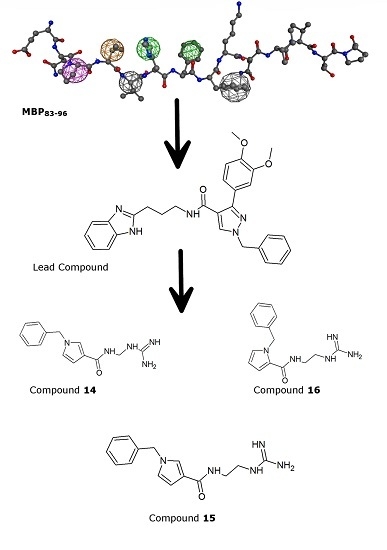
Design and Synthesis of Non-Peptide Mimetics Mapping the Immunodominant Myelin Basic Protein (MBP83–96) Epitope to Function as T-Cell Receptor Antagonists
Abstract
:1. Introduction
2. Results and Discussion
2.1. Pharmacophore Modeling and Virtual Screening
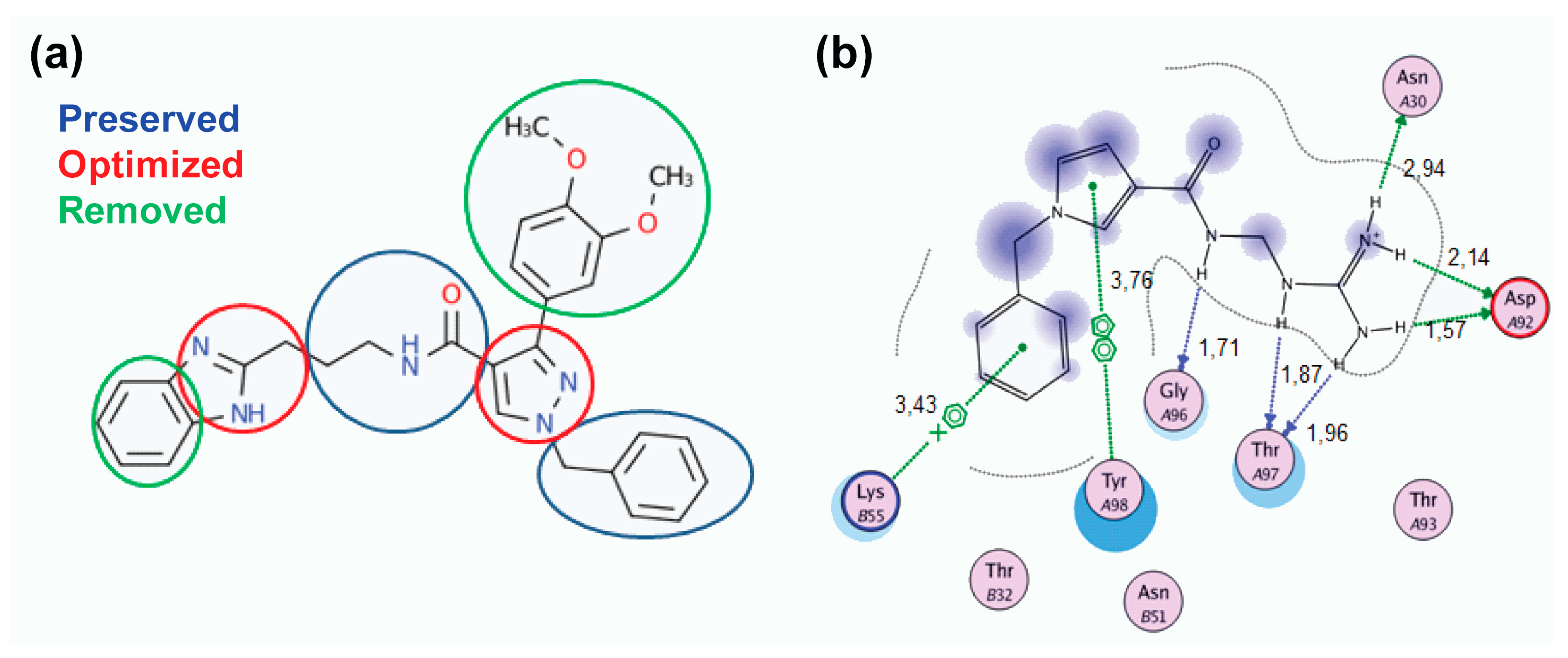
2.2. Lead Optimization and Molecular Docking Calculations
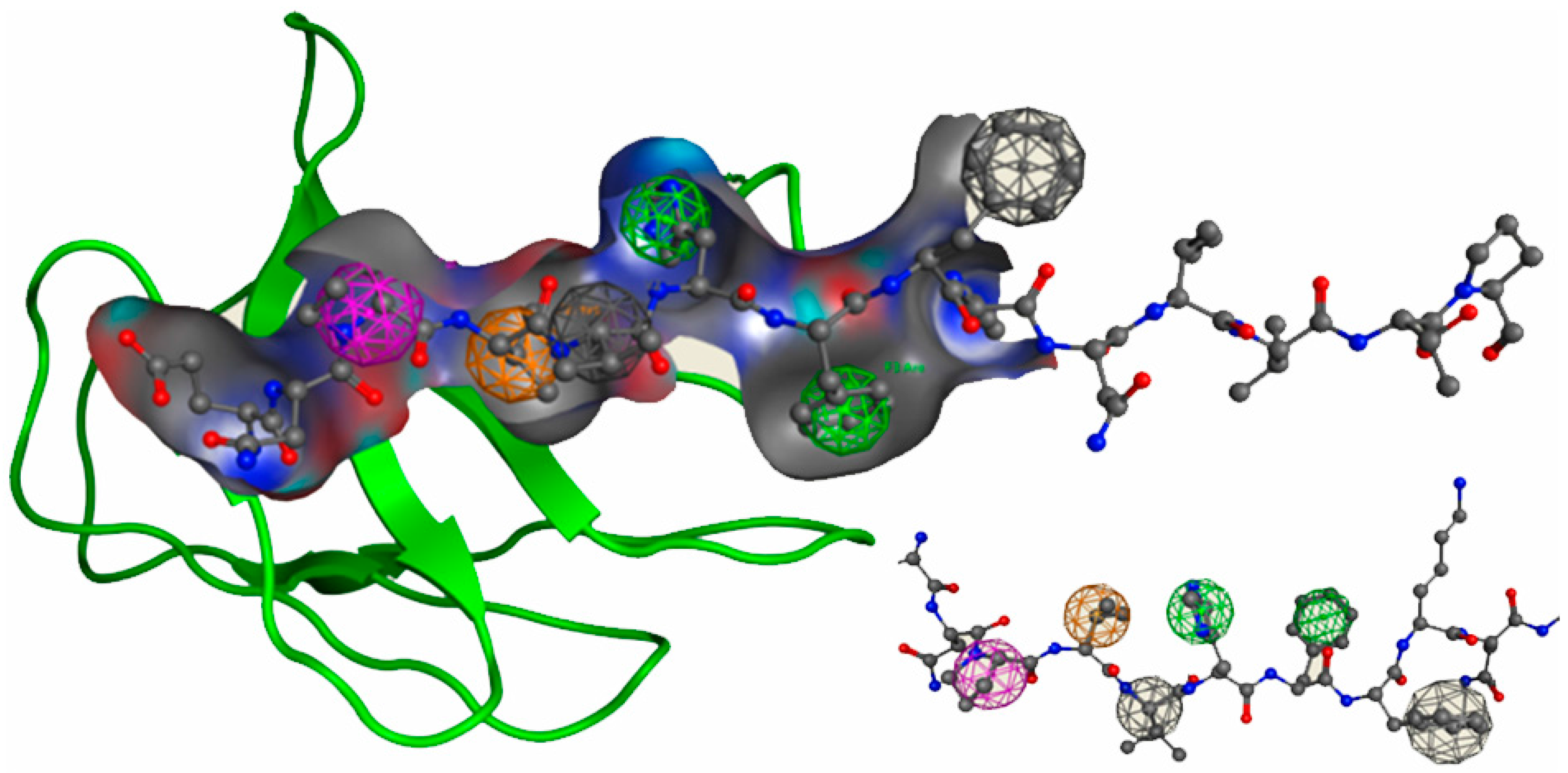
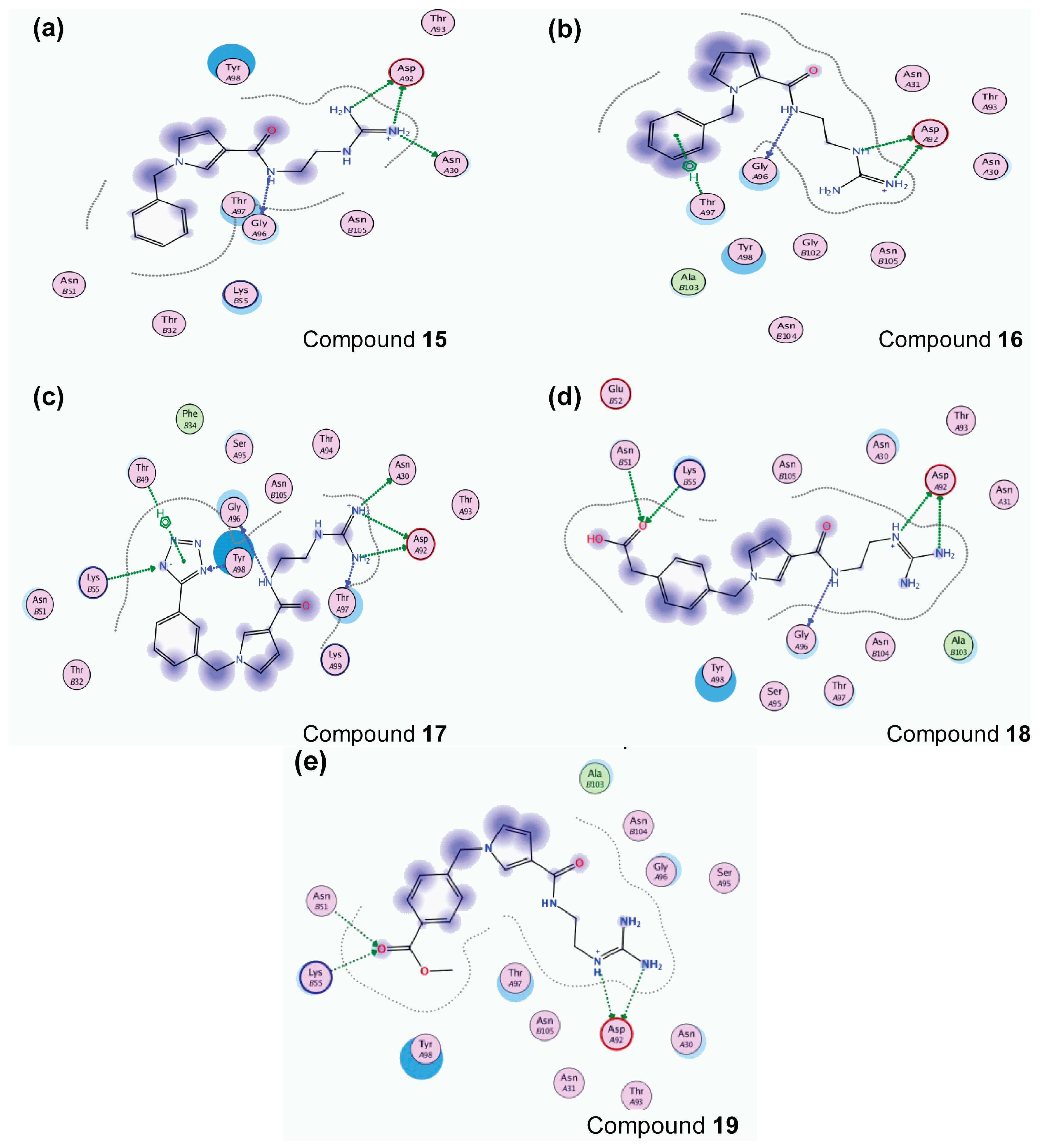
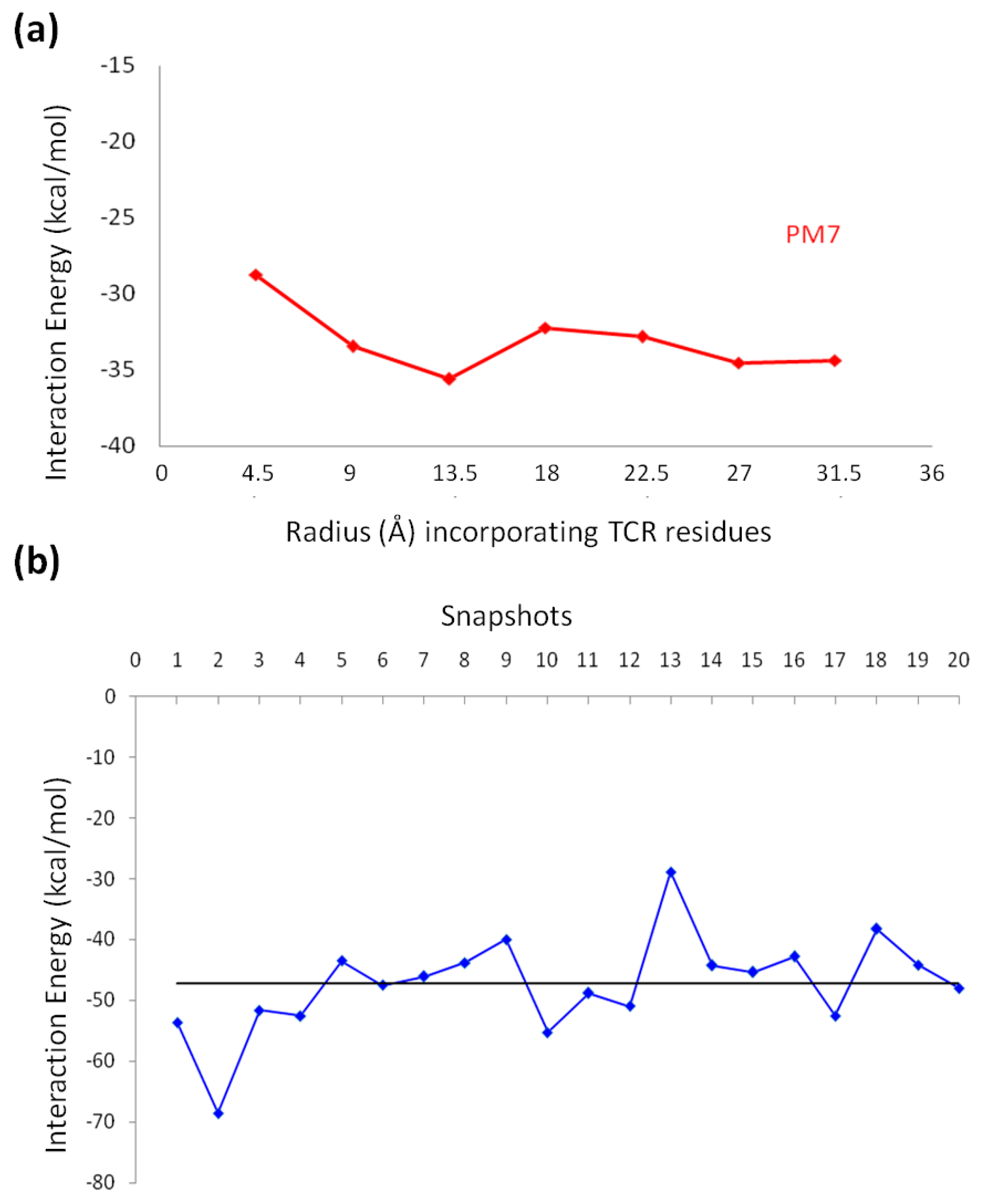
2.3. Molecular Dynamics Simulations
Hydrogen Bond Interactions
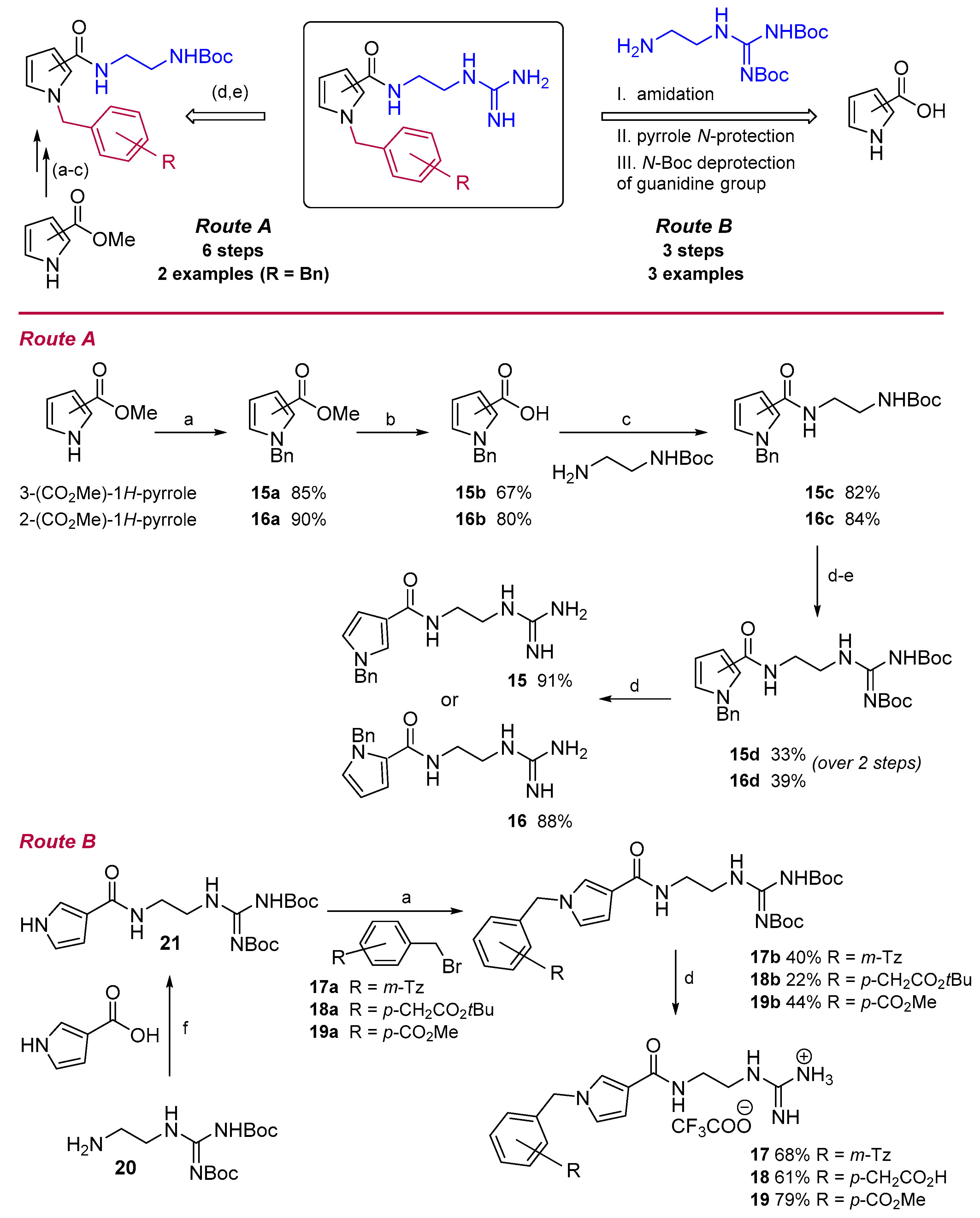
2.4. Chemistry
2.5. Molecular Orbital Calculations
2.5.1. Semi-Empirical Simulation Method
2.5.2. DFT Calculations
2.6. Biological Assays
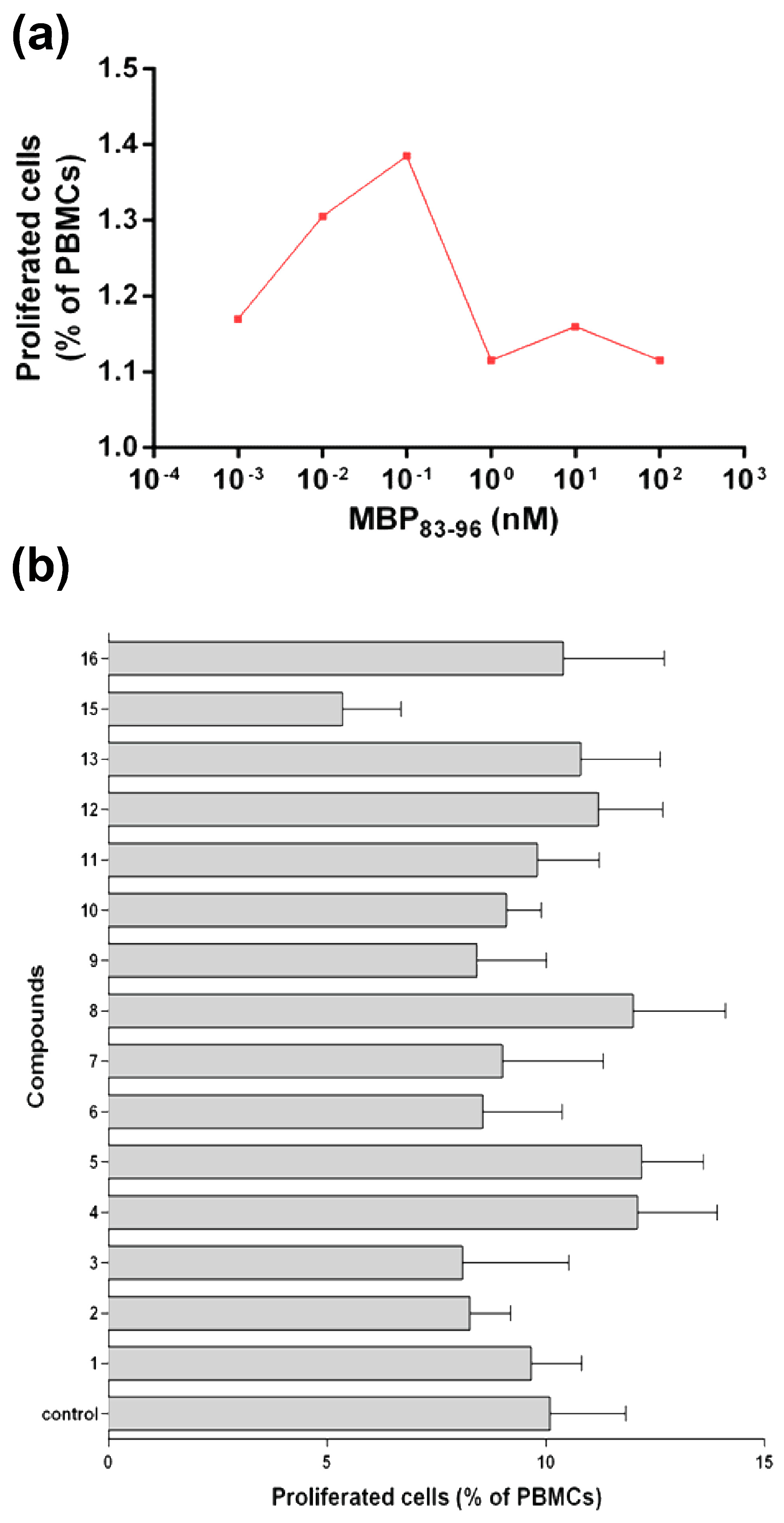
2.6.1. Human Peripheral Mononuclear Cells
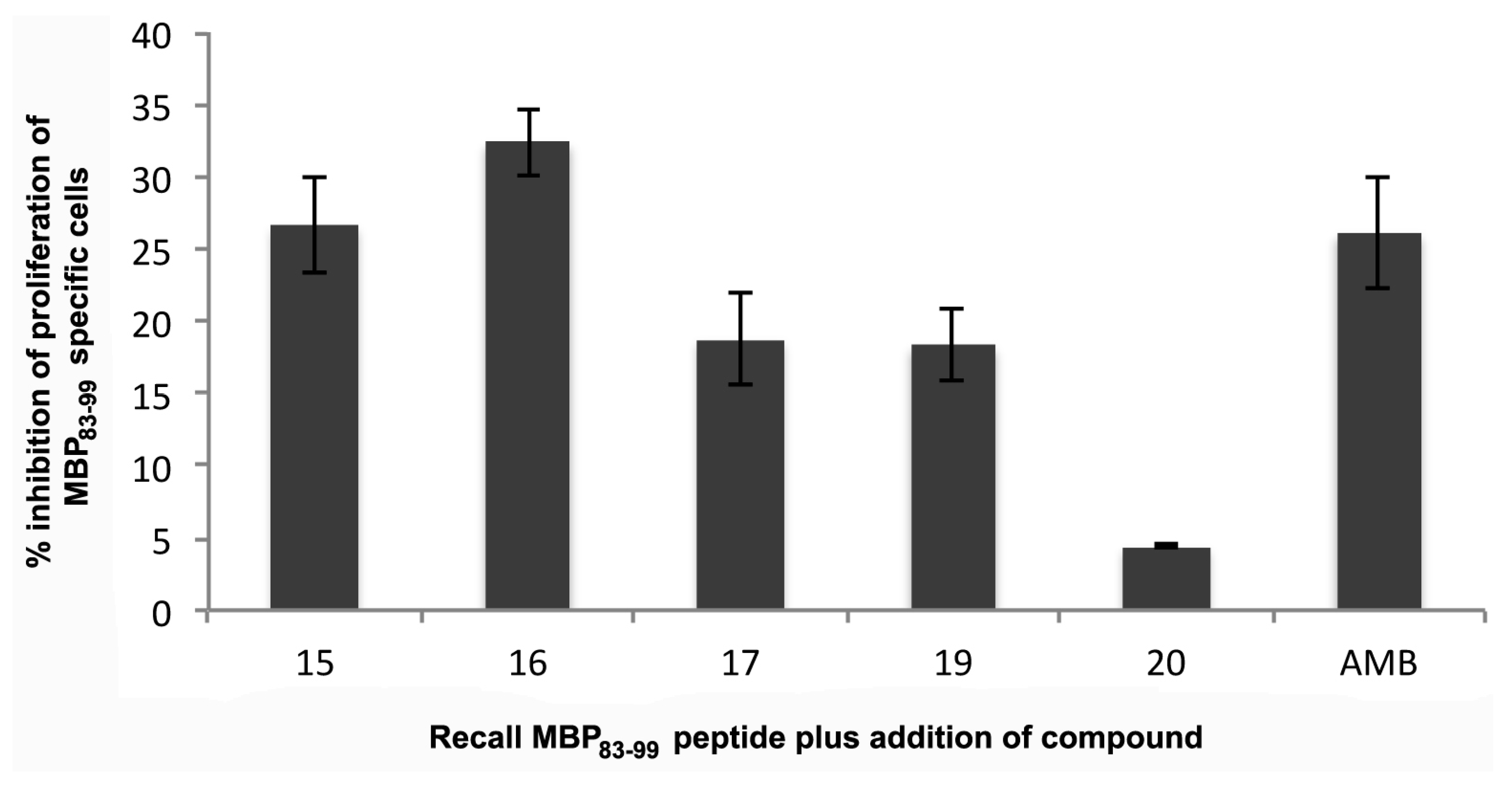
2.6.2. Mouse MBP83–99 Specific T Cell Assays
3. Materials and Methods
3.1. Structure Preparation
3.2. Pharmacophore Modeling
Virtual Screening
3.3. Molecular Docking
3.4. Lead Optimization
3.5. Molecular Dynamics (MD) Simulation
3.6. Chemistry
3.6.1. General Procedure A: N-Alkylation of Pyrroles
3.6.2. General Procedure B: Hydrolysis of Methyl Pyrrole-2/3-Carboxylates
3.6.3. General Procedure C: Amidation Reaction
3.6.4. General Procedure D: Removal of the Boc-Group
3.6.5. General Procedure E: Guanylation Reaction
3.6.6. Synthesis of Methyl 1-Benzyl-1H-Pyrrole-3-Carboxylate 15a [67]
3.6.7. Synthesis of 1-Benzyl-1H-Pyrrole-3-Carboxylic Acid 15b [68]
3.6.8. Synthesis of 1-Benzyl-1H-N-[2-(Tert-Butoxycarbonyl)Aminoethyl]Pyrrole-3-Carboxamide 15c
3.6.9. Synthesis of 1-Benzyl-1H-N-[2-(2,3-Di-Tert-Butoxycarbonyl)Guanidinoethyl]Pyrrole-3-Carboxamide 15d
3.6.10. Synthesis of 1-Benzyl-1H-N-(2-Guanidinoethyl)Pyrrole-3-Carboxamide 15
3.6.11. Synthesis of Methyl 1-Benzyl-1H-Pyrrole-2-Carboxylate 16a [67]
3.6.12. Synthesis of 1-Benzyl-1H-Pyrrol-2-Carboxylic Acid 16b
3.6.13. Synthesis of 1-Benzyl-1H-N-[2-(Tert-Butoxycarbonyl)Aminoethyl]Pyrrole-2-Carboxamide 16c
3.6.14. Synthesis of 1-Benzyl-1H-N-[2-(2,3-Di-Tert-Butoxycarbonyl)Guanidinoethyl]Pyrrole-2-Carboxamide 16d
3.6.15. Synthesis of 1-Benzyl-1H-N-(2-Guanidinoethyl)-Pyrrole-2-Carboxamide 16
3.6.16. Synthesis of N-[2-(2,3-Di-Tert-Butoxycarbonyl)Guanidinoethyl]-1-(m-(1-Trityl-Tetrazol-5-yl)Benzyl)-1H-Pyrrole-3-Carboxamide 17b [24]
3.6.17. Synthesis of 1-(2-(1-(m-(1H-Tetrazol-5-Yl)Benzyl)-1H-Pyrrole-3-Carboxamido)Ethyl) Guanidinium 2,2,2-Trifluoroacetate 17
3.6.18. Synthesis of N-[2-(2,3-Di-Tert-Butoxycarbonyl)Guanidinoethyl]-1-(p-Tert-Butoxycarbonyl Methyl)Benzyl-1H-Pyrrole-3-Carboxamide 18b
3.6.19. Synthesis of 1-(2-(1-(p-(Carboxymethyl)Benzyl)-1H-Pyrrole-3-Carboxamido)Ethyl) Guanidinium 2,2,2-Trifluoroacetate 18
3.6.20. Synthesis of N-[2-(2,3-Di-Tert-Butoxycarbonyl)Guanidinoethyl]-1-(P-Methoxycarbonyl) Benzyl-1H-Pyrrole-3-Carboxamide 19b
3.6.21. Synthesis of 1-(2-(1-(p-(Methoxycarbonyl)Benzyl)-1H-Pyrrole-3-Carboxamido)Ethyl) Guanidinium 2,2,2-Trifluoroacetate 19
3.6.22. Synthesis of N-(2,3-Di-(Tert-Butyloxycarbonyl)Guanidinoethyl)Pyrrole-3-Carboxamide 21
3.7. Molecular Orbital Calculations
3.8. In Vitro Evaluation of the Analogues Using Human PBMC
3.9. In Vitro Evaluation of the Analogues Using Mouse-Specific MBP83–99 T Cells
