossRef] [PubMed]
- Abuaf, N.; Rostane, H.; Rajoely, B.; Gaouar, H.; Autegarden, J.E.; Leynadier, F.; Girot, R. Comparison of two basophil activation markers CD63 and CD203c in the diagnosis of amoxicillin allergy. Clin. Exp. Allergy 2008, 38, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, T.D.; Ariza, A.; Palomares, F.; Montanez, M.I.; Salas, M.; Martin-Serrano, A.; Fernandez, R.; Ruiz, A.; Blanca, M.; Mayorga, C.; et al. Hypersensitivity to fluoroquinolones: The expression of basophil activation markers depends on the clinical entity and the culprit fluoroquinolone. Medicine 2016, 95, e3679. [Google Scholar] [CrossRef] [PubMed]
- Perkins, J.R.; Ariza, A.; Blanca, M.; Fernandez, T.D. Tests for evaluating non-immediate allergic drug reactions. Expert Rev. Clin. Immunol. 2014, 10, 1475–1486. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.J.; Padial, A.; Mayorga, C.; Fernandez, T.; Sanchez-Sabate, E.; Cornejo-Garcia, J.A.; Antunez, C.; Blanca, M. The diagnostic interpretation of basophil activation test in immediate allergic reactions to betalactams. Clin. Exp. Allergy 2004, 34, 1768–1775. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.J.; Ariza, A.; Mayorga, C.; Dona, I.; Blanca-Lopez, N.; Rondon, C.; Blanca, M. Clavulanic acid can be the component in amoxicillin-clavulanic acid responsible for immediate hypersensitivity reactions. J. Allergy Clin. Immunol. 2010, 125, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Eberlein, B.; Leon Suarez, I.; Darsow, U.; Rueff, F.; Behrendt, H.; Ring, J. A new basophil activation test using CD63 and CCR3 in allergy to antibiotics. Clin. Exp. Allergy 2010, 40, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Morillas, L.; Perez-Ezquerra, P.R.; Reano-Martos, M.; Laguna-Martinez, J.J.; Sanz, M.L.; Martinez, L.M. Selective allergic reactions to clavulanic acid: A report of 9 cases. J. Allergy Clin. Immunol. 2010, 126, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Ebo, D.G.; Bridts, C.H.; Hagendorens, M.M.; Mertens, C.H.; de Clerck, L.S.; Stevens, W.J. Flow-assisted diagnostic management of anaphylaxis from rocuronium bromide. Allergy 2006, 61, 935–939. [Google Scholar] [CrossRef] [PubMed]
- Kvedariene, V.; Kamey, S.; Ryckwaert, Y.; Rongier, M.; Bousquet, J.; Demoly, P.; Arnoux, B. Diagnosis of neuromuscular blocking agent hypersensitivity reactions using cytofluorimetric analysis of basophils. Allergy 2006, 61, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Sudheer, P.S.; Hall, J.E.; Read, G.F.; Rowbottom, A.W.; Williams, P.E. Flow cytometric investigation of peri-anaesthetic anaphylaxis using CD63 and CD203c. Anaesthesia 2005, 60, 251–256. [Google Scholar] [CrossRef] [PubMed]
- Hagau, N.; Gherman-Ionica, N.; Sfichi, M.; Petrisor, C. Threshold for basophil activation test positivity in neuromuscular blocking agents hypersensitivity reactions. Allergy Asthma Clin. Immunol. 2013, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Uyttebroek, A.P.; Sabato, V.; Leysen, J.; Bridts, C.H.; de Clerck, L.S.; Ebo, D.G. Flowcytometric diagnosis of atracurium-induced anaphylaxis. Allergy 2014, 69, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Abuaf, N.; Rajoely, B.; Ghazouani, E.; Levy, D.A.; Pecquet, C.; Chabane, H.; Leynadier, F. Validation of a flow cytometric assay detecting in vitro basophil activation for the diagnosis of muscle relaxant allergy. J. Allergy Clin. Immunol. 1999, 104, 411–418. [Google Scholar] [CrossRef]
