Subcellular Localization of Arabidopsis Pathogenesis-Related 1 (PR1) Protein
Abstract
:1. Introduction
2. Results
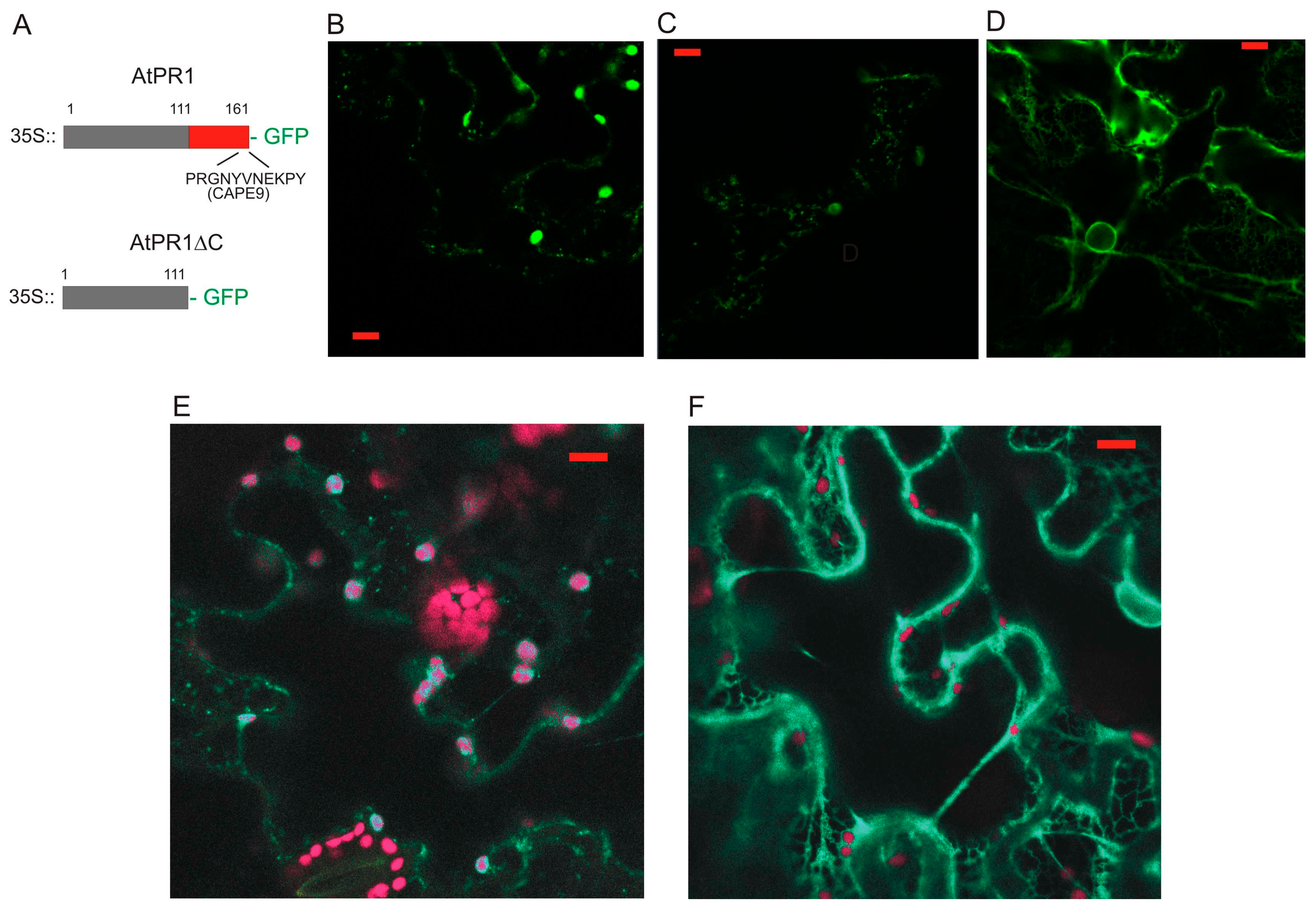
2.1. Localization of AtPR1 after the Transient Expression in N. benthamiana
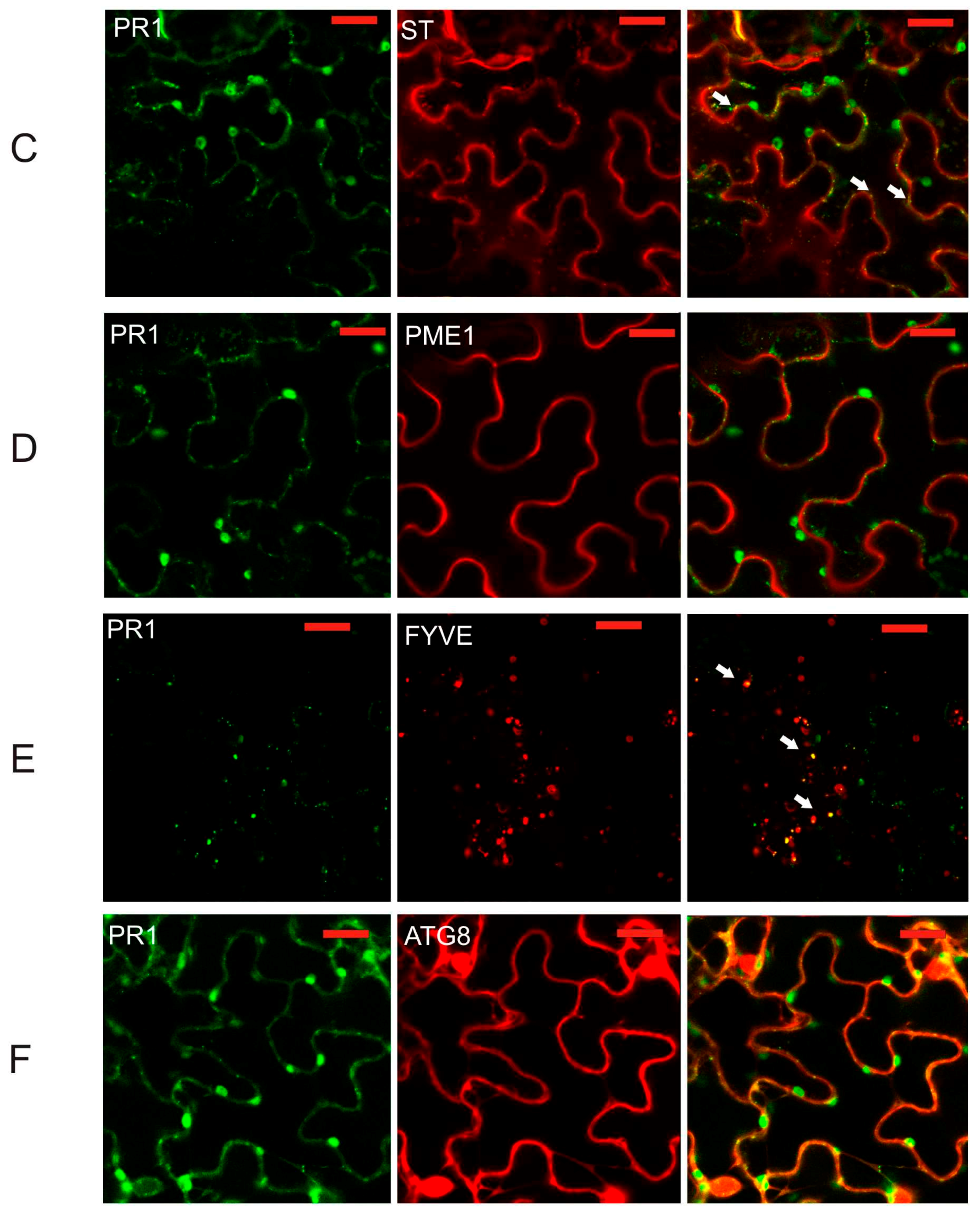
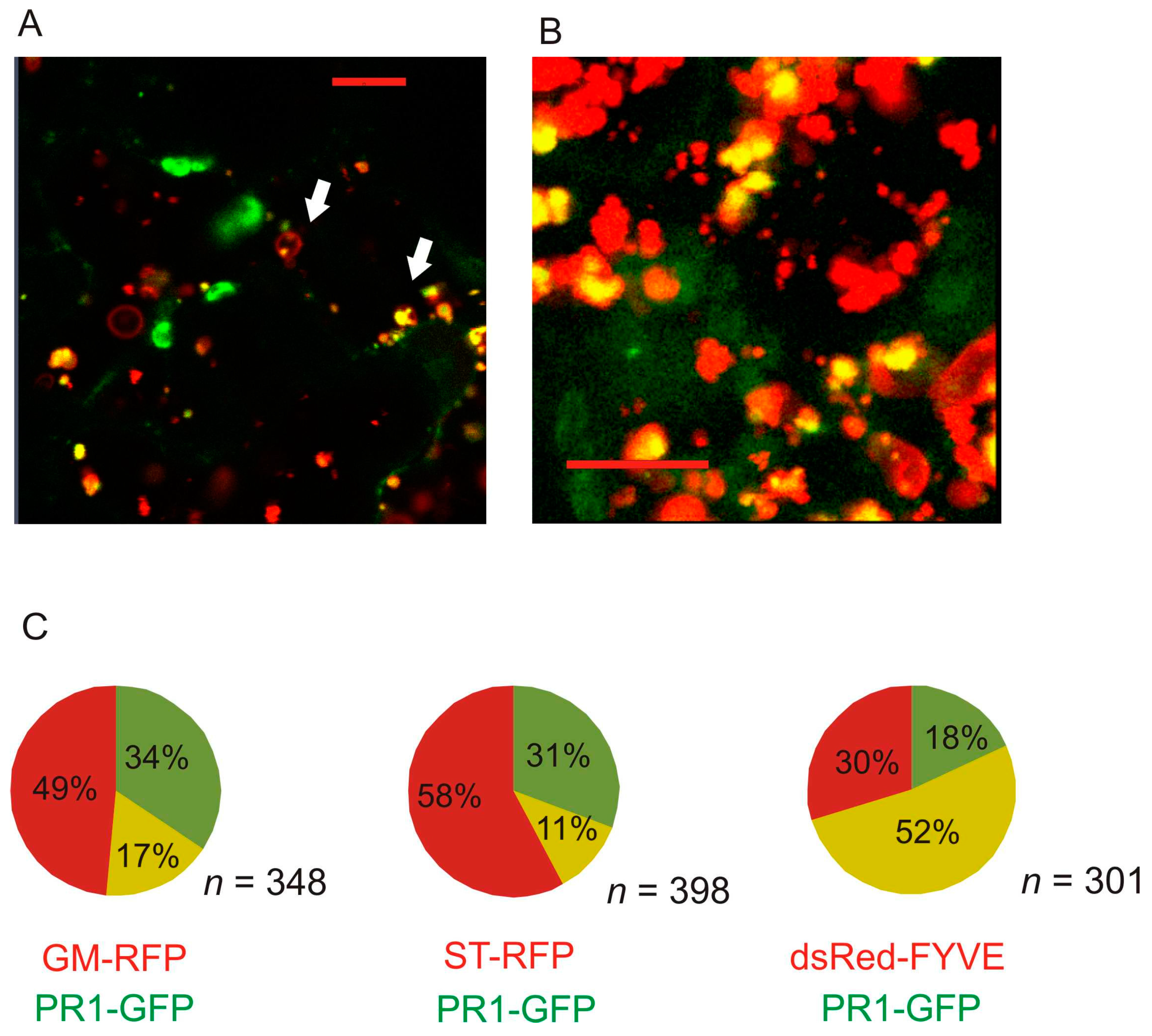
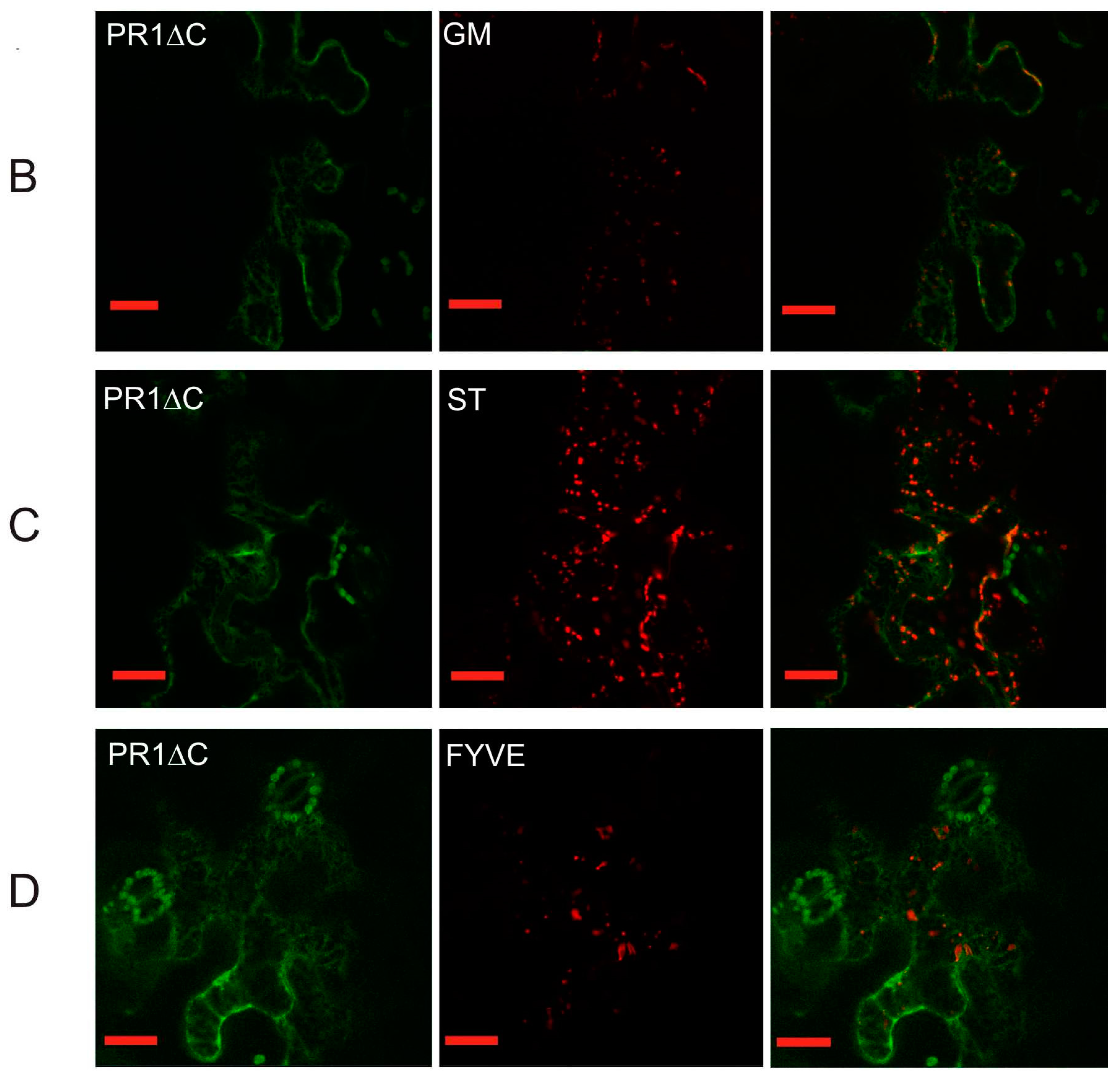
2.2. Co-Localization of PR1 with Compartmental Markers in N. benthamiana Transiently Transformed Cells
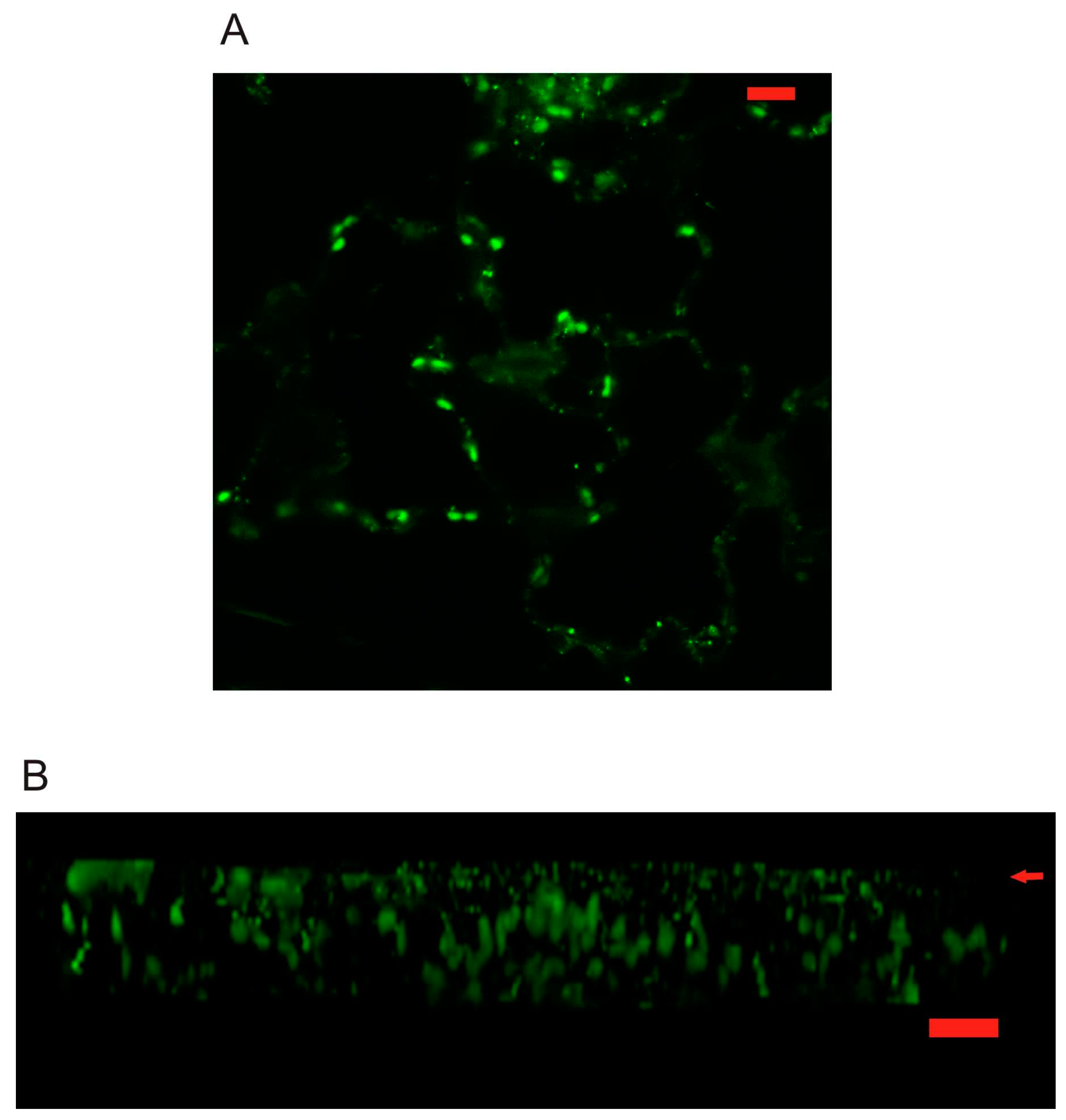
2.3. Localization of AtPR1 Expressed under the Native and 35S Promoter in Arabidopsis Cells
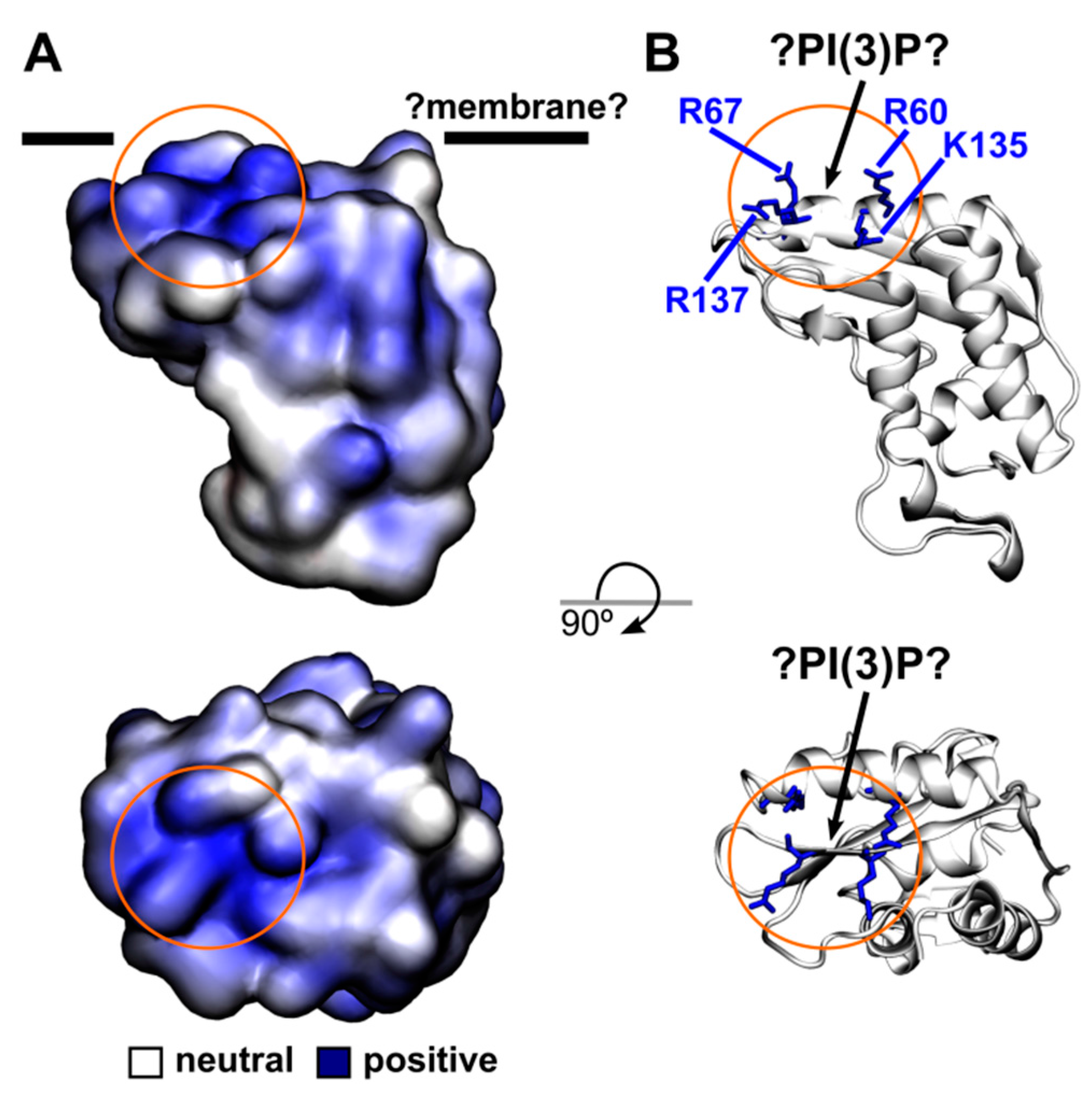
3. Discussion
4. Material and Methods
4.1. Plant Material
4.2. Cloning Procedure
4.3. Transient Expression in N. benthamiana
4.4. Stable Transformation of A. thaliana
4.5. Confocal Microscopy
4.6. Treatments of Seedlings
4.7. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis and Western Blot
4.8. Homology Modelling of the PR1 Structure and the Electrostatics Calculation
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stintzi, A.; Heitz, T.; Prasad, V.; Wiedemann-Merdinoglu, S.; Kauffmann, S.; Geoffroy, P.; Legrand, M.; Fritig, B. Plant ‘pathogenesis-related’ proteins and their role in defense against pathogens. Biochimie 1993, 75, 687–706. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Pierpoint, W.S.; Boller, T.; Conejero, V. Recommendations for naming plant pathogenesis-related proteins. Plant Mol. Biol. Rep. 1994, 12, 245–264. [Google Scholar] [CrossRef]