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| CDRs | complementarity determining regions |
| DCC | N,N′-dicyclohexylcarbodiimide |
| DCM | dichloromethane |
| DIPEA | N,N-diisopropylethylamine |
| DCU | dicyclohexylurea |
| DMAP | 4-dimethylaminopyridine |
| DMF | dimethylformamide |
| ESI MS | electrospray ionization mass spectrometry |
| HLA | human leukocyte antigen |
| HOBt | 1-hydroxybenzotriazole |
| MBP | myelin basic protein |
| MD | molecular dynamics |
| MHC | major histocompatibility complex |
| MS | multiple sclerosis |
| MW | molecular weight |
| 1Η NMR | proton nuclear magnetic resonance |
| 13C NMR | carbon-13 nuclear magnetic resonance |
| PBMC | peripheral blood mononuclear cells |
| RP-HPLC | reversed phase high-performance liquid chromatography |
| TCR | T cell receptor |
| TES | triethylsilane |
| TFA | trifluoroacetic acid |
| Th | T helper |
| TLC | thin layer chromatography |
| TPSA | total polar surface area |
References
- Steinman, L. Multiple sclerosis: A coordinated immunological attack against myelin in the central nervous system. Cell 1996, 85, 299–302. [Google Scholar] [CrossRef]
- Sospedra, M.; Martin, R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2005, 23, 683–747. [Google Scholar] [CrossRef] [PubMed]
- Mouzaki, A.; Rodi, M.; Dimisianos, N.; Emmanuil, A.; Kalavrizioti, D.; Lagoudaki, R.; Grigoriadis, N.C.; Papathanasopoulos, P. Immune parameters that distinguish multiple sclerosis patients from patients with other neurological disorders at presentation. PLoS ONE 2015, 10, e0135434. [Google Scholar] [CrossRef] [PubMed]
- Ben-Nun, A.; Kerlero de Rosbo, N.; Kaushansky, N.; Eisenstein, M.; Cohen, L.; Kaye, J.F.; Mendel, I. Anatomy of T cell autoimmunity to myelin oligodendrocyte glycoprotein (MOG): Prime role of MOG44F in selection and control of mog-reactive T cells in h-2b mice. Eur. J. Immunol. 2006, 36, 478–493. [Google Scholar] [CrossRef] [PubMed]
- Wucherpfennig, K.W.; Catz, I.; Hausmann, S.; Strominger, J.L.; Steinman, L.; Warren, K.G. Recognition of the immunodominant myelin basic protein peptide by autoantibodies and HLA-DR2-restricted T cell clones from multiple sclerosis patients. J. Clin. Investig. 1997, 100, 1114–1122. [Google Scholar] [CrossRef] [PubMed]
- International Multiple Sclerosis Genetics Consortium; Hafler, D.A.; Compston, A.; Sawcer, S.; Lander, E.S.; Daly, M.J.; De Jager, P.L.; de Bakker, P.I.; Gabriel, S.B.; Mirel, D.B.; et al. Risk alleles for multiple sclerosis identified by a genomewide study. N. Engl. J. Med. 2007, 357, 851–862. [Google Scholar]
- Moise, L.; Beseme, S.; Tassone, R.; Liu, R.; Kibria, F.; Terry, F.; Martin, W.; De Groot, A.S. T cell epitope redundancy: Cross-conservation of the TCR face between pathogens and self and its implications for vaccines and auto-immunity. Expert Rev. Vaccines 2016, 15, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Shahrizaila, N.; Yuki, N. Guillain-barre syndrome animal model: The first proof of molecular mimicry in human autoimmune disorder. J. Biomed. Biotechnol. 2011, 2011, 829129. [Google Scholar] [CrossRef] [PubMed]
- Madden, D.R. The three-dimensional structure of peptide-mhc complexes. Annu. Rev. Immunol. 1995, 13, 587–622. [Google Scholar] [CrossRef] [PubMed]