- Mayorga, C.; Andreu, I.; Aranda, A.; Dona, I.; Montanez, M.I.; Blanca-Lopez, N.; Ariza, A.; Nuin, E.; Blanca, M.; Miranda, M.A.; et al. Fluoroquinolone photodegradation influences specific basophil activation. Int. Arch. Allergy Immunol. 2013, 160, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Rouzaire, P.; Nosbaum, A.; Denis, L.; Bienvenu, F.; Berard, F.; Cozon, G.; Bienvenu, J. Negativity of the basophil activation test in quinolone hypersensitivity: A breakthrough for provocation test decision-making. Int. Arch. Allergy Immunol. 2012, 157, 299–302. [Google Scholar] [CrossRef] [PubMed]
- Hagau, N.; Longrois, D.; Petrisor, C. Threshold for positivity and optimal dipyrone concentration in flow cytometry-assisted basophil activation test. Allergy Asthma Immunol. Res. 2013, 5, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Gamboa, P.M.; Sanz, M.L.; Caballero, M.R.; Antepara, I.; Urrutia, I.; Jauregui, I.; Gonzalez, G.; Dieguez, I.; de Weck, A.L. Use of CD63 expression as a marker of in vitro basophil activation and leukotriene determination in metamizol allergic patients. Allergy 2003, 58, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.; Blanca-Lopez, N.; Torres, M.J.; Requena, G.; Rondon, C.; Canto, G.; Blanca, M.; Mayorga, C. Immunoglobulin E-mediated immediate allergic reactions to dipyrone: Value of basophil activation test in the identification of patients. Clin. Exp. Allergy 2009, 39, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Pinnobphun, P.; Buranapraditkun, S.; Kampitak, T.; Hirankarn, N.; Klaewsongkram, J. The diagnostic value of basophil activation test in patients with an immediate hypersensitivity reaction to radiocontrast media. Ann. Allergy Asthma Immunol. 2011, 106, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Salas, M.; Gomez, F.; Fernandez, T.D.; Dona, I.; Aranda, A.; Ariza, A.; Blanca-Lopez, N.; Mayorga, C.; Blanca, M.; Torres, M.J. Diagnosis of immediate hypersensitivity reactions to radiocontrast media. Allergy 2013, 68, 1203–1206. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ortega, P.; Marin, A. Usefulness of the basophil activation test (BAT) in the diagnosis of life-threatening drug anaphylaxis. Allergy 2010, 65, 1204. [Google Scholar] [CrossRef] [PubMed]
- Antunez, C.; Blanca-Lopez, N.; Torres, M.J.; Mayorga, C.; Perez-Inestrosa, E.; Montanez, M.I.; Fernandez, T.; Blanca, M. Immediate allergic reactions to cephalosporins: Evaluation of cross-reactivity with a panel of penicillins and cephalosporins. J. Allergy Clin. Immunol. 2006, 117, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Gueant-Rodriguez, R.M.; Viola, M.; Amoghly, F.; Gaeta, F.; Nicolas, J.P.; Gueant, J.L. Diagnosing immediate reactions to cephalosporins. Clin. Exp. Allergy 2005, 35, 1234–1242. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.J.; Romano, A.; Mayorga, C.; Moya, M.C.; Guzman, A.E.; Reche, M.; Juarez, C.; Blanca, M. Diagnostic evaluation of a large group of patients with immediate allergy to penicillins: The role of skin testing. Allergy 2001, 56, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, C.; Pena, R.R.; Blanca-Lopez, N.; Lopez, S.; Martin, E.; Torres, M.J. Monitoring the acute phase response in non-immediate allergic drug reactions. Curr. Opin. Allergy Clin. Immunol. 