Annexins as Overlooked Regulators of Membrane Trafficking in Plant Cells
Abstract
:1. Introduction
2. Annexin Characteristics
3. Membrane Flow in Eukaryotic Cells
The Role of Calcium in Plant Membrane Trafficking
4. Annexins in Membrane Trafficking
4.1. What Is Known from Vertebrate Annexins
4.2. Plant Annexins
4.3. Plant Annexins in Membrane Trafficking—Where We Are Now
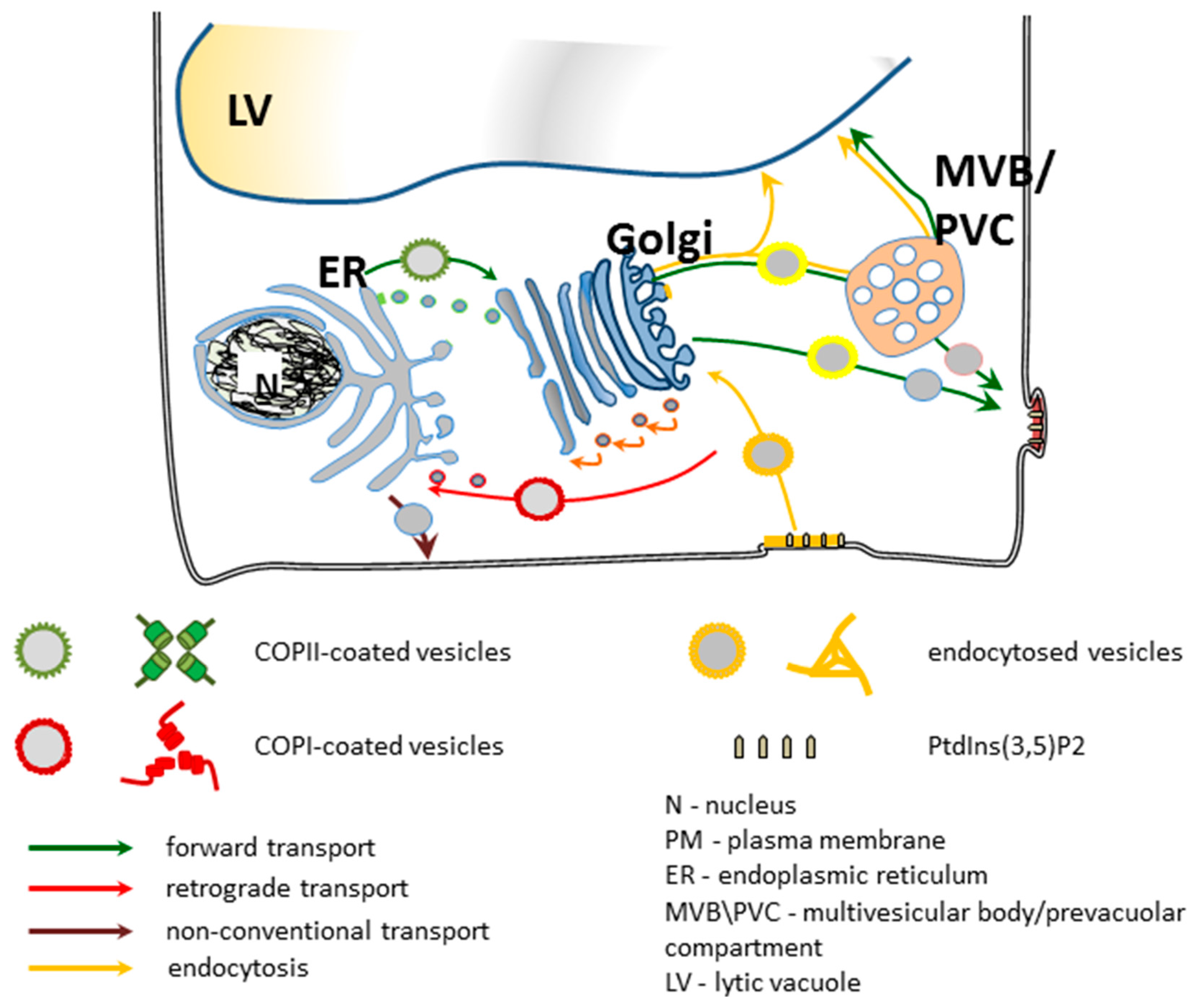
5. Secretory Trafficking Pathway
5.1. Between Golgi and Plasma Membrane: Forward Route and Exocytosis
5.2. Between Golgi and Plasma Membrane: Reverse Route and Endocytosis
5.3. Multivesicular Body/Prevacuolar Compartment
5.4. Between Plasma Membrane and Vacuoles
5.5. Leaderless Secretion
6. Future Perspectives-Potential Mechanisms of Annexins’ Effect on Cellular Trafficking
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Konopka-Postupolska, D.; Clark, G.; Hofmann, A. Structure, function and membrane interactions of plant annexins: An update. Plant Sci. 2011, 181, 230–241. [Google Scholar] [CrossRef] [PubMed]
- Cantacessi, C.; Seddon, J.M.; Miller, T.L.; Leow, C.Y.; Thomas, L.; Mason, L.; Willis, C.; Walker, G.; Loukas, A.; Gasser, R.B.; et al. A genome-wide analysis of annexins from parasitic organisms and their vectors. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.B.; Morgan, R.O.; Fernandez, M.-P.; Roux, S.J. Evolutionary adaptation of plant annexins has diversified their molecular structures, interactions and functional roles. New Phytol. 2012, 196, 695–712. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.O.; Martin-Almedina, S.; Iglesias, J.M.; Gonzalez-Florez, M.I.; Fernandez, M.P. Evolutionary perspective on annexin calcium-binding domains. Biochim. Biophys. Acta 2004, 1742, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Morgan, R.O.; Martin-Almedina, S.; Garcia, M.; Jhoncon-Kooyip, J.; Fernandez, M.-P. Deciphering function and mechanism of calcium-binding proteins from their evolutionary imprints. Biochim. Biophys. Acta 2006, 1763, 1238–1249. [Google Scholar] [CrossRef] [PubMed]
- Konopka-Postupolska, D.; Clark, G.; Goch, G.; Debski, J.; Floras, K.; Cantero, A.; Fijolek, B.; Roux, S.; Hennig, J. The role of annexin 1 in drought stress in Arabidopsis. Plant Physiol. 2009, 150, 1394–1410. [Google Scholar] [CrossRef] [PubMed]
- Laohavisit, A.; Davies, J.M. Multifunctional annexins. Plant Sci. 2009, 177, 532–539. [Google Scholar] [CrossRef]
- Laohavisit, A.; Davies, J.M. Annexins. New Phytol. 2011, 189, 40–53. [Google Scholar] [CrossRef] [PubMed]
- Laohavisit, A.; Brown, A.T.; Cicuta, P.; Davies, J.M. Annexins: Components of the calcium and reactive oxygen signaling network. Plant Physiol. 2010, 152, 1824–1829. [Google Scholar] [CrossRef] [PubMed]