- Adams, E.J.; Luoma, A.M. The adaptable major histocompatibility complex (MHC) fold: Structure and function of nonclassical and MHC class I-like molecules. Annu. Rev. Immunol. 2013, 31, 529–561. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; van der Veeken, J.; Shugay, M.; Putintseva, E.V.; Osmanbeyoglu, H.U.; Dikiy, S.; Hoyos, B.E.; Moltedo, B.; Hemmers, S.; Treuting, P.; et al. A mechanism for expansion of regulatory T-cell repertoire and its role in self-tolerance. Nature 2015, 528, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Gao, M.; Chen, G.; Pierce, B.G.; Lu, J.; Weng, N.P.; Mariuzza, R.A. Structural basis for clonal diversity of the public T cell response to a dominant human cytomegalovirus epitope. J. Biol. Chem. 2015, 290, 29106–29119. [Google Scholar] [CrossRef] [PubMed]
- Lessard, C.J.; Ice, J.A.; Adrianto, I.; Wiley, G.B.; Kelly, J.A.; Gaffney, P.M.; Montgomery, C.G.; Moser, K.L. The genomics of autoimmune disease in the era of genome-wide association studies and beyond. Autoimmun. Rev. 2012, 11, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Buckley, M.W.; Trampont, P.C.; Arandjelovic, S.; Fond, A.M.; Juncadella, I.J.; Ravichandran, K.S. Shca regulates late stages of T cell development and peripheral CD4+ T cell numbers. J. Immunol. 2015, 194, 1665–1676. [Google Scholar] [CrossRef] [PubMed]
- Hesnard, L.; Legoux, F.; Gautreau, L.; Moyon, M.; Baron, O.; Devilder, M.C.; Bonneville, M.; Saulquin, X. Role of the MHC restriction during maturation of antigen-specific human T cells in the thymus. Eur. J. Immunol. 2016, 46, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Valli, A.; Sette, A.; Kappos, L.; Oseroff, C.; Sidney, J.; Miescher, G.; Hochberger, M.; Albert, E.D.; Adorini, L. Binding of myelin basic protein peptides to human histocompatibility leukocyte antigen class II molecules and their recognition by T cells from multiple sclerosis patients. J. Clin. Investig. 1993, 91, 616–628. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.; Howell, M.D.; Jaraquemada, D.; Flerlage, M.; Richert, J.; Brostoff, S.; Long, E.O.; McFarlin, D.E.; McFarland, H.F. A myelin basic protein peptide is recognized by cytotoxic T cells in the context of four HLA-DR types associated with multiple sclerosis. J. Exp. Med. 1991, 173, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Ota, K.; Matsui, M.; Milford, E.L.; Mackin, G.A.; Weiner, H.L.; Hafler, D.A. T-cell recognition of an immuno-dominant myelin basic protein epitope in multiple sclerosis. Nature 1990, 346, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Bieganowska, K.D.; Ausubel, L.J.; Modabber, Y.; Slovik, E.; Messersmith, W.; Hafler, D.A. Direct ex vivo analysis of activated, fas-sensitive autoreactive t cells in human autoimmune disease. J. Exp. Med. 1997, 185, 1585–1594. [Google Scholar] [CrossRef] [PubMed]
- Mantzourani, E.D.; Platts, J.A.; Brancale, A.; Mavromoustakos, T.M.; Tselios, T.V. Molecular dynamics at the receptor level of immunodominant myelin basic protein epitope 87–99 implicated in multiple sclerosis and its antagonists altered peptide ligands: Triggering of immune response. J. Mol. Graph. Model. 2007, 26, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Spyranti, Z.; Dalkas, G.A.; Spyroulias, G.A.; Mantzourani, E.D.; Mavromoustakos, T.; Friligou, I.; Matsoukas, J.M.; Tselios, T.V. Putative bioactive conformations of amide linked cyclic myelin basic protein peptide analogues associated with experimental autoimmune encephalomyelitis. J. Med. Chem. 2007, 50, 6039–6047. [Google Scholar] [CrossRef] [PubMed]