2006, 6, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Barbaud, A. Skin testing and patch testing in non-IgE-mediated drug allergy. Curr. Allergy Asthma Rep. 2014, 14, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Whitaker, P.; Meng, X.; Lavergne, S.N.; El-Ghaiesh, S.; Monshi, M.; Earnshaw, C.; Peckham, D.; Gooi, J.; Conway, S.; Pirmohamed, M.; et al. Mass spectrometric characterization of circulating and functional antigens derived from piperacillin in patients with cystic fibrosis. J. Immunol. 2011, 187, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Schnyder, B.; Pichler, W.J. Skin and laboratory tests in amoxicillin- and penicillin-induced morbilliform skin eruption. Clin. Exp. Allergy 2000, 30, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Rozieres, A.; Hennino, A.; Rodet, K.; Gutowski, M.C.; Gunera-Saad, N.; Berard, F.; Cozon, G.; Bienvenu, J.; Nicolas, J.F. Detection and quantification of drug-specific T cells in penicillin allergy. Allergy 2009, 64, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Pena, R.; Lopez, S.; Mayorga, C.; Antunez, C.; Fernandez, T.D.; Torres, M.J.; Blanca, M. Potential involvement of dendritic cells in delayed-type hypersensitivity reactions to β-lactams. J. Allergy Clin. Immunol. 2006, 118, 949–956. [Google Scholar] [CrossRef] [PubMed]
- Orasch, C.E.; Helbling, A.; Zanni, M.P.; Yawalkar, N.; Hari, Y.; Pichler, W.J. T-cell reaction to local anaesthetics: Relationship to angioedema and urticaria after subcutaneous application—Patch testing and LTT in patients with adverse reaction to local anaesthetics. Clin. Exp. Allergy 1999, 29, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Nyfeler, B.; Pichler, W.J. The lymphocyte transformation test for the diagnosis of drug allergy: Sensitivity and specificity. Clin. Exp. Allergy 1997, 27, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Luque, I.; Leyva, L.; Jose Torres, M.; Rosal, M.; Mayorga, C.; Segura, J.M.; Blanca, M.; Juarez, C. In vitro T-cell responses to β-lactam drugs in immediate and nonimmediate allergic reactions. Allergy 2001, 56, 611–618. [Google Scholar] [CrossRef] [PubMed]
- Hari, Y.; Frutig-Schnyder, K.; Hurni, M.; Yawalkar, N.; Zanni, M.P.; Schnyder, B.; Kappeler, A.; von Greyerz, S.; Braathen, L.R.; Pichler, W.J. T cell involvement in cutaneous drug eruptions. Clin. Exp. Allergy 2001, 31, 1398–1408. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Miwa, S.; Shirai, M.; Ohba, H.; Murakami, M.; Fujita, K.; Suda, T.; Nakamura, H.; Hayakawa, H.; Chida, K. Drug lymphocyte stimulation test in the diagnosis of adverse reactions to antituberculosis drugs. Chest 2008, 134, 1027–1032. [Google Scholar] [CrossRef] [PubMed]