- Van Loon, L.C.; van Kammen, A. Polyacrylamide disc electrophoresis of the soluble leaf proteins from Nicotiana tabacum var. “Samsun” and “Samsun NN”. II. Changes in protein constitution after infection with tobacco mosaic virus. Virology 1970, 40, 190–211. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Van Strien, E.A. The families of pathogenesis-related proteins, their activities, and comparative analysis of PR-1 type proteins. Physiol. Mol. Plant Pathol. 1999, 55, 85–97. [Google Scholar] [CrossRef]
- Schreiber, M.C.; Karlo, J.C.; Kovalick, G.E. A novel cDNA from Drosophila encoding a protein with similarity to mammalian cysteine-rich secretory proteins, wasp venom antigen 5, and plant group 1 pathogenesis-related proteins. Gene 1997, 191, 135–141. [Google Scholar] [CrossRef]
- Van Loon, L.C.; Rep, M.; Pieterse, C.M. Significance of inducible defense-related proteins in infected plants. Annu. Rev. Phytopathol. 2006, 44, 135–162. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhara, I.; Iwai, T.; Seo, S.; Yanagawa, Y.; Kawahigasi, H.; Hirose, S.; Ohkawa, Y.; Ohashi, Y. Characteristic expression of twelve rice PR1 family genes in response to pathogen infection, wounding, and defense-related signal compounds (121/180). Mol. Genet. Genom. 2008, 279, 415–427. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Innes, R.W. The KEEP ON GOING protein of Arabidopsis regulates intracellular protein trafficking and is degraded during fungal infection. Plant Cell 2012, 24, 4717–4730. [Google Scholar] [CrossRef] [PubMed]
- Stone, S.L.; Williams, L.A.; Farmer, L.M.; Vierstra, R.D.; Callis, J. KEEP ON GOING, a RING E3 ligase essential for Arabidopsis growth and development, is involved in abscisic acid signaling. Plant Cell 2006, 18, 3415–3428. [Google Scholar] [CrossRef] [PubMed]
- Haseloff, J.; Siemering, K.R.; Prasher, D.C.; Hodge, S. Removal of a cryptic intron and subcellular localization of green fluorescent protein are required to mark transgenic Arabidopsis plants brightly. Proc. Natl. Acad. Sci. USA 1997, 94, 2122–2127. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, Y.; Yamada, K.; Shimada, T.; Matsushima, R.; Nishizawa, N.K.; Nishimura, M.; Hara-Nishimura, I. A proteinase-storing body that prepares for cell death or stresses in the epidermal cells of Arabidopsis. Plant Cell Physiol. 2001, 42, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Shimada, T.L.; Hiruma, K.; Takano, Y. Pathogen infection trial increases the secretion of proteins localized in the endoplasmic reticulum body of Arabidopsis. Plant Physiol. 2013, 163, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Broekaert, W.F.; Terras, F.R.G.; Cammue, B.P.A. Induced and preformed antimicrobial proteins. In Mechanisms of Resistance to Plant Diseases; Slusarenko, A.J., Fraser, R.S.S., Van Loon, L.C., Eds.; Dordrecht: Kluwer, The Netherlands, 2000; pp. 371–477. [Google Scholar]
