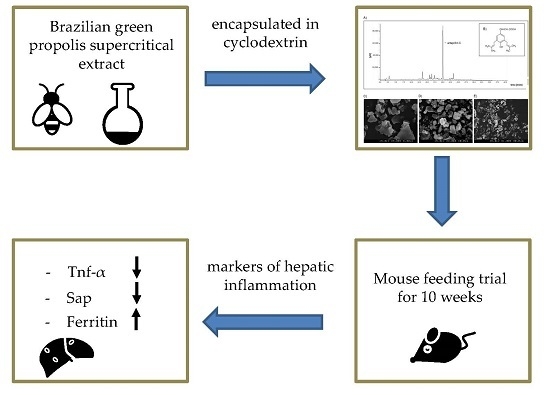
Anti-Inflammatory Properties of Brazilian Green Propolis Encapsulated in a γ-Cyclodextrin Complex in Mice Fed a Western-Type Diet
Abstract
:1. Introduction
2. Results
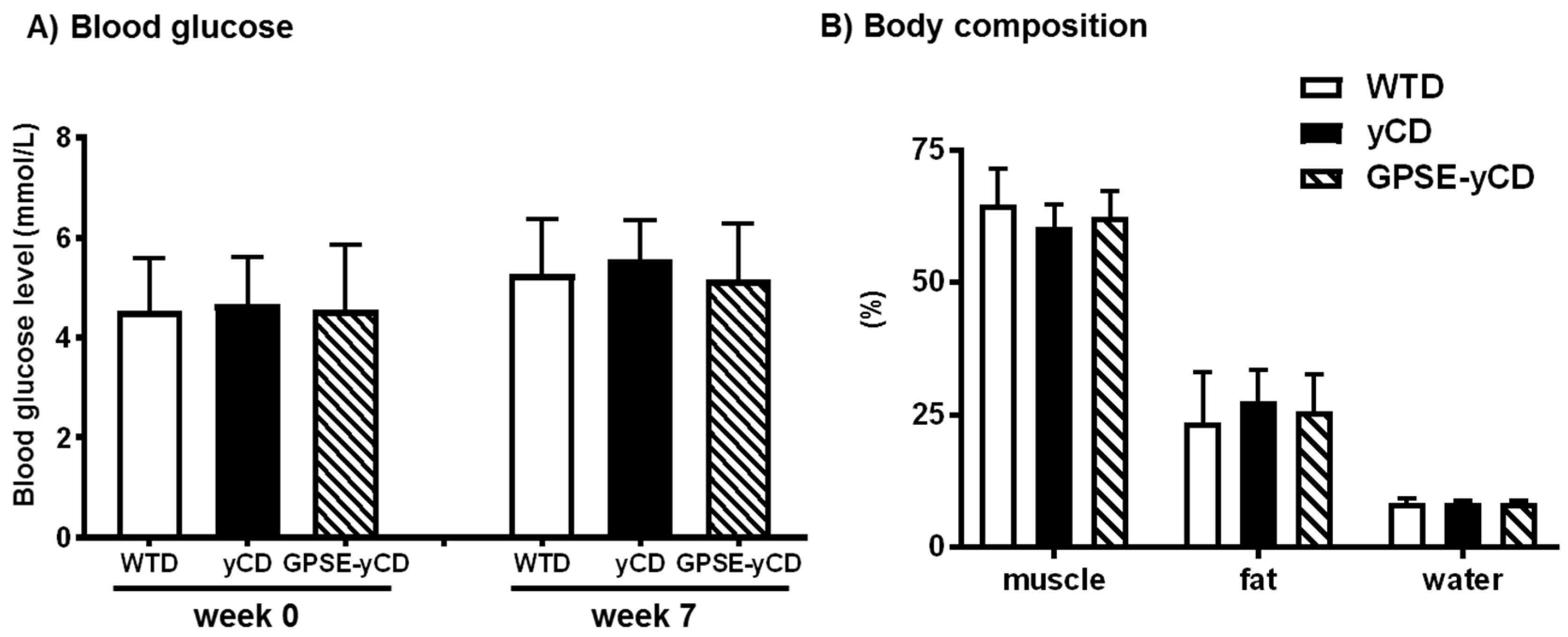
2.1. Body Weight, Food Intake, Blood Glucose and Body Composition
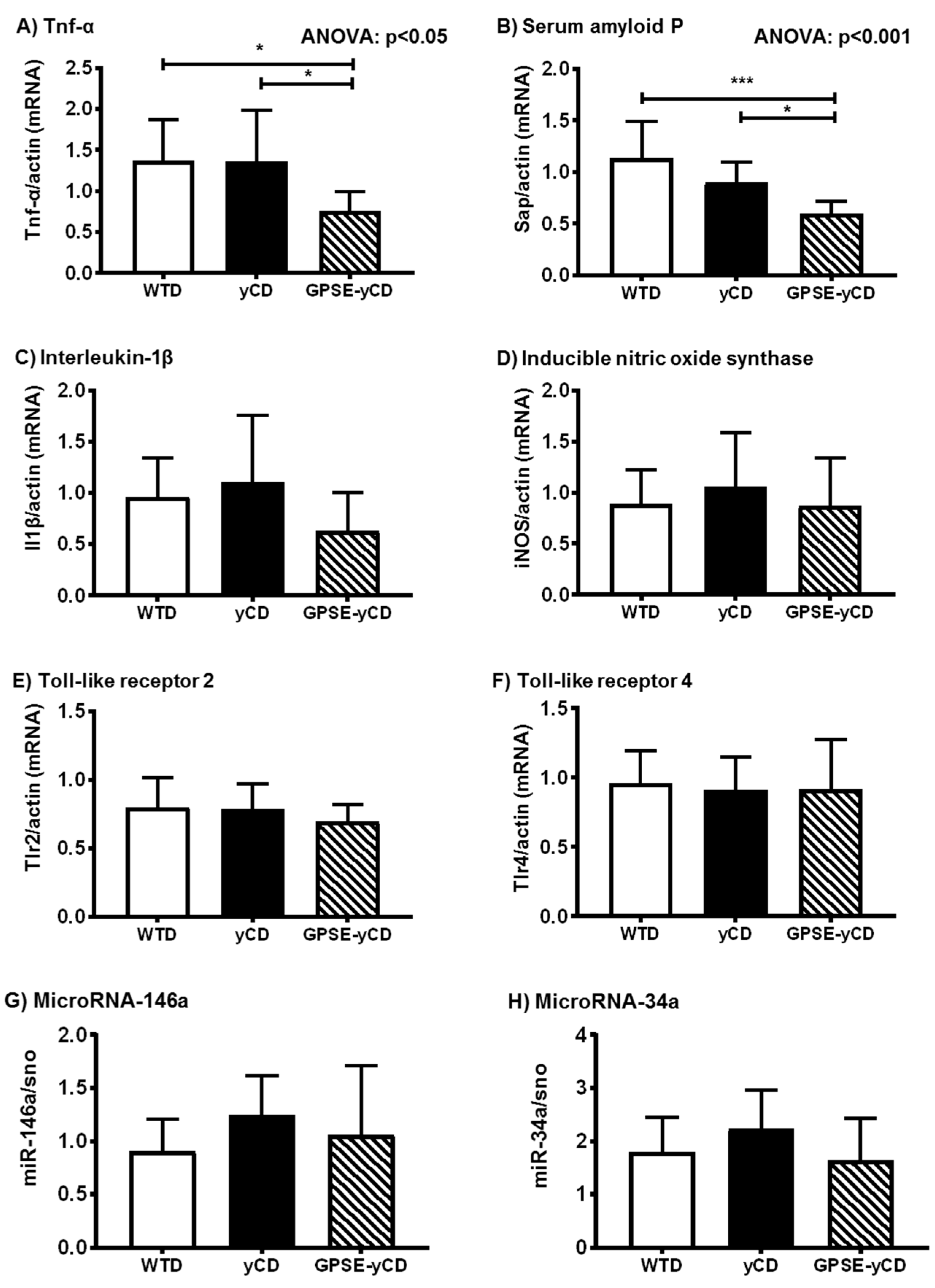
2.2. Inflammatory Gene Expression
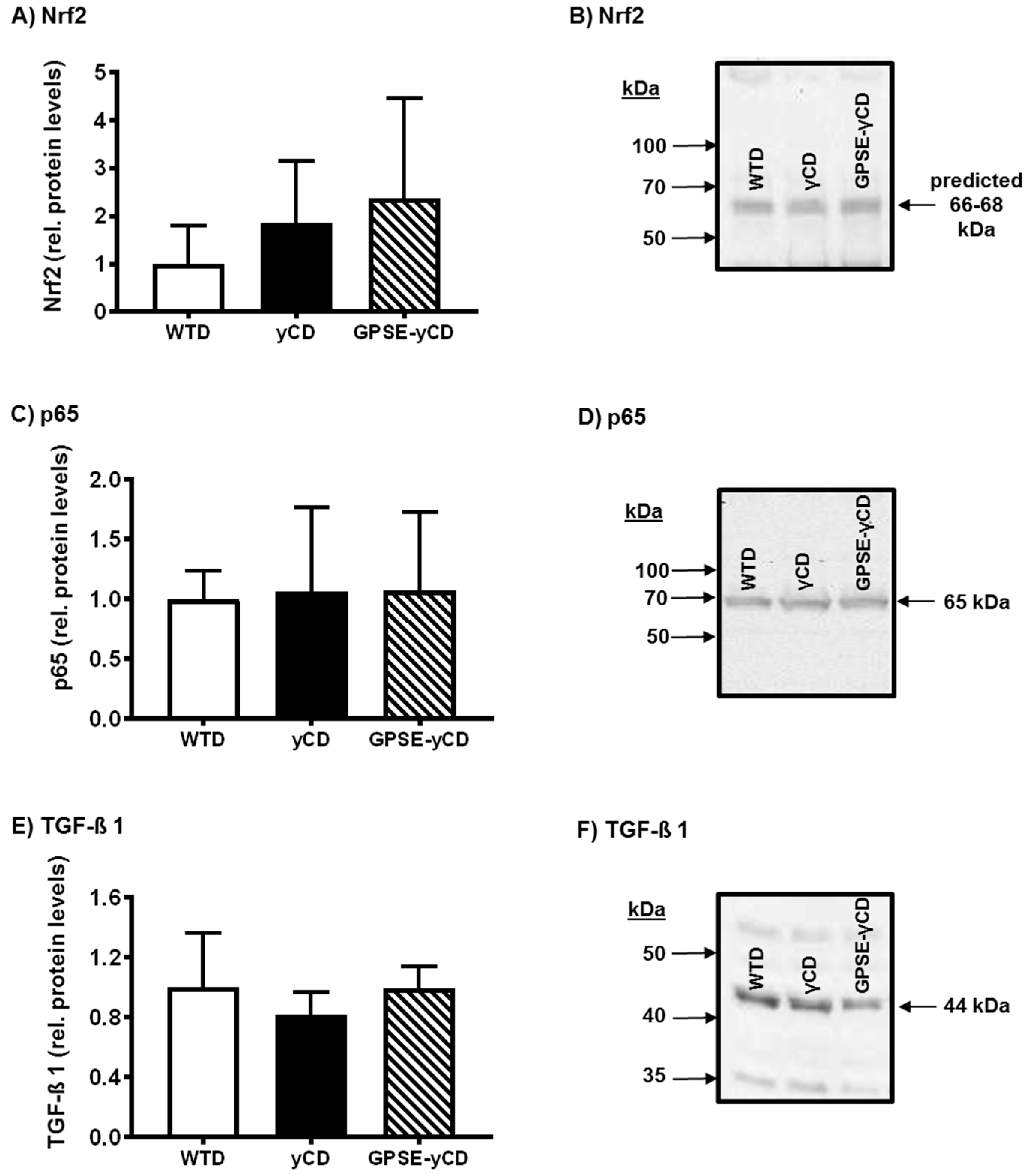
2.3. Antioxidant Defence
2.4. Inflammatory Protein Expression
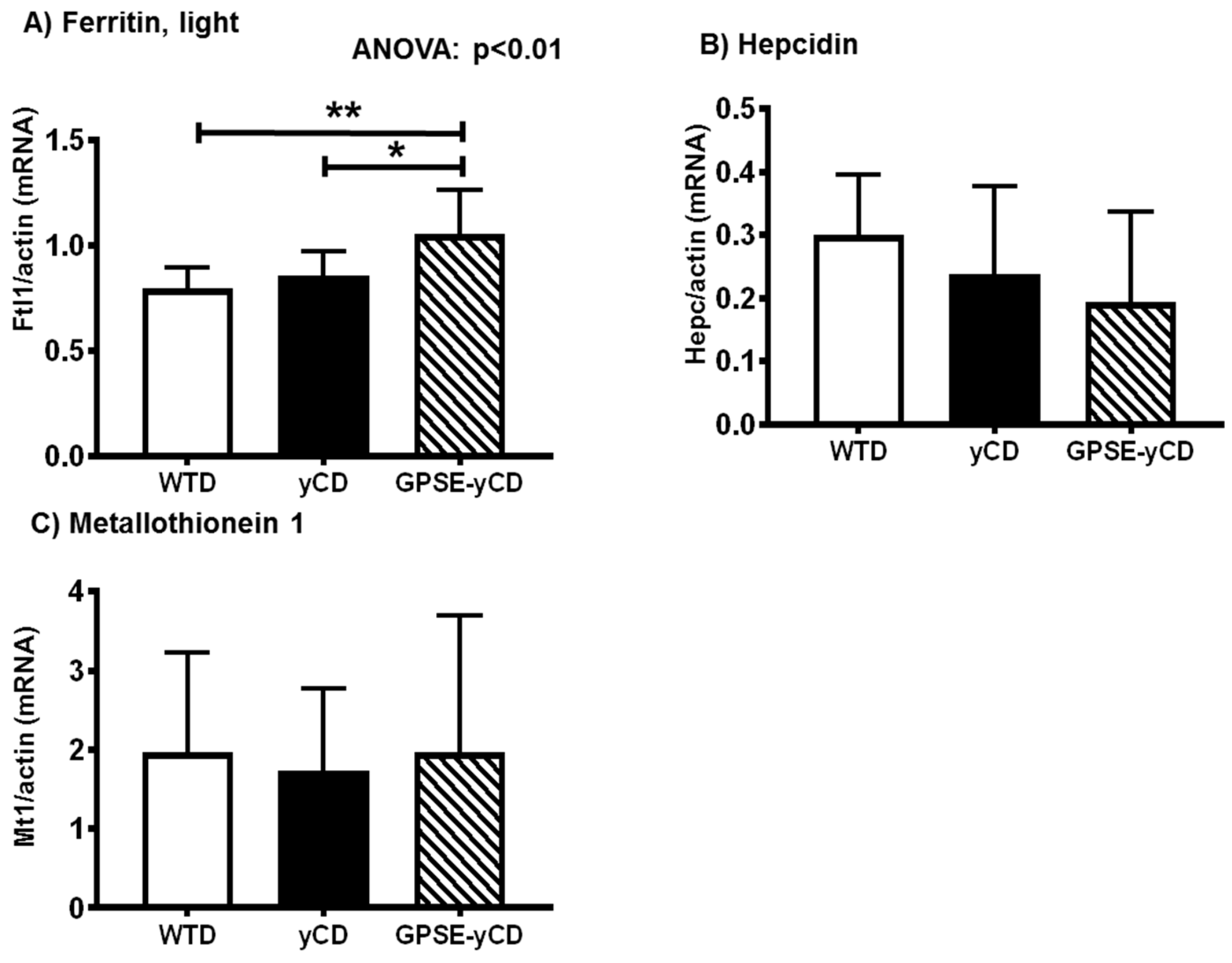
2.5. Ferritin, Hepcidin, and Metallothionein
3. Discussion
4. Materials and Methods
4.1. Experimental Animals and Diets
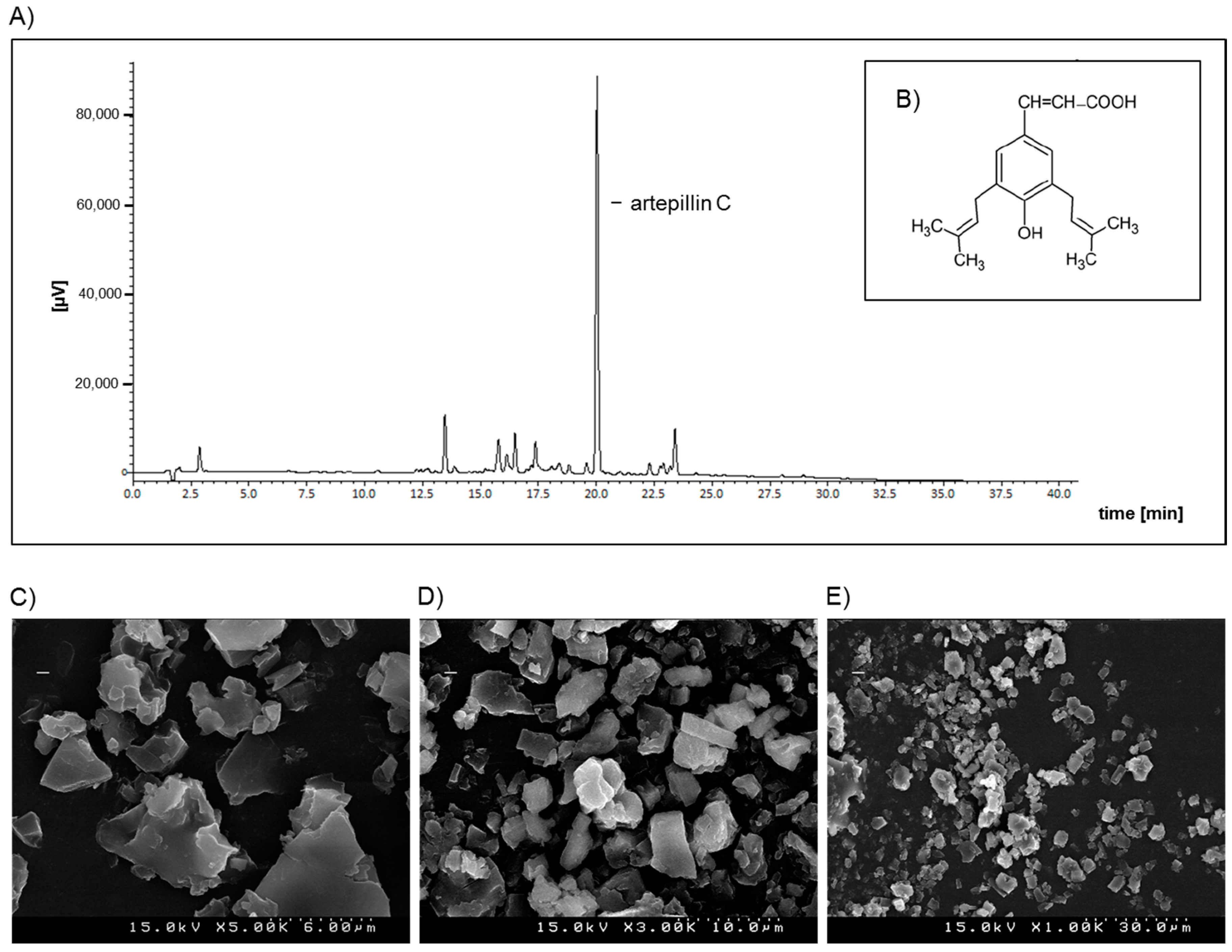
4.2. Preparation of GPSE/γ-Cyclodextrin Complex and Artepillin C for Analysis by HPLC
4.3. L C-APCI-IT-MS/MS Analysis of the Green Propolis Supercritical Extract (GPSE)
4.4. Morphological Characterization of the GPSE-γCD Complex by Scanning Electron Microscopy
4.5. Blood Glucose and Body Composition
4.6. Hepatic Gene Expression Using Quantitative Reverse Transcriptase PCR
4.7. MicroRNA Determination
4.8. Determination of Hepatic Protein Expression Levels by Western Blotting
4.9. Statistical Analyses
5. Conclusions
Supplementary Materials
Acknowledgements
Author Contributions
Conflicts of Interest
Abbreviations
| CAPE | Caffeic ester phenethyl ester |
| γCD | γ-Cyclodextrin |
| GPSE | Brazilian green propolis supercritical extract |
| Sap | Serum amyloid P |
| SD | Standard deviation |
| LPS | Lipopolysaccharide |
| Tlr | Toll-like receptor |
| TGF-β1 | Transforming growth factor β1 |
| TNF-α | Tumour necrosis factor α |
| WTD | Western-type diet |
References