- Sachs, B.; Erdmann, S.; Malte Baron, J.; Neis, M.; Al Masaoudi, T.; Merk, H.F. Determination of interleukin-5 secretion from drug-specific activated ex vivo peripheral blood mononuclear cells as a test system for the in vitro detection of drug sensitization. Clin. Exp. Allergy 2002, 32, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Roujeau, J.C.; Albengres, E.; Moritz, S.; Piacentino, A.; Cuny, M.; Revuz, J.; Touraine, R. Lymphocyte transformation test in drug-induced toxic epidermal necrolysis. Int. Arch. Allergy Appl. Immunol. 1985, 78, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Porebski, G.; Pecaric-Petkovic, T.; Groux-Keller, M.; Bosak, M.; Kawabata, T.T.; Pichler, W.J. In vitro drug causality assessment in Stevens-Johnson syndrome-alternatives for lymphocyte transformation test. Clin. Exp. Allergy 2013, 43, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Polak, M.E.; Belgi, G.; McGuire, C.; Pickard, C.; Healy, E.; Friedmann, P.S.; Ardern-Jones, M.R. In vitro diagnostic assays are effective during the acute phase of delayed-type drug hypersensitivity reactions. Br. J. Dermatol. 2013, 168, 539–549. [Google Scholar] [CrossRef] [PubMed]
- Antunez, C.; Barbaud, A.; Gomez, E.; Audonnet, S.; Lopez, S.; Gueant-Rodriguez, R.M.; Aimone-Gastin, I.; Gomez, F.; Blanca, M.; Gueant, J.L. Recognition of iodixanol by dendritic cells increases the cellular response in delayed allergic reactions to contrast media. Clin. Exp. Allergy 2011, 41, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Quintero, M.J.; Torres, M.J.; Blazquez, A.B.; Gomez, E.; Fernandez, T.D.; Dona, I.; Ariza, A.; Andreu, I.; Melendez, L.; Blanca, M.; et al. Synergistic effect between amoxicillin and TLR ligands on dendritic cells from amoxicillin-delayed allergic patients. PLoS ONE 2013, 8, e74198. [Google Scholar] [CrossRef] [PubMed]
- Czerkinsky, C.; Moldoveanu, Z.; Mestecky, J.; Nilsson, L.A.; Ouchterlony, O. A novel two colour ELISPOT assay: I. Simultaneous detection of distinct types of antibody-secreting cells. J. Immunol. Methods 1988, 115, 31–37. [Google Scholar] [CrossRef]
- Lochmatter, P.; Beeler, A.; Kawabata, T.T.; Gerber, B.O.; Pichler, W.J. Drug-specific in vitro release of IL-2, IL-5, IL-13 and IFN-γ in patients with delayed-type drug hypersensitivity. Allergy 2009, 64, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Naisbitt, D.J.; Farrell, J.; Wong, G.; Depta, J.P.; Dodd, C.C.; Hopkins, J.E.; Gibney, C.A.; Chadwick, D.W.; Pichler, W.J.; Pirmohamed, M.; et al. Characterization of drug-specific T cells in lamotrigine hypersensitivity. J. Allergy Clin. Immunol. 2003, 111, 1393–1403. [Google Scholar] [CrossRef] [PubMed]
- Zawodniak, A.; Lochmatter, P.; Yerly, D.; Kawabata, T.; Lerch, M.; Yawalkar, N.; Pichler, W.J. In vitro detection of cytotoxic T and NK cells in peripheral blood of patients with various drug-induced skin diseases. Allergy 2010, 65, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Rozieres, A.; Vocanson, M.; Said, B.B.; Nosbaum, A.; Nicolas, J.F. Role of T cells in nonimmediate allergic drug reactions. Curr. Opin. Allergy Clin. Immunol. 2009, 9, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Tanvarasethee, B.; Buranapraditkun, S.; Klaewsongkram, J. The potential of using enzyme-linked immunospot to diagnose cephalosporin-induced maculopapular exanthems. Acta Derm. Venereol. 2013, 93, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Klaewsongkram, J.; Thantiworasit, P.; Suthumchai, N.; Rerknimitr, P.; Sukasem, C.; Tuchinda, P.; Chularojanamontri, L.; Srinoulprasert, Y.; Rerkpattanapipat, T.; Chanprapaph, K.; et al. In vitro test to confirm diagnosis of allopurinol-induced severe cutaneous adverse reactions. Br. J. Dermatol. 