- Szalonek, M.; Sierpien, B.; Rymaszewski, W.; Gieczewska, K.; Garstka, M.; Lichocka, M.; Sass, L.; Paul, K.; Vass, I.; Vankova, R.; et al. Potato annexin STANN1 promotes drought tolerance and mitigates light stress in transgenic Solanum tuberosum L. Plants. PLoS ONE 2015, 10, e0132683. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.; Chen, H.; Zhou, Y.; Li, Y.; Ding, Y.; Jiang, L.; Tsang, E.W.T.; Wu, K.; Huang, S. Proteomic and functional analyses of Nelumbo nucifera annexins involved in seed thermotolerance and germination vigor. Planta 2012, 235, 1271–1288. [Google Scholar] [CrossRef] [PubMed]
- Jami, S.K.; Clark, G.B.; Turlapati, S.A.; Handley, C.; Roux, S.J.; Kirti, P.B. Ectopic expression of an annexin from Brassica juncea confers tolerance to abiotic and biotic stress treatments in transgenic tobacco. Plant Physiol. Biochem. 2008, 46, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Lizarbe, M.; Barrasa, J.; Olmo, N.; Gavilanes, F.; Turnay, J. Annexin-phospholipid interactions. Functional implications. Int. J. Mol. Sci. 2013, 14, 2652–2683. [Google Scholar] [CrossRef] [PubMed]
- Surpin, M.; Zheng, H.; Morita, M.T.; Saito, C.; Avila, E.; Blakeslee, J.J.; Bandyopadhyay, A.; Kovaleva, V.; Carter, D.; Murphy, A.; et al. The VTI family of SNARE proteins is necessary for plant viability and mediates different protein transport pathways. Plant Cell 2003, 15, 2885–2899. [Google Scholar] [CrossRef] [PubMed]
- Bassil, E.; Coku, A.; Blumwald, E. Cellular ion homeostasis: Emerging roles of intracellular NHX Na+/H+ antiporters in plant growth and development. J. Exp. Bot. 2012, 63, 5727–5740. [Google Scholar] [CrossRef] [PubMed]
- Pittman, J.K. Multiple transport pathways for mediating intracellular pH homeostasis: The vontribution of H+/ion exchangers. Front. Plant Sci. 2012, 3. [Google Scholar] [CrossRef] [PubMed]
- Hamaji, K.; Nagira, M.; Yoshida, K.; Ohnishi, M.; Oda, Y.; Uemura, T.; Goh, T.; Sato, M.H.; Morita, M.T.; Tasaka, M.; et al. Dynamic aspects of ion accumulation by vesicle traffic under salt stress in Arabidopsis. Plant Cell Physiol. 2009, 50, 2023–2033. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Gong, Z.; Zhang, C.; Song, C.-P.; Damsz, B.; Inan, G.; Koiwa, H.; Zhu, J.-K.; Hasegawa, P.M.; Bressan, R.A. OSM1/SYP61: A syntaxin protein in Arabidopsis controls abscisic acid-mediated and non-abscisic acid-mediated responses to abiotic stress. Plant Cell 2002, 14, 3009–3028. [Google Scholar] [CrossRef] [PubMed]
- Kalde, M.; Nühse, T.S.; Findlay, K.; Peck, S.C. The syntaxin SYP132 contributes to plant resistance against bacteria and secretion of pathogenesis-related protein 1. Proc. Natl. Acad. Sci. USA 2007, 104, 11850–11855. [Google Scholar] [CrossRef] [PubMed]
- Leborgne-Castel, N.; Bouhidel, K. Plasma membrane protein trafficking in plant-microbe interactions: A plant cell point of view. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Grefen, C.; Blatt, M.R. SNAREs—Molecular governors in signalling and development. Curr. Opin. Plant Biol. 2008, 11, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Hachez, C.; Laloux, T.; Reinhardt, H.; Cavez, D.; Degand, H.; Grefen, C.; de Rycke, R.; Inzé, D.; Blatt, M.R.; Russinova, E.; et al. Arabidopsis SNAREs SYP61 and SYP121 coordinate the trafficking of plasma membrane aquaporin PIP2;7 to modulate the cell membrane water permeability. Plant Cell 2014, 26, 3132–3147. [Google Scholar] [CrossRef] [PubMed]