- Bankova, V.; Popova, M.; Trusheva, B. Propolis volatile compounds: chemical diversity and biological activity: A review. Chem. Cent. J. 2014, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Washio, K.; Shimamoto, Y.; Kitamura, H. Brazilian propolis extract increases leptin expression in mouse adipocytes. Biomed. Res. 2015, 36, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Nafady, A.M.; El-Shanawany, M.A.; Mohamed, M.H.; Hassanean, H.A.; Nohara, T.; Yoshimitsu, H.; Ono, M.; Sugimoto, H.; Doi, S.; Sasaki, K.; et al. Cyclodextrin-enclosed substances of Brazilian propolis. Chem. Pharm. Bull. 2003, 51, 984–985. [Google Scholar] [CrossRef] [PubMed]
- Machado, B.A.; Silva, R.P.; Barreto Gde, A.; Costa, S.S.; Silva, D.F.; Brandao, H.N.; Rocha, J.L.; Dellagostin, O.A.; Henriques, J.A.; Umsza-Guez, M.A.; et al. Chemical Composition and Biological Activity of Extracts Obtained by Supercritical Extraction and Ethanolic Extraction of Brown, Green and Red Propolis Derived from Different Geographic Regions in Brazil. PLoS ONE 2016, 11, e0145954. [Google Scholar] [CrossRef] [PubMed]
- Catchpole, O.; Mitchell, K.; Bloor, S.; Davis, P.; Suddes, A. Antiproliferative activity of New Zealand propolis and phenolic compounds vs human colorectal adenocarcinoma cells. Fitoterapia 2015, 106, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Wadhwa, R.; Nigam, N.; Bhargava, P.; Dhanjal, J.K.; Goyal, S.; Grover, A.; Sundar, D.; Ishida, Y.; Terao, K.; Kaul, S.C. Molecular Characterization and Enhancement of Anticancer Activity of Caffeic Acid Phenethyl Ester by γ Cyclodextrin. J. Cancer 2016, 7, 1755–1771. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, J.P.; Bueno, P.C.; Gregorio, L.E.; da Silva Filho, A.A.; Furtado, N.A.; de Sousa, M.L.; Bastos, J.K. A reliable quantitative method for the analysis of phenolic compounds in Brazilian propolis by reverse phase high performance liquid chromatography. J. Sep. Sci. 2007, 30, 2656–2665. [Google Scholar] [CrossRef] [PubMed]