2016, 175, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Kawase, A.; Azukizawa, H.; Hanafusa, T.; Nakagawa, Y.; Murota, H.; Sakaguchi, S.; Asada, H.; Katayama, I. Novel interferon-γ enzyme-linked immunoSpot assay using activated cells for identifying hypersensitivity-inducing drug culprits. J. Dermatol. Sci. 2017, 86, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Mauri-Hellweg, D.; Bettens, F.; Mauri, D.; Brander, C.; Hunziker, T.; Pichler, W.J. Activation of drug-specific CD4+ and CD8+ T cells in individuals allergic to sulfonamides, phenytoin, and carbamazepine. J. Immunol. 1995, 155, 462–472. [Google Scholar] [PubMed]
- Yawalkar, N.; Shrikhande, M.; Hari, Y.; Nievergelt, H.; Braathen, L.R.; Pichler, W.J. Evidence for a role for IL-5 and eotaxin in activating and recruiting eosinophils in drug-induced cutaneous eruptions. J. Allergy Clin. Immunol. 2000, 106, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Beeler, A.; Zaccaria, L.; Kawabata, T.; Gerber, B.O.; Pichler, W.J. CD69 upregulation on T cells as an in vitro marker for delayed-type drug hypersensitivity. Allergy 2008, 63, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Cornejo-Garcia, J.A.; Fernandez, T.D.; Torres, M.J.; Carballo, M.; Hernan, I.; Antunez, C.; Blanca, M.; Mayorga, C. Differential cytokine and transcription factor expression in patients with allergic reactions to drugs. Allergy 2007, 62, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, T.D.; Mayorga, C.; Torres, M.J.; Cornejo-Garcia, J.A.; Lopez, S.; Chaves, P.; Rondon, C.; Blanca, M. Cytokine and chemokine expression in the skin from patients with maculopapular exanthema to drugs. Allergy 2008, 63, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Wurpts, G.; Ott, H.; Baron, J.M.; Erdmann, S.; Merk, H.F.; Sachs, B. In vitro detection and characterization of drug hypersensitivity using flow cytometry. Allergy 2010, 65, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Khalil, G.; El-Sabban, M.; Al-Ghadban, S.; Azzi, S.; Shamra, S.; Khalife, S.; Maroun, R. Cytokine expression profile of sensitized human T lymphocytes following in vitro stimulation with amoxicillin. Eur. Cytokine Netw. 2008, 19, 131–141. [Google Scholar] [PubMed]
- Halevy, S.; Grossman, N. Multiple drug allergy in patients with cutaneous adverse drug reactions diagnosed by in vitro drug-induced interferon-γ release. Isr. Med. Assoc. J. 2008, 10, 865–868. [Google Scholar] [PubMed]
| Paper | Patients | Drugs | Diag | Method | Sens (%) | Spec (%) | NPV (%) | PPV (%) |
|---|---|---|---|---|---|---|---|---|
| Betalactams | ||||||||
| Garcia 1997 [15] | 30 Pat 30 Cont | PG | ST | RAST | 86.66 | 90 | 87.09 | 89.65 |
| Blanca 2001 [16] | 74 Pat 55 Cont | AX, PG | ST, DPT | CAP | 50 | 96 | 58.8 | 94.4 |
| Sanz 2002 [18] | 58 Pat 30 Cont | PG, AX, AMP, CEFU, CEFAZ | ST | CAP | 37.9 | 86.7 | 41.9 | 84.6 |
| Garcia-Aviles 2005 [21] | 67 Pat 30 Cont | AX, PG CEFU, CEPHA | ST, DPT | CAP | 37.8 | 83.3 | 37.5 | 83.5 |
| Fontaine 2007 [20] | 30 Pat 15 Cont | AX, PG, AMP, CEFOT, CEFT, CEPH, CEFAC | ST, DPT | RAST | 50 | 73.3 | 42.3 | 78.9 |
| CAP | 16.6 | 93.3 | 35.9 | 83.2 | ||||
| De Weck 2009 [19] | 178 Pat 81 Cont | BP, AX, AMP, CEFs | ST, DPT | CAP | 28.3 | 86.5 | 35.4 | 82.1 |