- Rivière, M.P.; Marais, A.; Ponchet, M.; Willats, W.; Galiana, E. Silencing of acidic pathogenesis-related PR-1 genes increases extracellular β-(1–>3)-glucanase activity at the onset of tobacco defence reactions. J. Exp. Bot. 2008, 59, 1225–1239. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Lee, C.Y.; Cheng, K.T.; Chang, W.H.; Huang, R.N.; Nam, H.G.; Chen, Y.R. Quantitative peptidomics study reveals that a wound-induced peptide from PR-1 regulates immune signaling in tomato. Plant Cell 2014, 26, 4135–4148. [Google Scholar] [CrossRef] [PubMed]
- Chien, P.S.; Nam, H.G.; Chen, Y.R. A salt-regulated peptide derived from the CAP superfamily protein negatively regulates salt-stress tolerance in Arabidopsis. J. Exp. Bot. 2015, 66, 5301–5313. [Google Scholar] [CrossRef] [PubMed]
- Nelson, B.K.; Cai, X.; Nebenführ, A. A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J. 2007, 51, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Saint-Jore-Dupas, C.; Nebenführ, A.; Boulaflous, A.; Follet-Gueye, M.L.; Plasson, C.; Hawes, C.; Driouich, A.; Faye, L.; Gomord, V. Plant N-glycan processing enzymes employ different targeting mechanisms for their spatial arrangement along the secretory pathway. Plant Cell 2006, 18, 3182–3200. [Google Scholar] [CrossRef] [PubMed]