- Hata, T.; Tazawa, S.; Ohta, S.; Rhyu, M.R.; Misaka, T.; Ichihara, K. Artepillin C, a major ingredient of Brazilian propolis, induces a pungent taste by activating TRPA1 channels. PLoS ONE 2012, 7, e48072. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Emerging Adjuvant Therapy for Cancer: Propolis and its Constituents. J. Diet. Suppl. 2016, 13, 245–268. [Google Scholar] [CrossRef] [PubMed]
- Neiva, K.G.; Catalfamo, D.L.; Holliday, S.; Wallet, S.M.; Pileggi, R. Propolis decreases lipopolysaccharide-induced inflammatory mediators in pulp cells and osteoclasts. Dent. Traumatol. 2014, 30, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Kucharska, A.Z.; Sokol-Letowska, A.; Mertas, A.; Czuba, Z.P.; Krol, W. Chemical Composition and Anti-Inflammatory Effect of Ethanolic Extract of Brazilian Green Propolis on Activated J774A.1 Macrophages. Evid.-Based Complement. Altern. Med. 2013, 2013, 976415. [Google Scholar] [CrossRef] [PubMed]
- Korish, A.A.; Arafa, M.M. Propolis derivatives inhibit the systemic inflammatory response and protect hepatic and neuronal cells in acute septic shock. Braz. J. Infect. Dis. 2011, 15, 332–338. [Google Scholar] [CrossRef]
- Ishikawa, H.; Goto, M.; Matsuura, N.; Murakami, Y.; Goto, C.; Sakai, T.; Kanazawa, K. A pilot, randomized, placebo-controlled, double-blind phase 0/biomarker study on effect of artepillin C-rich extract of Brazilian propolis in frequent colorectal adenoma polyp patients. J. Am. Coll. Nutr. 2012, 31, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, S.; McCullough, R.L.; Sanz-Garcia, C.; Saikia, P.; Alkhouri, N.; Matloob, A.; Pollard, K.A.; McMullen, M.R.; Croniger, C.M.; Nagy, L.E. Receptor interacting protein 3 protects mice from high-fat diet-induced liver injury. Hepatology 2016, 64, 1518–1533. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, S.A.; Kountouras, J.; Zavos, C.; Tsiaousi, E. The role of adiponectin in the pathogenesis and treatment of non-alcoholic fatty liver disease. Diabetes Obes. Metab. 2010, 12, 365–383. [Google Scholar] [CrossRef] [PubMed]