| Vultaggio 2009 [13] | 61 Pat 115 Cont | PG, PV, AX, AMP | ST | CAP | 85 | 54 | 87.2 | 49.5 |
| Vultaggio 2015 [22] | 171 Pat 122 Cont | PG, PV, AX, AMP | ST | CAP | 66 | 52 | 52.2 | 65.8 |
| CAP | 43 | 95 | 54.3 | 92.3 | ||||
| Mean Values | 50.1 | 81.01 | 53.3 | 80.4 | ||||
| SD | 23.0 | 16.2 | 19.6 | 13.5 | ||||
| Quinolones | ||||||||
| Manfredi 2004 [32] | 55 Pat 32 Cont | CIPRO, LOME, NORFL, OFLO, PIP, RUFL, PEFL, NALI | CH | SEPH | 54.5 | 100 | 56.1 | 100 |
| Aranda 2011 [31] | 38 Pat 25 Cont | CIPRO, MOXI, LEVO | CH, DPT | SEPH | 31.6 | 100 | 49.0 | 100 |
| Mean Values | 43.1 | 100 | 52.6 | 100 | ||||
| SD | 16.19 | 0 | 5.0 | 0 | ||||
| NMBAs | ||||||||
| Guilloux 1992 [26] | 31 Pat 34 Cont | MOR, SUC, ALCU, TMA, TEA | ST | RIA | 96.7 | 97.2 | 96.9 | 96.9 |
| Mata 1992 [28] | 40 Pat 44 Cont | SUX, VECU, PANCU, ALCU, ATRAC, GALLA | CH, ST | SEPH | 82.5 | 100 | 86.2 | 100 |
| Monneret 2002 [25] | 39 Pat 17 Cont | ROC, SUX, ATRAC | CH, ST | RIA | 62 | 100 | 53.4 | 100 |
| Ebo 2007 [23] | 25 Pat 30 Cont | ROC, SUX, MOR, PHO | ST | CAP | ROC: 92 | ROC: 93 | ROC: 93.3 | ROC: 92 |
| SUX: 72 | SUX: 100 | SUX: 81.1 | SUX: 100 | |||||
| MOR: 88 | MOR: 100 | MOR: 90.9 | MOR: 100 | |||||
| PHO: 86 | PHO: 100 | PHO: 89.5 | PHO: 100 | |||||
| Leysen 2011 [24] | 41 Pat 25 Cont | ROC | CH, ST | CAP | 82.9 | 72.0 | 72.1 | 82.9 |
| Laroche 2011 [29] | 57 Pat 54 Cont | MOR | CH, ST | CAP | 84.2 | 90.7 | 84.2 | 90.7 |
| Rouzaire 2012 [27] | 11 Pat 20 Cont | ROC, SUX, MOR | CH, ST | CAP | SUX: 44 | SUX: 100 | SUX: 76.4 | SUX: 100 |
| ROC: 83 | ROC: 68 | ROC: 87.9 | ROC: 59 | |||||
| MOR: 78 | MOR: 85 | MOR: 87.5 | MOR: 74 | |||||
| Mean Values | 79.3 | 92.2 | 83.3 | 91.3 | ||||
| SD | 14.3 | 11.4 | 11.7 | 13.1 | ||||
| Clorhexidine | ||||||||
| Garvey 2007 [30] | 12 Pat 10 Cont | CHLOR | ST | CAP | 91.6 | 100 | 90.8 | 100 |
| Biological Agents | ||||||||
| Chung 2008 [33] | 26 Pat 512 Cont | CETUX | CH, DPT | CAP | 68.0 | 98.0 | 98.4 | 63.3 |
| Vultaggio 2010 [34] | 11 Pat 20 Cont | INFLIX | CH | CAP | 27.2 | 100 | 71.4 | 100 |
| Mariotte 2011 [35] | 14 Pat 195 Cont | CETUX | CH | ELISA | 71.4 | 82.1 | 97.6 | 22.3 |
| Matucci 2013 [36] | 30 Pat 50 Cont | INFLIX | CH, ST | CAP | 26.0 | 90.0 | 66.9 | 60.9 |
| Mean Values | 48.2 | 92.5 | 83.6 | 61.6 | ||||
| SD | 24.9 | 8.2 | 16.8 | 31.7 | ||||
| Paper | Patients | Drugs | Diag | Method | Sens (%) | Spec (%) | NPV (%) | PPV (%) |
|---|---|---|---|---|---|---|---|---|
| Betalactam | ||||||||
| Sanz 2002 [18] | 58 Pat 30 Cont | PG, AX, AMP, CEFU, CEFAZ | ST | BAT | 50 | 93.3 | 49.1 | 93.5 |
| Torres 2004 [42] | 70 Pat 40 Cont | PG, AX, AMP, CEFU, CEFAZ, CEFAC | ST, DPT | BAT | 48.6 | 93 | 50.8 | 92.4 |
| Abuaf 2008 [39] | 27 Pat 14 Cont | AX, AMP, CEFU | ST | BAT | 63 | 79 | 52.5 | 85.2 |
| De Weck 2009 [19] | 178 Pat 81 Cont | BP, AX, AMP, CEFs | ST, DPT | BAT | 48.3 | 88.9 | 43.8 | 90.5 |
| Torres 2010 [43] | 55 Pat 30 Cont | PG, AX, AX-CLV, CLV | ST | BAT | 52.7 | 90 | 50.9 | 90.6 |
| Eberlein 2010 [44] | 24 Pat 15 Cont | PG, PV, AMP, AX, CEFU | ST | BAT | 55 | 80 | 52.6 | 81.5 |
| Sanchez-Morillas 2010 [45] | 9 Pat 5 Cont | CLV | ST, DPT | BAT | 44.4 | 100 | 49.9 | 100 |
| Mean Values | 51.7 | 89.2 | 50.0 | 90.5 | ||||
| SD | 6.0 | 7.5 | 3.0 | 5.9 | ||||