- Dorokhov, Y.L.; Skurat, E.V.; Frolova, O.Y.; Gasanova, T.V.; Ivanov, P.A.; Ravin, N.V.; Skryabin, K.G.; Mäkinen, K.M.; Klimyuk, V.I.; Gleba, Y.Y.; et al. Role of the leader sequence in tobacco pectin methylesterase secretion. FEBS Lett. 2006, 580, 3329–3334. [Google Scholar] [CrossRef] [PubMed]
- Honig, A.; Avin-Wittenberg, T.; Ufaz, S.; Galili, G. A new type of compartment, defined by plant-specific Atg8-interacting proteins, is induced upon exposure of Arabidopsis plants to carbon starvation. Plant Cell 2012, 24, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Voigt, B.; Timmers, A.C.; Samaj, J.; Hlavacka, A.; Ueda, T.; Preuss, M.; Nielsen, E.; Mathur, J.; Emans, N.; Stenmark, H.; et al. Actin-based motility of endosomes is linked to the polar tip growth of root hairs. Eur. J. Cell Biol. 2005, 84, 609–621. [Google Scholar] [CrossRef] [PubMed]
- Sessa, G.; Yang, X.Q.; Raz, V.; Eyal, Y.; Fluhr, R. Dark induction and subcellular localization of the pathogenesis-related PRB-1b protein. Plant Mol. Biol. 1995, 28, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Berson, T.; von Wangenheim, D.; Takáč, T.; Šamajová, O.; Rosero, A.; Ovečka, M.; Komis, G.; Stelzer, E.H.; Šamaj, J. Trans-Golgi network localized small GTPase RabA1d is involved in cell plate formation and oscillatory root hair growth. BMC Plant Biol. 2014, 14, 252. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Eu, Y.J.; Yoo, C.M.; Kim, Y.W.; Pih, K.T.; Jin, J.B.; Kim, S.J.; Stenmark, H.; Hwang, I. Trafficking of phosphatidylinositol 3-phosphate from the trans-Golgi network to the lumen of the central vacuole in plant cells. Plant Cell 2001, 13, 287–301. [Google Scholar] [CrossRef] [PubMed]
- Kolb, C.; Nagel, M.K.; Kalinowska, K.; Hagmann, J.; Ichikawa, M.; Anzenberger, F.; Alkofer, A.; Sato, M.H.; Braun, P.; Isono, E. FYVE1 is essential for vacuole biogenesis and intracellular trafficking in Arabidopsis. Plant Physiol. 2015, 167, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Van Galen, J.; van Balkom, B.W.; Serrano, R.L.; Kaloyanova, D.; Eerland, R.; Stüven, E.; Helms, J.B. Binding of GAPR-1 to negatively charged phospholipid membranes: Unusual binding characteristics to phosphatidylinositol. Mol. Membr. Biol. 2010, 27, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Van Galen, J.; Olrichs, N.K.; Schouten, A.; Serrano, R.L.; Nolte Hoen, E.N.; Eerland, R.; Kaloyanova, D.; Gros, P.; Helms, J.B. Interaction of GAPR-1 with lipid bilayers is regulated by alternative homodimerization. Biochim. Biophys. Acta 2012, 1818, 2175–2183. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, J.C.; Sturm, S.; Langhans, M.; Hillmer, S.; Marcote, M.J.; Robinson, D.G.; Aniento, F. Coupled transport of Arabidopsis p24 proteins at the ER-Golgi interface. J. Exp. Bot. 2012, 63, 4243–4261. [Google Scholar] [CrossRef] [PubMed]