- Hahn, M.; Nicholson, M.J.; Pyrdol, J.; Wucherpfennig, K.W. Unconventional topology of self peptide-major histocompatibility complex binding by a human autoimmune T cell receptor. Nat. Immunol. 2005, 6, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Sethi, D.K.; Schubert, D.A.; Anders, A.K.; Heroux, A.; Bonsor, D.A.; Thomas, C.P.; Sundberg, E.J.; Pyrdol, J.; Wucherpfennig, K.W. A highly tilted binding mode by a self-reactive T cell receptor results in altered engagement of peptide and mhc. J. Exp. Med. 2011, 208, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Li, Y.; Kerzic, M.C.; Martin, R.; Mariuzza, R.A. Structure of a TCR with high affinity for self-antigen reveals basis for escape from negative selection. EMBO J. 2011, 30, 1137–1148. [Google Scholar] [CrossRef] [PubMed]
- Apostolopoulos, V.; Deraos, G.; Matsoukas, M.-T.; Day, S.; Stojanovska, L.; Tselios, T.; Androutsou, M.-E.; Matsoukas, J. Cyclic citrullinated MBP87–99 peptide stimulates t cell responses: Implications in triggering disease. Bioorg. Med. Chem. 2017, 25, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Katsara, M.; Deraos, G.; Tselios, T.; Matsoukas, J.; Apostolopoulos, V. Design of novel cyclic altered peptide ligands of myelin basic protein MBP87–99 that modulate immune responses in SJL/J mice. J. Med. Chem. 2008, 51, 3971–3978. [Google Scholar] [CrossRef] [PubMed]
- Katsara, M.; Matsoukas, J.; Deraos, G.; Apostolopoulos, V. Towards immunotherapeutic drugs and vaccines against multiple sclerosis. Acta Biochim. Biophys. Sin. 2008, 40, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Katsara, M.; Minigo, G.; Plebanski, M.; Apostolopoulos, V. The good, the bad and the ugly: How altered peptide ligands modulate immunity. Exp. Opin. Biol. Ther. 2008, 8, 1873–1884. [Google Scholar] [CrossRef] [PubMed]
- Katsara, M.; Tselios, T.; Deraos, S.; Deraos, G.; Matsoukas, M.T.; Lazoura, E.; Matsoukas, J.; Apostolopoulos, V. Round and round we go: Cyclic peptides in disease. Curr. Med. Chem. 2006, 13, 2221–2232. [Google Scholar] [PubMed]
- Katsara, M.; Yuriev, E.; Ramsland, P.A.; Deraos, G.; Tselios, T.; Matsoukas, J.; Apostolopoulos, V. A double mutation of MBP87–99 peptide induces IL-4 responses and antagonizes IFN-γ responses. J. Neuroimmunol. 2008, 200, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Katsara, M.; Yuriev, E.; Ramsland, P.A.; Deraos, G.; Tselios, T.; Matsoukas, J.; Apostolopoulos, V. Mannosylation of mutated MBP87–99 peptides diverts immune responses from Th1 to Th2. Mol. Immunol. 2008, 45, 3661–3670. [Google Scholar] [CrossRef] [PubMed]
- Katsara, M.; Yuriev, E.; Ramsland, P.A.; Tselios, T.; Deraos, G.; Lourbopoulos, A.; Grigoriadis, N.; Matsoukas, J.; Apostolopoulos, V. Altered peptide ligands of myelin basic protein (MBP87–99) conjugated to reduced mannan modulate immune responses in mice. Immunology 2009, 128, 521–533. [Google Scholar] [CrossRef] [PubMed]
- Matsoukas, J.; Apostolopoulos, V.; Kalbacher, H.; Papini, A.M.; Tselios, T.; Chatzantoni, K.; Biagioli, T.; Lolli, F.; Deraos, S.; Papathanassopoulos, P.; et al. Design and synthesis of a novel potent myelin basic protein epitope 87–99 cyclic analogue: Enhanced stability and biological properties of mimics render them a potentially new class of immunomodulators. J. Med. Chem. 2005, 48, 1470–1480. [Google Scholar] [CrossRef] [PubMed]