- Krecek, P.; Skupa, P.; Libus, J.; Naramoto, S.; Tejos, R.; Friml, J.; Zazímalová, E. The PIN-FORMED (PIN) protein family of auxin transporters. Genome Biol. 2009, 10, 249. [Google Scholar] [CrossRef] [PubMed]
- Sutter, J.-U.; Sieben, C.; Hartel, A.; Eisenach, C.; Thiel, G.; Blatt, M.R. Abscisic acid triggers the endocytosis of the Arabidopsis KAT1 K+ channel and its recycling to the plasma membrane. Curr. Biol. 2007, 17, 1396–1402. [Google Scholar] [CrossRef] [PubMed]
- Nibau, C.; Tao, L.; Levasseur, K.; Wu, H.-M.; Cheung, A.Y. The Arabidopsis small GTPase AtRAC7/ROP9 is a modulator of auxin and abscisic acid signalling. J. Exp. Bot. 2013, 64, 3425–3437. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, Q.; Qi, L.; Jiang, H.; Li, S.; Xu, Y.; Liu, F.; Zhou, W.; Pan, J.; Li, X.; et al. Jasmonate modulates endocytosis and plasma membrane accumulation of the Arabidopsis PIN2 protein. New Phytol. 2011, 191, 360–375. [Google Scholar] [CrossRef] [PubMed]
- Ghanashyam, C.; Jain, M. Role of auxin-responsive genes in biotic stress responses. Plant Signal. Behav. 2009, 4, 846–848. [Google Scholar] [CrossRef] [PubMed]
- Janmey, P.A.; Lindberg, U. Cytoskeletal regulation: Rich in lipids. Nat. Rev. Mol. Cell Biol. 2004, 5, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Creutz, C.E.; Kambouris, N.G.; Snyder, S.L.; Hamman, H.C.; Nelson, M.R.; Liu, W.; Rock, P. Effects of the expression of mammalian annexins in yeast secretory mutants. J. Cell Sci. 1992, 103, 1177–1192. [Google Scholar] [PubMed]
- Novick, P.; Field, C.; Schekman, R. Identification of 23 complementation groups required for post-translational events in the yeast secretory pathway. Cell 1980, 21, 205–215. [Google Scholar] [CrossRef]
- Draeger, A.; Wray, S.; Babiychuk, E.B. Domain architecture of the smooth-muscle plasma membrane: Regulation by annexins. Biochem. J. 2005, 387, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Monastyrskaya, K.; Babiychuk, E.B.; Hostettler, A.; Rescher, U.; Draeger, A. Annexins as intracellular calcium sensors. Cell Calcium 2007, 41, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Swairjo, M.A.; Concha, N.O.; Kaetzel, M.A.; Dedman, J.R.; Seaton, B.A. Ca2+-bridging mechanism and phospholipid head group recognition in the membrane-binding protein annexin V. Nat. Struct. Biol. 1995, 2, 968–974. [Google Scholar] [CrossRef] [PubMed]
- Bitto, E.; Cho, W. Roles of individual domains of annexin I in its vesicle binding and vesicle aggregation: A comprehensive mutagenesis study. Biochemistry 1998, 37, 10231–10237. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Ossa, C.G. Binding to phosphatidyl serine membranes causes a conformational change in the concave face of annexin I. Biophys. J. 1997, 72, 383–387. [Google Scholar] [CrossRef]