| Fluoroquinolones | ||||||||
| Aranda 2011 [31] | 38 Pat 25 Cont | CIPRO, MOXI, LEVO | CH, DPT | BAT | 71.1 | 88 | 66.7 | 90.1 |
| Rouzaire 2012 [53] | 17 Pat 15 Cont | CIPRO, MOXI, LEVO, OFLOX, LOME, FLUME, NORFLO, PIPEMI | ST, DPT | BAT | 76.5 | 100 | 78.9 | 100 |
| Mayorga 2013 [52] | 28 Pat 20 Cont | CIPRO, MOXI | CH, DPT | BAT | 57.1 | 90 | 59.9 | 88.9 |
| Fernandez 2016 [40] | 17 Pat 18 Cont | CIPRO, MOXI | CH, DPT | BAT | 52.9 | 88.9 | 66.7 | 81.8 |
| Mean Values | 64.4 | 91.7 | 68.1 | 90.2 | ||||
| SD | 11.2 | 5.6 | 7.9 | 7.5 | ||||
| NMBAs | ||||||||
| Abuaf 1999 [51] | 21 Pat 29 Cont | SUX, GALLA, VECU, PAN | CH, ST | BAT | 64.0 | 93.0 | 78.1 | 86.9 |
| Monneret 2002 [25] | 39 Pat 17 Cont | ROC, SUX, ATRAC | CH, ST | BAT | 54.0 | 100 | 48.6 | 100 |
| Sudheer 2005 [48] | 14 Pat 10 Cont | ROC, ATRAC, SUX, VECU | CH, ST | BAT | 78.6 | 100 | 76.9 | 100 |
| Ebo 2006 [46] | 14 Pat 8 Cont | ROC | ST | BAT | 91.7 | 100 | 87.3 | 100 |
| Kvedariene 2006 [47] | 47 Pat 45 Cont | ROC, VECU, ATRA, PAN, SUX | ST, DPT | BAT | 36.1 | 93.3 | 58.3 | 84.9 |
| Leysen 2011 [24] | 41 Pat 25 Cont | ROC | CH, ST | BAT | 80.5 | 96.0 | 74.5 | 97.0 |
| Hagau 2013 [49] | 22 Pat 34 Cont | ATRAC, ROC, SUX, PAN | ST | BAT | 68.2 | 100 | 82.9 | 100 |
| Uyttebroek 2014 [50] | 8 Pat 7 Cont | ATRA | ST | BAT | 62.5 | 100 | 70.0 | 100 |
| Mean Values | 66.9 | 97.8 | 72.1 | 96.1 | ||||
| SD | 17.2 | 3.2 | 12.9 | 6.4 | ||||
| Pyrazolones | ||||||||
| Gamboa 2003 [55] | 26 Pat 30 Cont | META | ST, DPT | BAT | 42.3 | 100 | 66.7 | 100 |
| Gomez 2009 [56] | 51 Pat 56 Cont | META | CH, ST, DPT | BAT | 54.9 | 85.7 | 65.1 | 79.6 |
| Hagau 2013 [54] | 20 Pat 10 Cont | DIP | ST | BAT | 65.0 | 100 | 58.8 | 100 |
| Mean Values | 54.1 | 95.2 | 63.5 | 93.2 | ||||
| SD | 11.4 | 8.3 | 4.2 | 11.8 | ||||
| Radio Contrast Media | ||||||||
| Pinnobphun 2011 [57] | 26 Pat 43 Cont | IOXIT, IOPR, IOPA, IOH, IOB | CH, ST | BAT | 57.7 | 97.7 | 79.3 | 93.8 |
| Salas 2013 [58] | 8 Pat 20 Cont | IOD, IOH, IOM, IOB | ST, DPT | BAT | 62.5 | 100 | 86.9 | 100 |
| Mean Values | 60.1 | 98.9 | 83.1 | 96.9 | ||||
| SD | 3.4 | 1.6 | 5.4 | 4.4 | ||||
| Paper | Patients | Clinical Entity | Drugs | Diag | Method | Sens (%) | Spec (%) | NPV (%) | PPV (%) |
|---|---|---|---|---|---|---|---|---|---|
| LTT | |||||||||
| Roujeau 1985 [75] | 12 Pats 8 Cont | TEN | Ant-Con, NSAID | CH | Thymidine | 44.0 | 63.0 | 42.9 | 64.1 |
| Nyfeler 1997 [70] | 100 Pat 102 Cont | ND | BLs | CH, ST | Thymidine | 74.4 | 85.0 | 77.2 | 82.9 |
| Orasch 1999 [69] | 10 Pat 6 Cont | URT/ANG, EXANT | LA | CH, ST | Thymidine | 60.0 | 100 | 60.0 | 100 |
| Schnyder 2000 [66] | 12 Pat 6 Cont | EXANT | BLs | CH | Thymidine | 83.3 | 100 | 74.9 | 100 |
| Luque 2001 [71] | 19 Pat 28 Cont | URT, EXANT | BLs | ST, DPT | Thymidine | 57.9 | 92.8 | 76.5 | 84.5 |
| Hari 2001 [72] | 21 Pat 16 Cont | MPE, BULL-EXANT, URT | Ant-Con, Ant-hyp, others | CH | Thymidine | 66.6 | 93.8 | 68.2 | 93.4 |
| Sachs 2002 [74] | 10 Pat 10 Cont | MPE, AGEP, TEN | BLs, Ant-Con | CH, ST | Thymidine | 75.0 | 100 | 80.0 | 100 |
| Rodriguez-Pena 2006 [68] | 9 Pat 8 Cont | MPE | BLs | CH, ST | Thymidine | 22.2 | 100 | 53.3 | 100 |
| + DC | 88.8 | 100 | 88.8 | 100 | |||||
| Suzuki 2008 [73] | 69 Pat 50 Cont | BULL- EXANT, DILI | Ant-tub | CH, DPT | Thymidine | 28.9 | 90.7 | 48.03 | 81.1 |