- Axe, E.L.; Walker, S.A.; Manifava, M.; Chandra, P.; Roderick, H.L.; Habermann, A.; Griffiths, G.; Ktistakis, N.T. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J. Cell Biol. 2008, 182, 685–701. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, S.; Speicher, KD.; Stahelin, R.V.; Speicher, D.W.; Haldar, K. PI(3)P-independent and -dependent pathways function together in a vacuolar translocation sequence to target malarial proteins to the host erythrocyte. Mol. Biochem. Parasitol. 2012, 185, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Kurose, T.; Hino, T.; Tanaka, K.; Kawamukai, M.; Niwa, Y.; Toyooka, K.; Matsuoka, K.; Jinbo, T.; Kimura, T. Development of series of gateway binary vectors, pGWBs, for realizing efficient construction of fusion genes for plant transformation. J. Biosci. Bioeng. 2007, 104, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Hála, M.; Cole, R.; Synek, L.; Drdová, E.; Pecenková, T.; Nordheim, A.; Lamkemeyer, T.; Madlung, J.; Hochholdinger, F.; Fowler, J.E.; et al. An exocyst complex functions in plant cell growth in Arabidopsis and tobacco. Plant Cell 2008, 20, 1330–1345. [Google Scholar] [CrossRef] [PubMed]