- Moss, S.E.; Morgan, R.O. The annexins. Genome Biol. 2004, 5, 219. [Google Scholar] [CrossRef] [PubMed]
- Rosengarth, A.; Gerke, V.; Luecke, H. X-ray structure of full-length annexin 1 and implications for membrane aggregation. J. Mol. Biol. 2001, 306, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Sparkes, I.A.; Frigerio, L.; Tolley, N.; Hawes, C. The plant endoplasmic reticulum: A cell-wide web. Biochem. J. 2009, 423, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Uemura, T.; Nakano, A. Formation and maintenance of the Golgi apparatus in plant cells. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2014; Volume 310, pp. 221–287. [Google Scholar]
- Gendre, D.; Jonsson, K.; Boutté, Y.; Bhalerao, R.P. Journey to the cell surface—The central role of the trans-Golgi network in plants. Protoplasma 2015, 252, 385–398. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Shen, J.; Gao, C.; Zhuang, X.; Wang, J.; Jiang, L. Biogenesis of plant prevacuolar multivesicular bodies. Mol. Plant 2016, 9, 774–786. [Google Scholar] [CrossRef] [PubMed]
- Marty, F. Plant vacuoles. Plant Cell 1999, 11, 587–600. [Google Scholar] [CrossRef] [PubMed]
- Neuhaus, J.-M.; Paris, N. Plant vacuoles: From biogenesis to function. In Plant Endocytosis; Šamaj, J., Baluška, F., Menzel, D., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; Volume 1, pp. 63–82. [Google Scholar]
- Fan, L.; Li, R.; Pan, J.; Ding, Z.; Lin, J. Endocytosis and its regulation in plants. Trends Plant Sci. 2015, 20, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Paez Valencia, J.; Goodman, K.; Otegui, M.S. Endocytosis and endosomal trafficking in plants. Annu. Rev. Plant Biol. 2016, 67, 309–335. [Google Scholar] [CrossRef] [PubMed]
- Tse, Y.C.; Mo, B.; Hillmer, S.; Zhao, M.; Lo, S.W.; Robinson, D.G.; Jiang, L. Identification of multivesicular bodies as prevacuolar compartments in Nicotiana tabacum BY-2 cells. Plant Cell 2004, 16, 672–693. [Google Scholar] [CrossRef] [PubMed]
- Tse, Y.C.; Lam, S.K.; Jiang, L. Organelle identification and characterization in plant cells: Using a combinational approach of confocal immunofluorescence and electron microscope. J. Plant Biol. 2009, 52, 1–9. [Google Scholar] [CrossRef]
- Lam, S.K.; Tse, Y.C.; Miao, Y.; Li, H.-Y.; Wang, J.; Lo, S.W.; Jiang, L. Molecular characterization of plant prevacuolar and endosomal compartments. J. Integr. Plant Biol. 2007, 49, 1119–1128. [Google Scholar] [CrossRef]