- Tapeinou, A.; Androutsou, M.E.; Kyrtata, K.; Vlamis-Gardikas, A.; Apostolopoulos, V.; Matsoukas, J.; Tselios, T. Conjugation of a peptide to mannan and its confirmation by tricine sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Anal. Biochem. 2015, 485, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Tselios, T.; Probert, L.; Daliani, I.; Matsoukas, E.; Troganis, A.; Gerothanassis, I.P.; Mavromoustakos, T.; Moore, G.J.; Matsoukas, J.M. Design and synthesis of a potent cyclic analogue of the myelin basic protein epitope MBP87–99: Importance of the ala81 carboxyl group and of a cyclic conformation for induction of experimental allergic encephalomyelitis. J. Med. Chem. 1999, 42, 1170–1177. [Google Scholar] [CrossRef] [PubMed]
- Koehler, N.K.; Yang, C.Y.; Varady, J.; Lu, Y.; Wu, X.W.; Liu, M.; Yin, D.; Bartels, M.; Xu, B.Y.; Roller, P.P.; et al. Structure-based discovery of nonpeptidic small organic compounds to block the T cell response to myelin basic protein. J. Med. Chem. 2004, 47, 4989–4997. [Google Scholar] [CrossRef] [PubMed]
- Mochona, B.; Le, L.; Gangapuram, M.; Mateeva, N.; Ardley, T.; Redda, K.K. Synthesis of 2-(N-benzylpyrrolyl)-benzimidazoles using polyphosphoric acid prompted cyclocondensation. J. Heterocycl. Chem. 2010, 47, 1367–1371. [Google Scholar] [CrossRef] [PubMed]
- Ohara, K.; Vasseur, J.-J.; Smietana, M. Nis-promoted guanylation of amines. Tetrahedron Lett. 2009, 50, 1463–1465. [Google Scholar] [CrossRef]
- Exposito, A.; Fernandez-Suarez, M.; Iglesias, T.; Munoz, L.; Riguera, R. Total synthesis and absolute configuration of minalemine a, a guanidine peptide from the marine tunicate didemnum rodriguesi. J. Org. Chem. 2001, 66, 4206–4213. [Google Scholar] [CrossRef] [PubMed]
- Aldulaijan, S.; Platts, J.A. Theoretical prediction of a peptide binding to major histocompatibility complex II. J. Mol. Graph. Model. 2010, 29, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A. Ab initio quantum chemistry: Methodology and applications. Proc. Natl. Acad. Sci. USA 2005, 102, 6648–6653. [Google Scholar] [CrossRef] [PubMed]
- Orio, M.; Pantazis, D.A.; Neese, F. Density functional theory. Photosynth. Res. 2009, 102, 443–453. [Google Scholar] [CrossRef] [PubMed]
- Goerigk, L.; Grimme, S. A thorough benchmark of density functional methods for general main group thermochemistry, kinetics, and noncovalent interactions. Phys. Chem. Chem. Phys. PCCP 2011, 13, 6670–6688. [Google Scholar] [CrossRef] [PubMed]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic interaction of a solute with a continuum. A direct utilization of ab initio molecular potentials for the prevision of solvent effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Carvalho, A.T.; Gouveia, M.L.; Raju Kanna, C.; Warmlander, S.K.; Platts, J.; Kamerlin, S.C. Theoretical modelling of epigenetically modified DNA sequences. F1000 Res. 2015, 4, 52. [Google Scholar] [CrossRef] [PubMed]
- Raulf-Heimsoth, M. T cell—Primary culture from peripheral blood. Methods Mol. Med. 2008, 138, 17–30. [Google Scholar] [PubMed]
- Zamvil, S.; Nelson, P.; Trotter, J.; Mitchell, D.; Knobler, R.; Fritz, R.; Steinman, L. T-cell clones specific for myelin basic protein induce chronic relapsing paralysis and demyelination. Nature 1985, 317, 355–358. [Google Scholar] [CrossRef] [PubMed]