- Meydani, S.N.; Wu, D. Age-associated inflammatory changes: Role of nutritional intervention. Nutr. Rev. 2007, 65, S213–S216. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Cruces, J.; Hernandez, O.; Ortega, E. Strategies to improve the functions and redox state of the immune system in aged subjects. Curr. Pharm. Des. 2011, 17, 3966–3993. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Nicolas, J.M.; Rodriguez-Bonilla, P.; Garcia-Carmona, F. Cyclodextrins and antioxidants. Crit. Rev. Food Sci. Nutr. 2014, 54, 251–276. [Google Scholar] [CrossRef] [PubMed]
- Fenyvesi, E.; Vikmon, M.; Szente, L. Cyclodextrins in Food Technology and Human Nutrition: Benefits and Limitations. Crit. Rev. Food sci. Nutr. 2016, 56, 1981–2004. [Google Scholar] [CrossRef] [PubMed]
- Schloesser, A.; Esatbeyoglu, T.; Piegholdt, S.; Dose, J.; Ikuta, N.; Okamoto, H.; Ishida, Y.; Terao, K.; Matsugo, S.; Rimbach, G. Dietary Tocotrienol/γ-Cyclodextrin Complex Increases Mitochondrial Membrane Potential and ATP Concentrations in the Brains of Aged Mice. Oxid. Med. Cell. Longev. 2015, 2015, 789710. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, N.; Sugiyama, H.; Shimosegawa, H.; Nakane, R.; Ishida, Y.; Uekaji, Y.; Nakata, D.; Pallauf, K.; Rimbach, G.; Terao, K.; et al. Analysis of the enhanced stability of R(+)-α lipoic Acid by the complex formation with cyclodextrins. Int. J. Mol. Sci. 2013, 14, 3639–3655. [Google Scholar] [CrossRef] [PubMed]
- Nikolai, S.; Huebbe, P.; Metges, C.C.; Schloesser, A.; Dose, J.; Ikuta, N.; Terao, K.; Matsugo, S.; Rimbach, G. R-α lipoic acid γ-cyclodextrin complex increases energy expenditure: A 4-month feeding study in mice. Nutrition 2014, 30, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Terao, K.; Nakata, D.; Fukumi, H.; Schmid, G.; Arima, H.; Hirayama, F.; Uekama, K. Enhancement of oral bioavailability of coenzyme Q10 by complexation with gamma-cyclodextrin in healthy adults. Nutr. Res. 2006, 26, 503–508. [Google Scholar] [CrossRef]