- Rojo, E.; Denecke, J. What is moving in the secretory pathway of plants? Plant Physiol. 2008, 147, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Donohoe, B.S.; Kang, B.-H.; Staehelin, L.A. Identification and characterization of COPIa- and COPIb-type vesicle classes associated with plant and algal Golgi. Proc. Natl. Acad. Sci. USA 2007, 104, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Suda, Y.; Kurokawa, K.; Nakano, A. COPI is essential for Golgi cisternal maturation and dynamics. J. Cell Sci. 2016, 129, 3251–3261. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-J.; Brandizzi, F. The plant secretory pathway for the trafficking of cell wall polysaccharides and glycoproteins. Glycobiology 2016, 26, 940–949. [Google Scholar] [CrossRef] [PubMed]
- Leucci, M.R.; Di Sansebastiano, G.-P.; Gigante, M.; Dalessandro, G.; Piro, G. Secretion marker proteins and cell-wall polysaccharides move through different secretory pathways. Planta 2007, 225, 1001–1017. [Google Scholar] [CrossRef] [PubMed]
- Harholt, J.; Suttangkakul, A.; Vibe Scheller, H. Biosynthesis of pectin. Plant Physiol. 2010, 153, 384–395. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, A.; Lund, C.H.; Sakuragi, Y.; Scheller, H.V. Golgi-localized enzyme complexes for plant cell wall biosynthesis. Trends Plant Sci. 2013, 18, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Parsons, H.T.; Christiansen, K.; Knierim, B.; Carroll, A.; Ito, J.; Batth, T.S.; Smith-Moritz, A.M.; Morrison, S.; McInerney, P.; Hadi, M.Z.; et al. Isolation and proteomic characterization of the Arabidopsis Golgi defines functional and novel components involved in plant cell wall biosynthesis. Plant Physiol. 2012, 159, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Spang, A. On vesicle formation and tethering in the ER–Golgi shuttle. Curr. Opin. Cell Biol. 2009, 21, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Drakakaki, G.; Dandekar, A. Protein secretion: How many secretory routes does a plant cell have? Plant Sci. 2013, 203–204, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.; Voss, U.; Jürgens, G. Post-Golgi traffic in plants. Traffic 2009, 10, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Niemes, S.; Labs, M.; Scheuring, D.; Krueger, F.; Langhans, M.; Jesenofsky, B.; Robinson, D.G.; Pimpl, P. Sorting of plant vacuolar proteins is initiated in the ER: Vacuolar protein sorting. Plant J. 2010, 62, 601–614. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.G.; Pimpl, P. Clathrin and post-Golgi trafficking: A very complicated issue. Trends Plant Sci. 2014, 19, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.G.; Pimpl, P. Receptor-mediated transport of vacuolar proteins: A critical analysis and a new model. Protoplasma 2014, 251, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Viotti, C.; Krüger, F.; Krebs, M.; Neubert, C.; Fink, F.; Lupanga, U.; Scheuring, D.; Boutté, Y.; Frescatada-Rosa, M.; Wolfenstetter, S.; et al. The endoplasmic reticulum is the main membrane source for biogenesis of the lytic vacuole in Arabidopsis. Plant Cell 2013, 25, 3434–3449. [Google Scholar] [CrossRef] [PubMed]