- Kalbus, M.; Fleckenstein, B.T.; Offenhausser, M.; Bluggel, M.; Melms, A.; Meyer, H.E.; Rammensee, H.G.; Martin, R.; Jung, G.; Sommer, N. Ligand motif of the autoimmune disease-associated mouse MHC class II molecule h2-a(s). Eur. J. Immunol. 2001, 31, 551–562. [Google Scholar] [CrossRef]
- Chemical Computing Group Inc. Molecular Operating Environment (MOE), 2013.08; Chemical Computing Group Inc.: Montreal, QC, Canada, 2016. [Google Scholar]
- Cornell, W.D.; Cieplak, P.; Bayly, C.I.; Gould, I.R.; Merz, K.M., Jr.; Ferguson, D.M.; Spellmeyer, D.C.; Fox, T.; Caldwell, J.W.; Kollman, P.A. A second generation force field for the simulation of proteins, nucleic acids, and organic molecules. J. Am. Chem. Soc. 1995, 117, 5179–5197. [Google Scholar] [CrossRef]
- Jensen, J.H.; Li, H.; Robertson, A.D.; Molina, P.A. Prediction and rationalization of protein pka values using qm and qm/mm methods. J. Phys. Chem. A 2005, 109, 6634–6643. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Robertson, A.D.; Jensen, J.H. Very fast empirical prediction and rationalization of protein pka values. Proteins 2005, 61, 704–721. [Google Scholar] [CrossRef] [PubMed]
- Wucherpfennig, K.W.; Sethi, D. T cell receptor recognition of self and foreign antigens in the induction of autoimmunity. Semin. Immunol. 2011, 23, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Wucherpfennig, K.W.; Gagnon, E.; Call, M.J.; Huseby, E.S.; Call, M.E. Structural biology of the t-cell receptor: Insights into receptor assembly, ligand recognition, and initiation of signaling. Cold Spring Harb. Perspect. Biol. 2010, 2, a005140. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, Y.; Lue, J.; Quandt, J.A.; Martin, R.; Mariuzza, R.A. Structure of a human autoimmune tcr bound to a myelin basic protein self-peptide and a multiple sclerosis-associated mhc class II molecule. EMBO J. 2005, 24, 2968–2979. [Google Scholar] [CrossRef] [PubMed]
- Irwin, J.J.; Sterling, T.; Mysinger, M.M.; Bolstad, E.S.; Coleman, R.G. Zinc: A free tool to discover chemistry for biology. J. Chem. Inf. Model. 2012, 52, 1757–1768. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Corbeil, C.R.; Williams, C.I.; Labute, P. Variability in docking success rates due to dataset preparation. J. Comput. Aided Mol. Des. 2012, 26, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Martinez, C.; Kasavajhala, K.; Wickstrom, L.; Hauser, K.E.; Simmerling, C. Ff14sb: Improving the accuracy of protein side chain and backbone parameters from FF99SB. J. Chem. Theory Comput. 2015, 11, 3696–3713. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wolf, R.M.; Caldwell, J.W.; Kollman, P.A.; Case, D.A. Development and testing of a general amber force field. J. Comput. Chem. 2004, 25, 1157–1174. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of simple potential functions for simulating liquid water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Izaguirre, J.A.; Catarello, D.P.; Wozniak, J.M.; Skeel, R.D. Langevin stabilization of molecular dynamics. J. Chem. Phys. 2001, 114, 2090–2098. [Google Scholar] [CrossRef]