- Grau, T.; Bonet, A. Caloric intake and liver dysfunction in critically ill patients. Curr. Opin. Clin. Nutr. Metabol. Care 2009, 12, 175–179. [Google Scholar] [CrossRef] [PubMed]
- Oh, A.R.; Bae, J.S.; Lee, J.; Shin, E.; Oh, B.C.; Park, S.C.; Cha, J.Y. Ursodeoxycholic acid decreases age-related adiposity and inflammation in mice. BMB Rep. 2016, 49, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Bayram, B.; Nikolai, S.; Huebbe, P.; Ozcelik, B.; Grimm, S.; Grune, T.; Frank, J.; Rimbach, G. Biomarkers of oxidative stress, antioxidant defence and inflammation are altered in the senescence-accelerated mouse prone 8. Age 2013, 35, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Meli, R.; Mattace Raso, G.; Irace, C.; Simeoli, R.; di Pascale, A.; Paciello, O.; Pagano, T.B.; Calignano, A.; Colonna, A.; Santamaria, R. High Fat Diet Induces Liver Steatosis and Early Dysregulation of Iron Metabolism in Rats. PLoS ONE 2013, 8, e66570. [Google Scholar] [CrossRef] [PubMed]
- Pagliarone, A.C.; Orsatti, C.L.; Bufalo, M.C.; Missima, F.; Bachiega, T.F.; Junior, J.P.; Sforcin, J.M. Propolis effects on pro-inflammatory cytokine production and Toll-like receptor 2 and 4 expression in stressed mice. Int. Immunopharmacol. 2009, 9, 1352–1356. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; French, B.A.; Li, J.; Tillman, B.; French, S.W. Altered regulation of miR-34a and miR-483-3p in alcoholic hepatitis and DDC fed mice. Exp. Mol. Pathol. 2015, 99, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Kong, L.; Ni, Q.; Lu, Y.; Ding, W.; Liu, G.; Pu, L.; Tang, W.; Kong, L. miR-146a ameliorates liver ischemia/reperfusion injury by suppressing IRAK1 and TRAF6. PLoS ONE 2014, 9, e101530. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Garcia, J.C.; Cardona, F.; Tinahones, F.J. Inflammation, Oxidative Stress and Metabolic Syndrome: Dietary Modulation. Curr. Vasc. Pharmacol. 2013, 11, 906–919. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Jiang, Y.Z.; Liu, P.; He, W.; Xiong, Z.; Chang, W.; Zhu, J.; Cui, Q. Toll-like 4 receptor /NFκB inflammatory/miR-146a pathway contributes to the ART-correlated preterm birth outcome. Oncotarget 2016, 7, 72475–72485. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lian, F.; Liu, C.; Hu, K.Q.; Wang, X.D. Isocaloric Pair-Fed High-Carbohydrate Diet Induced More Hepatic Steatosis and Inflammation than High-Fat Diet Mediated by miR-34a/SIRT1 Axis in Mice. Sci. Rep. 2015, 5, 16774. [Google Scholar] [CrossRef] [PubMed]
- Song, L.; Zhou, F.; Cheng, L.; Hu, M.; He, Y.; Zhang, B.; Liao, D.; Xu, Z. MicroRNA-34a Suppresses Autophagy in Alveolar Type II Epithelial Cells in Acute Lung Injury by Inhibiting FoxO3 Expression. Inflammation 2017, 40, 927–936. [Google Scholar] [CrossRef] [PubMed]
- Fuse, Y.; Kobayashi, M. Conservation of the Keap1-Nrf2 System: An Evolutionary Journey through Stressful Space and Time. Molecules 2017, 22, 436. [Google Scholar] [CrossRef] [PubMed]
- Esatbeyoglu, T.; Ewald, P.; Yasui, Y.; Yokokawa, H.; Wagner, A.E.; Matsugo, S.; Winterhalter, P.; Rimbach, G. Chemical Characterization, Free Radical Scavenging, and Cellular Antioxidant and Anti-Inflammatory Properties of a Stilbenoid-Rich Root Extract of Vitis vinifera. Oxid. Med. Cell. Longev. 2016, 2016, 8591286. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.G.; Oakley, F. Nuclear factor-βB1: Regulation and function. Int. J. Biochem. Cell Biol. 2008, 40, 1425–1430. [Google Scholar] [CrossRef] [PubMed]