- Avila, E.L.; Zouhar, J.; Agee, A.E.; Carter, D.G.; Chary, S.N.; Raikhel, N.V. Tools to study plant organelle biogenesis. point mutation lines with disrupted vacuoles and high-speed confocal screening of green fluorescent protein-tagged organelles. Plant Physiol. 2003, 133, 1673–1676. [Google Scholar] [CrossRef] [PubMed]
- Foresti, O.; Gershlick, D.C.; Bottanelli, F.; Hummel, E.; Hawes, C.; Denecke, J. A recycling-defective vacuolar sorting receptor reveals an intermediate compartment situated between prevacuoles and vacuoles in Tobacco. Plant Cell 2010, 22, 3992–4008. [Google Scholar] [CrossRef] [PubMed]
- De Marchis, F.; Bellucci, M.; Pompa, A. Unconventional pathways of secretory plant proteins from the endoplasmic reticulum to the vacuole bypassing the Golgi complex. Plant Signal. Behav. 2013, 8, e25129. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.; Moore, I. The Arabidopsis Rab GTPase family: Another enigma variation. Curr. Opin. Plant Biol. 2002, 5, 518–528. [Google Scholar] [CrossRef]
- Rapoport, T.A. Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature 2007, 450, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, J. Endoplasmic reticulum-mediated protein quality control in Arabidopsis. Front. Plant Sci. 2014. [Google Scholar] [CrossRef] [PubMed]
- Marti, L.; Fornaciari, S.; Renna, L.; Stefano, G.; Brandizzi, F. COPII-mediated traffic in plants. Trends Plant Sci. 2010, 15, 522–528. [Google Scholar] [CrossRef] [PubMed]
- Rojo, E.; Gillmor, C.S.; Kovaleva, V.; Somerville, C.R.; Raikhel, N.V. VACUOLELESS1 is an essential gene required for vacuole formation and morphogenesis in Arabidopsis. Dev. Cell 2001, 1, 303–310. [Google Scholar] [CrossRef]
- Surpin, M.; Raikhel, N. Plant cell biology: Traffic jams affect plant development and signal transduction. Nat. Rev. Mol. Cell Biol. 2004, 5, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Kolb, C.; Nagel, M.-K.; Kalinowska, K.; Hagmann, J.; Ichikawa, M.; Anzenberger, F.; Alkofer, A.; Sato, M.H.; Braun, P.; Isono, E. FYVE1 is essential for vacuole biogenesis and intracellular trafficking in Arabidopsis. Plant Physiol. 2015, 167, 1361–1373. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Zhao, Q.; Gao, C.; Ding, Y.; Zeng, Y.; Ueda, T.; Nakano, A.; Jiang, L. Activation of the Rab7 GTPase by the MON1-CCZ1 complex is essential for PVC-to-vacuole trafficking and plant growth in Arabidopsis. Plant Cell 2014, 26, 2080–2097. [Google Scholar] [CrossRef] [PubMed]
- Lukowitz, W.; Mayer, U.; Jürgens, G. Cytokinesis in the Arabidopsis embryo involves the syntaxin-related KNOLLE gene product. Cell 1996, 84, 61–71. [Google Scholar] [CrossRef]
- Park, M.; Song, K.; Reichardt, I.; Kim, H.; Mayer, U.; Stierhof, Y.-D.; Hwang, I.; Jurgens, G. Arabidopsis-adaptin subunit AP1M of adaptor protein complex 1 mediates late secretory and vacuolar traffic and is required for growth. Proc. Natl. Acad. Sci. USA 2013, 110, 10318–10323. [Google Scholar] [CrossRef] [PubMed]
- Sanderfoot, A.A.; Pilgrim, M.; Adam, L.; Raikhel, N.V. Disruption of individual members of Arabidopsis syntaxin gene families indicates each has essential functions. Plant Cell 2001, 13, 659–666. [Google Scholar] [CrossRef] [PubMed]