- Ryckaert, J.P.; Ciccotti, G.; Berendsen, H.J.C. Numerical integration of the cartesian equations of motion of a system with constraints: Molecular dynamics of n-alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle mesh ewald: An N⋅log(n) method for ewald sums in large systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Case, D.A.; Darden, T.; Cheatham, T.E., III; Simmerling, C.; Wang, J.; Duke, R.E.; Luo, R.; Walker, R.C.; Zhang, W.; Merz, K.M.; et al. Amber12; University of California: Oakland, CA, USA, 2012. [Google Scholar]
- Roe, D.R.; Cheatham, T.E., 3rd. Ptraj and cpptraj: Software for processing and analysis of molecular dynamics trajectory data. J. Chem. Theory Comput. 2013, 9, 3084–3095. [Google Scholar] [CrossRef] [PubMed]
- Padwa, A.; Chen, Y.Y.; Dent, W.; Nimmesgern, H. Synthetic application of cyanoaminosilanes as azomethine ylide equivalents. J. Org. Chem. 1985, 50, 4006–4014. [Google Scholar] [CrossRef]
- Villarreal, C.; Martínez, R. Synthesis of novel furo-, thieno-, and pyrroloazepines. Synthesis 2010, 2010, 3346–3352. [Google Scholar]
- Agelis, G.; Resvani, A.; Matsoukas, M.-T.; Tselios, T.; Kelaidonis, K.; Kalavrizioti, D.; Vlahakos, D.; Matsoukas, J. Towards non-peptide Ang II AT1 receptor antagonists based on urocanic acid: Rational design, synthesis and biological evaluation. Amino Acids 2011, 40, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Stewart, J.J. Optimization of parameters for semiempirical methods VI: More modifications to the nddo approximations and re-optimization of parameters. J. Mol. Model. 2013, 19, 1–32. [Google Scholar] [CrossRef] [PubMed]
- McNamara, J.P.; Hillier, I.H. Semi-empirical molecular orbital methods including dispersion corrections for the accurate prediction of the full range of intermolecular interactions in biomolecules. Phys. Chem. Chem. Phys. PCCP 2007, 9, 2362–2370. [Google Scholar] [CrossRef] [PubMed]
- McNamara, J.P.; Sharma, R.; Vincent, M.A.; Hillier, I.H.; Morgado, C.A. The non-covalent functionalisation of carbon nanotubes studied by density functional and semi-empirical molecular orbital methods including dispersion corrections. Phys. Chem. Chem. Phys. PCCP 2008, 10, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09; revision e.01; Gaussian, Inc.: Wallingford CT, USA, 2009. [Google Scholar]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods V: Modification of nddo approximations and application to 70 elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef] [PubMed]
- Klamt, A.; Schüürmann, G. Cosmo: A new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J. Chem. Soc. 1993, 799–805. [Google Scholar] [CrossRef]
- Day, S.; Tselios, T.; Androutsou, M.E.; Tapeinou, A.; Frilligou, I.; Stojanovska, L.; Matsoukas, J.; Apostolopoulos, V. Mannosylated linear and cyclic single amino acid mutant peptides using a small 10 amino acid linker constitute promising candidates against multiple sclerosis. Front. Immunol. 2015, 6, 136. [Google Scholar] [CrossRef] [PubMed]
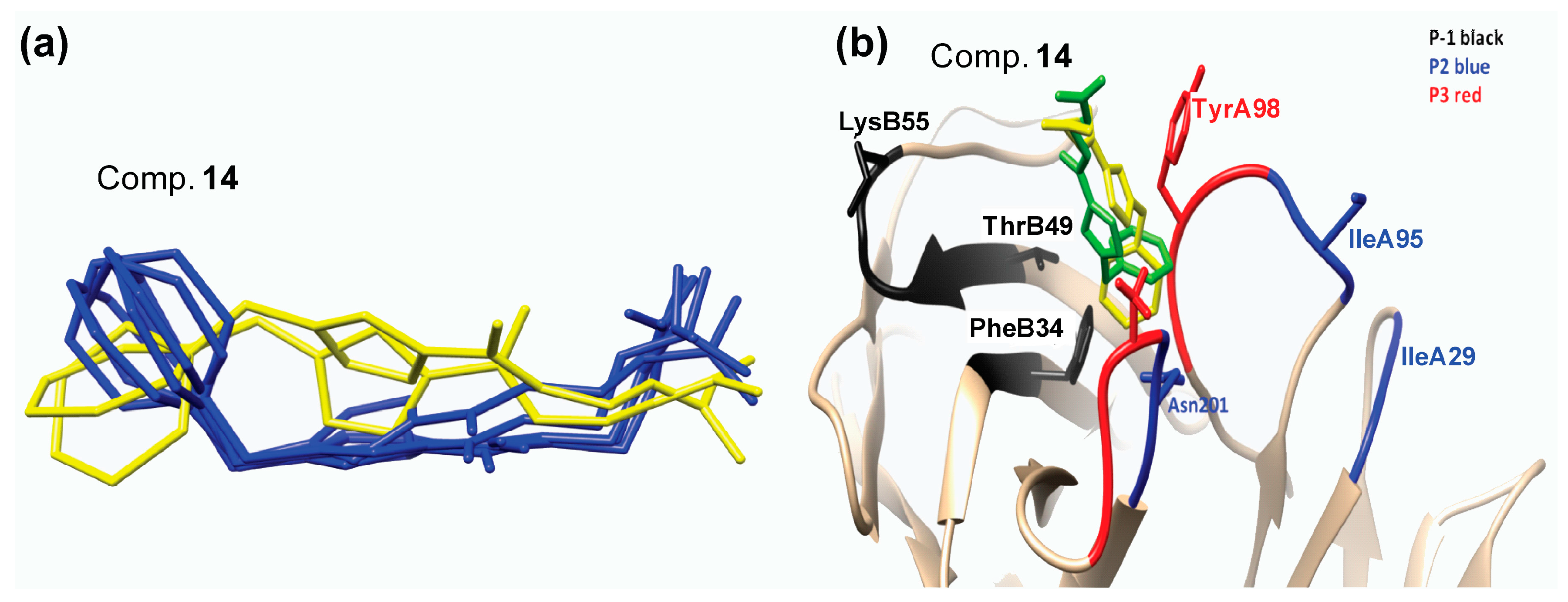
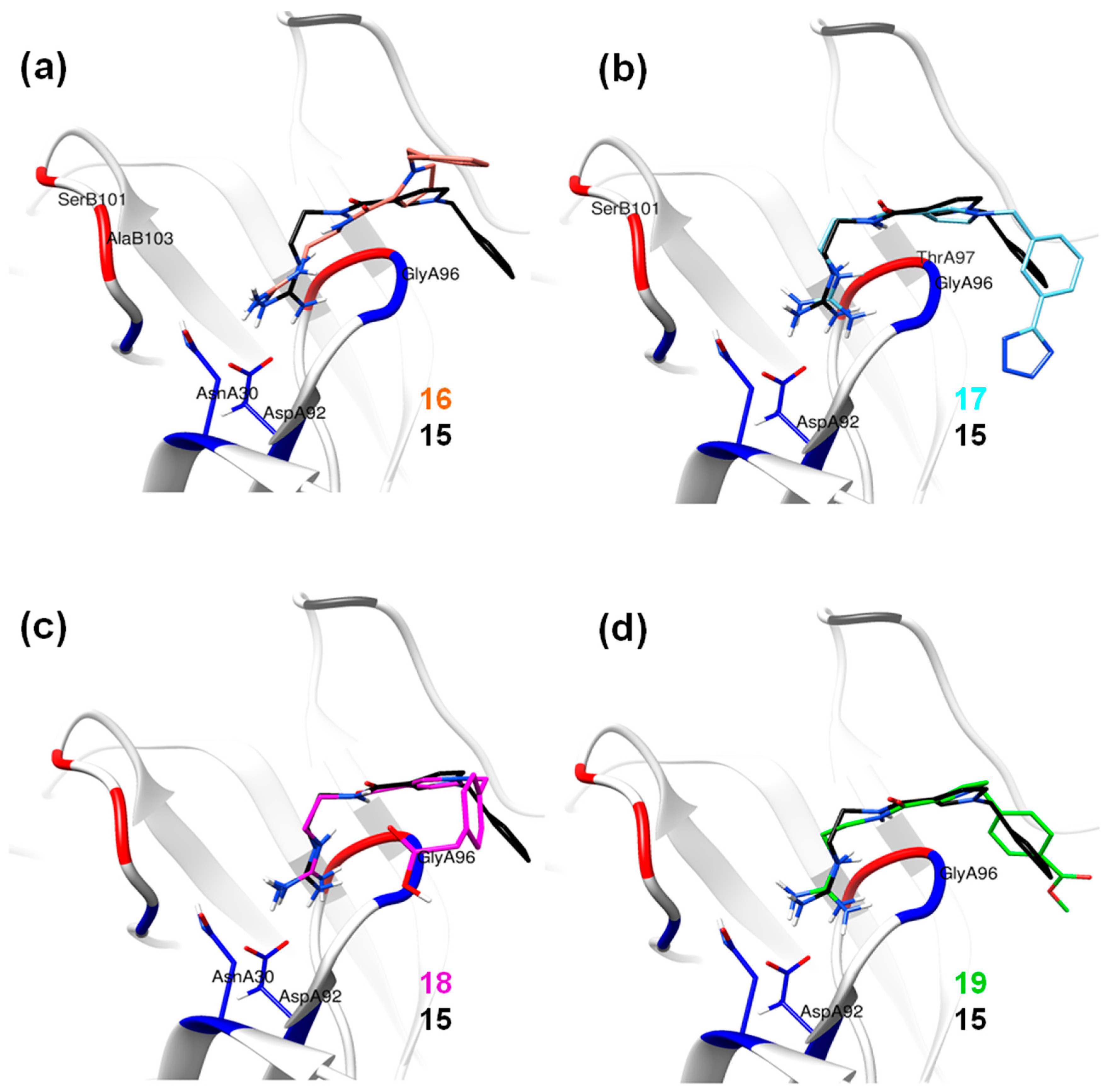
| Compound Number | Structure | ΔG d (kcal/mol) |
|---|---|---|
| MBP83–96 | Seq: ENPVVHFFKNIVTP | −11.89 |
| 1 a |  | −15.87 |
| 2 (* S/** R) | 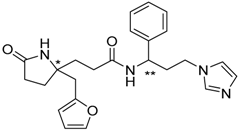 | −19.71 |
| 3 (* S/** S) | 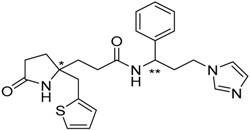 | −14.46 |
| 4 (* R) | 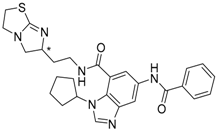 | −14.43 |
| 5 | 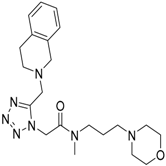 | −10.32 |
| 6 | 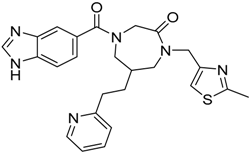 | −15.34 |