- Richter, K.; Konzack, A.; Pihlajaniemi, T.; Heljasvaara, R.; Kietzmann, T. Redox-fibrosis: Impact of TGFbeta1 on ROS generators, mediators and functional consequences. Redox Biol. 2015, 6, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Kano, Y.; Horie, N.; Doi, S.; Aramaki, F.; Maeda, H.; Hiragami, F.; Kawamura, K.; Motoda, H.; Koike, Y.; Akiyama, J.; et al. Artepillin C derived from propolis induces neurite outgrowth in PC12m3 cells via ERK and p38 MAPK pathways. Neurochem. Res. 2008, 33, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Hitomi, Y.; Yoshida, M.; Yoshioka, E. Absorption and bioavailability of artepillin C in rats after oral administration. J. Agric. Food Chem. 2005, 53, 9928–9933. [Google Scholar] [CrossRef] [PubMed]
- Rushdi, A.I.; Adgaba, N.; Bayaqoob, N.I.; Al-Khazim, A.; Simoneit, B.I.; El-Mubarak, A.H.; Al-Mutlaq, K.F. Characteristics and chemical compositions of propolis from Ethiopia. SpringerPlus 2014, 3, 253. [Google Scholar] [CrossRef] [PubMed]
- Tazawa, S.; Warashina, T.; Noro, T. Studies on the constituents of Brazilian propolis. II. Chem. Pharm. Bull. 1999, 47, 1388–1392. [Google Scholar] [CrossRef]
- Machado, C.S.; Mokochinski, J.B.; de Lira, T.O.; de Oliveira, F.D.C.E.; Cardoso, M.V.; Ferreira, R.G.; Sawaya, A.C.H.; Ferreira, A.G.; Pessoa, C.; Cuesta-Rubio, O.; et al. Comparative Study of Chemical Composition and Biological Activity of Yellow, Green, Brown, and Red Brazilian Propolis. Evid. Based Complement. Alternat. Med. 2016, 2016, 6057650. [Google Scholar] [CrossRef] [PubMed]
- Garnier, N.; Cren-Olive, C.; Rolando, C.; Regert, M. Characterization of archaeological beeswax by electron ionization and electrospray ionization mass spectrometry. Anal. Chem. 2002, 74, 4868–4877. [Google Scholar] [CrossRef] [PubMed]
- Gurtler, A.; Kunz, N.; Gomolka, M.; Hornhardt, S.; Friedl, A.A.; McDonald, K.; Kohn, J.E.; Posch, A. Stain-Free technology as a normalization tool in Western blot analysis. Anal. Biochem. 2013, 433, 105–111. [Google Scholar] [CrossRef] [PubMed]