| Rozieres 2009 [67] | 22 Pat 11 Cont | MPE | BLs | ST | Thymidine | 68.2 | 100 | 61.1 | 100 |
| Whitaker 2011 [65] | 28 Pat | URT/ANG, MPE, others | BLs | CH | Thymidine | 64.3 | ND | ND | ND |
| Polak 2013 [77] | 43 Pat 14 Cont | MPE, DRESS, TEN, FDE, ECZ | Various | CH | Thymidine | 25.0 | 95.1 | 29.2 | 94.0 |
| Porebski 2013 [76] | 15 Pat 18 Cont | SJS/TEN | Ant-Con | CH | Thymidine | 26.6 | 100 | 62.0 | 100 |
| Mean Values | 56.1 | 93.9 | 63.2 | 92.3 | |||||
| SD | 22.7 | 10.4 | 16.8 | 11.1 | |||||
| ELISpot | |||||||||
| Rozieres 2009 [67] | 22 Pat 11 Cont | MPE | BLs | ST | IFN-γ | 90.9 | 100 | 84.6 | 100 |
| Polak 2013 [77] | 43 Pat 14 Cont | MPE, DRESS, TEN, FDE, ECZ | Various | CH | IFN-γ, IL-4 | IFNγ: 50.0 IL-4: 50.0 | IFNγ: 82.9 IL-4: 92.0 | IFNγ: 35.1 IL-4: 37.5 | IFNγ:90.0 IL-4: 95.0 |
| Porebski 2013 [76] | 15 Pat 18 Cont | SJS/TEN | Ant-Con | CH | GranzymeB | 33.3 | 98.0 | 63.8 | 93.3 |
| Tanvarasethee 2013 [85] | 25 Pat 20 Cont | MPE | BLs | CH | IFN-γ, IL-5 | 40.0 | 100 | 57.1 | 100 |
| Klaewsongkram 2016 [86] | 24 Pat 21 Cont | DRESS, SJS/TEN | Allop | CH | IFN-γ | 79.2 | 95.2 | 80.0 | 95.0 |
| Kato 2017 [87] | 16 Pat 3 Cont | EXANT, DRESS, TEN, SJS | Ant-Con | CH | IFN-γ | 85.0 | 100 | 55.5 | 100 |
| Mean Values | 61.2 | 98.6 | 59.1 | 96.2 | |||||
| SD | 23.3 | 6.3 | 19.0 | 3.9 | |||||
| ELISA+Flow Cytomety | |||||||||
| Sachs 2002 [74] | 10 Pat 10 Cont | MPE, AGEP, TEN | BLs, Ant-Con | CH, ST | IL-5, IFN-γ, IL-10 | IL5: 91.6 IFNγ: 36.4 IL10: 50.0 | IL5: 100 IFNγ: 60.0 IL10: 100 | IL5: 92.3 IFNγ: 48.5 IL10: 66.7 | IL5: 100 IFNγ: 47.6 IL10: 100 |
| Khalil 2008 [94] | 15 Pat 12 Cont | URT/ANG, MPE | BLs | ST | IL-2, IL-5, IFN-γ | IL-2: 86.7 IL-5: 100 IFNγ: 78.5 | IL-2: 100 IL-5: 62.5 IFNγ: 90.0 | IL-2: 85.7 IL-5: 100 IFNγ: 77.1 | IL-2: 100 IL-5: 76.9 IFNγ: 90.8 |
| Halevy 2008 [95] | 12 Pat 11 Cont | VASC, URT, MPE, TEN, FDE, Others | Various | CH | IFN-γ | 80.0 | 62.0 | 74.0 | 70.0 |
| Martin 2010 [93] | 19 Pat 10 Cont | URT, MPE, TEN, others | BLs, Ant-Con, RCM, others | CH | IL-2, IL-5, IFN-γ | IL2: 43.0 IL5: 43.0 IFNγ: 57.0 | 100 | IL2: 48.0 IL5: 48.0 IFNγ: 55.0 | 100 |
| Mean Values | 66.6 | 87.5 | 69.5 | 88.5 | |||||
| SD | 23.2 | 18.2 | 19.4 | 18.1 | |||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayorga, C.; Doña, I.; Perez-Inestrosa, E.; Fernández, T.D.; Torres, M.J. The Value of In Vitro Tests to Diminish Drug Challenges. Int. J. Mol. Sci. 2017, 18, 1222. https://doi.org/10.3390/ijms18061222
Mayorga C, Doña I, Perez-Inestrosa E, Fernández TD, Torres MJ. The Value of In Vitro Tests to Diminish Drug Challenges. International Journal of Molecular Sciences. 2017; 18(6):1222. https://doi.org/10.3390/ijms18061222
Chicago/Turabian StyleMayorga, Cristobalina, Inmaculada Doña, Ezequiel Perez-Inestrosa, Tahia D. Fernández, and Maria J. Torres. 2017. "The Value of In Vitro Tests to Diminish Drug Challenges" International Journal of Molecular Sciences 18, no. 6: 1222. https://doi.org/10.3390/ijms18061222
APA StyleMayorga, C., Doña, I., Perez-Inestrosa, E., Fernández, T. D., & Torres, M. J. (2017). The Value of In Vitro Tests to Diminish Drug Challenges. International Journal of Molecular Sciences, 18(6), 1222. https://doi.org/10.3390/ijms18061222