- Biasini, M.; Bienert, S.; Waterhouse, A.; Arnold, K.; Studer, G.; Schmidt, T.; Kiefer, F.; Cassarino, T.G.; Bertoni, M.; Bordoli, L.; Schwede, T. SWISS-MODEL: Modelling protein tertiary and quaternary structure using evolutionary information. Nucleic Acids Res. 2014, 42, W252–W258. [Google Scholar] [CrossRef] [PubMed]
- Wiederstein, M.; Sippl, M.J. ProSA-web: Interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res. 2007, 35, W407–W410. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.A.; Sept, D.; Joseph, S.; Holst, M.J.; McCammon, J.A. Electrostatics of nanosystems: Application to microtubules and the ribosome. Proc. Natl. Acad. Sci. USA 2001, 98, 10037–10041. [Google Scholar] [CrossRef] [PubMed]
- Humphrey, W.; Dalke, A.; Schulten, K. VMD: Visual molecular dynamics. J. Mol. Graph. 1996, 14, 33–38. [Google Scholar] [CrossRef]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pečenková, T.; Pleskot, R.; Žárský, V. Subcellular Localization of Arabidopsis Pathogenesis-Related 1 (PR1) Protein. Int. J. Mol. Sci. 2017, 18, 825. https://doi.org/10.3390/ijms18040825
Pečenková T, Pleskot R, Žárský V. Subcellular Localization of Arabidopsis Pathogenesis-Related 1 (PR1) Protein. International Journal of Molecular Sciences. 2017; 18(4):825. https://doi.org/10.3390/ijms18040825
Chicago/Turabian StylePečenková, Tamara, Roman Pleskot, and Viktor Žárský. 2017. "Subcellular Localization of Arabidopsis Pathogenesis-Related 1 (PR1) Protein" International Journal of Molecular Sciences 18, no. 4: 825. https://doi.org/10.3390/ijms18040825