| 7 |  | −16.38 |
| 8 (* S) | 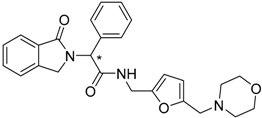 | −13.26 |
| 9 |  | −15.86 |
| 10 (Lead Compound) |  | −21.56 |
| 11 (* R) | 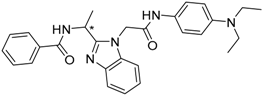 | −20.85 |
| 12 | 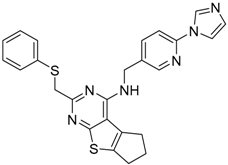 | −16.05 |
| 13 (* S) |  | −20.65 |
| 14 b | 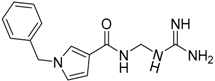 | −23.76 |
| 15 | 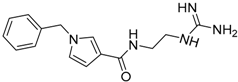 | −18.13 |
| 16 | 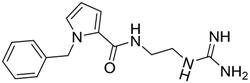 | −18.03 |
| 17 c | 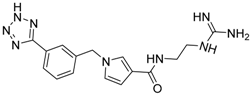 | −18.49 |
| 18 | 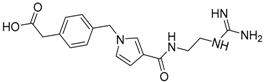 | −20.70 |
| 19 |  | −21.32 |
| Compound | MW (g/mol) | TPSA (Å2) | logP | Docking Score (kcal/mol) |
|---|---|---|---|---|
| 10 | 495.57 | 94.06 a | 5.25 b | −21.56 |
| 14 | 272.33 | 97.67 | −0.84 | −23.76 |
| 15 | 286.36 | 97.67 | −0.84 | −18.13 |
| 16 | 286.36 | 97.67 | −0.71 | −18.03 |
| 17 c | 354.40 | 152.13 | −1.62 | −18.49 |
| 18 | 344.39 | 137.34 | −1.49 | −20.70 |
| 19 | 344.39 | 126.34 | −1.42 | −21.32 |
| TCR Residues | Compounds | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 14 | 15 | 16 | 17 | 18 | 19 | |||||||
| Dock | MD | Dock | MD | Dock | MD | Dock | MD | Dock | MD | Dock | MD | |
| AsnA30 | ✓ a | ✓ | ✓ | ✓ | ✓ | ✓ | ||||||
| AspA92 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| ThrA93 | ✓ | |||||||||||
| GlyA96 | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| ThrA97 | ✓ | ✓ | ✓ | ✓ | ||||||||
| TyrA98 | ✓ | ✓ | ||||||||||
| TyrA100 | ✓ | |||||||||||
| AsnB51 | ✓ | ✓ | ||||||||||
| LysB55 | ✓ | ✓ | ||||||||||
| SerB101 | ✓ | ✓ | ||||||||||
| AlaB103 | ✓ | |||||||||||
| AsnB104 | ✓ | |||||||||||
| GluB106 | ✓ | |||||||||||
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yannakakis, M.-P.; Simal, C.; Tzoupis, H.; Rodi, M.; Dargahi, N.; Prakash, M.; Mouzaki, A.; Platts, J.A.; Apostolopoulos, V.; Tselios, T.V. Design and Synthesis of Non-Peptide Mimetics Mapping the Immunodominant Myelin Basic Protein (MBP83–96) Epitope to Function as T-Cell Receptor Antagonists. Int. J. Mol. Sci. 2017, 18, 1215. https://doi.org/10.3390/ijms18061215
Yannakakis M-P, Simal C, Tzoupis H, Rodi M, Dargahi N, Prakash M, Mouzaki A, Platts JA, Apostolopoulos V, Tselios TV. Design and Synthesis of Non-Peptide Mimetics Mapping the Immunodominant Myelin Basic Protein (MBP83–96) Epitope to Function as T-Cell Receptor Antagonists. International Journal of Molecular Sciences. 2017; 18(6):1215. https://doi.org/10.3390/ijms18061215
Chicago/Turabian StyleYannakakis, Mary-Patricia, Carmen Simal, Haralambos Tzoupis, Maria Rodi, Narges Dargahi, Monica Prakash, Athanasia Mouzaki, James A. Platts, Vasso Apostolopoulos, and Theodore V. Tselios. 2017. "Design and Synthesis of Non-Peptide Mimetics Mapping the Immunodominant Myelin Basic Protein (MBP83–96) Epitope to Function as T-Cell Receptor Antagonists" International Journal of Molecular Sciences 18, no. 6: 1215. https://doi.org/10.3390/ijms18061215
APA StyleYannakakis, M.-P., Simal, C., Tzoupis, H., Rodi, M., Dargahi, N., Prakash, M., Mouzaki, A., Platts, J. A., Apostolopoulos, V., & Tselios, T. V. (2017). Design and Synthesis of Non-Peptide Mimetics Mapping the Immunodominant Myelin Basic Protein (MBP83–96) Epitope to Function as T-Cell Receptor Antagonists. International Journal of Molecular Sciences, 18(6), 1215. https://doi.org/10.3390/ijms18061215