| Ingredients | Western-Type Diet (WTD) |
|---|---|
| Macronutrients | |
| Crude protein | 17.5% |
| Crude fat | 21.2% |
| Crude fibre | 5.0% |
| Crude ash | 4.5% |
| Nitrogen-free extracts | 48.8% |
| Starch | 14.6% |
| Sugar | 33.2% |
| Cholesterol | 2.1 mg/kg |
| Supplements | |
| γ-Cyclodextrin (WTD+γCD) | 2.2 g/kg |
| Green propolis supercritical extract-γCD (WTD+GPSE-γCD) 1 | 2.3 g/kg |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rimbach, G.; Fischer, A.; Schloesser, A.; Jerz, G.; Ikuta, N.; Ishida, Y.; Matsuzawa, R.; Matsugo, S.; Huebbe, P.; Terao, K. Anti-Inflammatory Properties of Brazilian Green Propolis Encapsulated in a γ-Cyclodextrin Complex in Mice Fed a Western-Type Diet. Int. J. Mol. Sci. 2017, 18, 1141. https://doi.org/10.3390/ijms18061141
Rimbach G, Fischer A, Schloesser A, Jerz G, Ikuta N, Ishida Y, Matsuzawa R, Matsugo S, Huebbe P, Terao K. Anti-Inflammatory Properties of Brazilian Green Propolis Encapsulated in a γ-Cyclodextrin Complex in Mice Fed a Western-Type Diet. International Journal of Molecular Sciences. 2017; 18(6):1141. https://doi.org/10.3390/ijms18061141
Chicago/Turabian StyleRimbach, Gerald, Alexandra Fischer, Anke Schloesser, Gerold Jerz, Naoko Ikuta, Yoshiyuki Ishida, Ryota Matsuzawa, Seiichi Matsugo, Patricia Huebbe, and Keiji Terao. 2017. "Anti-Inflammatory Properties of Brazilian Green Propolis Encapsulated in a γ-Cyclodextrin Complex in Mice Fed a Western-Type Diet" International Journal of Molecular Sciences 18, no. 6: 1141. https://doi.org/10.3390/ijms18061141
APA StyleRimbach, G., Fischer, A., Schloesser, A., Jerz, G., Ikuta, N., Ishida, Y., Matsuzawa, R., Matsugo, S., Huebbe, P., & Terao, K. (2017). Anti-Inflammatory Properties of Brazilian Green Propolis Encapsulated in a γ-Cyclodextrin Complex in Mice Fed a Western-Type Diet. International Journal of Molecular Sciences, 18(6), 1141. https://doi.org/10.3390/ijms18061141