- Chapman, E.R. How does synaptotagmin trigger neurotransmitter release? Annu. Rev. Biochem. 2008, 77, 615–641. [Google Scholar] [CrossRef] [PubMed]
- Di Giovanni, J.; Iborra, C.; Maulet, Y.; Leveque, C.; El Far, O.; Seagar, M. Calcium-dependent regulation of SNARE-mediated membrane fusion by calmodulin. J. Biol. Chem. 2010, 285, 23665–23675. [Google Scholar] [CrossRef] [PubMed]
- Mahal, L.K.; Sequeira, S.M.; Gureasko, J.M.; Söllner, T.H. Calcium-independent stimulation of membrane fusion and SNARE pin formation by synaptotagmin I. J. Cell Biol. 2002, 158, 273–282. [Google Scholar] [CrossRef] [PubMed]
- De Haro, L.; Quetglas, S.; Iborra, C.; Lévêque, C.; Seagar, M. Calmodulin-dependent regulation of a lipid binding domain in the v-SNARE synaptobrevin and its role in vesicular fusion. Biol. Cell 2003, 95, 459–464. [Google Scholar] [CrossRef]
- Südhof, T.C. The synaptic vesicle. Annu. Rev. Neurosci. 2004, 27, 509–547. [Google Scholar] [CrossRef] [PubMed]
- Südhof, T.C. Membrane fusion as a team effort. Proc. Natl. Acad. Sci. USA 2007, 104, 13541–13542. [Google Scholar] [CrossRef] [PubMed]
- Swulius, M.T.; Waxham, M.N. Ca2+/Calmodulin-dependent protein kinases. Cell. Mol. Life Sci. 2008, 65, 2637–2657. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Lledo, P.M.; Roa, M.; Vincent, J.D.; Henry, J.P.; Darchen, F. The GTPase Rab3a negatively controls calcium-dependent exocytosis in neuroendocrine cells. EMBO J. 1994, 13, 2029–2037. [Google Scholar] [PubMed]
- Augustin, I.; Rosenmund, C.; Südhof, T.C.; Brose, N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature 1999, 400, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Beckers, C.J.; Balch, W.E. Calcium and GTP: Essential components in vesicular trafficking between the endoplasmic reticulum and Golgi apparatus. J. Cell Biol. 1989, 108, 1245–1256. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.I.; Beron, W.; Stahl, P.D. Calmodulin regulates endosome fusion. J. Biol. Chem. 1997, 272, 7707–7712. [Google Scholar] [CrossRef] [PubMed]
- Hay, J.C. Calcium: A fundamental regulator of intracellular membrane fusion? EMBO Rep. 2007, 8, 236–240. [Google Scholar] [CrossRef] [PubMed]
- Peters, C.; Mayer, A. Ca2+/calmodulin signals the completion of docking and triggers a late step of vacuole fusion. Nature 1998, 396, 575–580. [Google Scholar] [PubMed]
- Porat, A.; Elazar, Z. Regulation of intra-Golgi membrane transport by calcium. J. Biol. Chem. 2000, 275, 29233–29237. [Google Scholar] [CrossRef] [PubMed]
- Pryor, P.R.; Mullock, B.M.; Bright, N.A.; Gray, S.R.; Luzio, J.P. The role of intraorganellar Ca2+ in late endosome-lysosome heterotypic fusion and in the reformation of lysosomes from hybrid organelles. J. Cell Biol. 2000, 149, 1053–1062. [Google Scholar] [CrossRef] [PubMed]
- Rexach, M.F.; Schekman, R.W. Distinct biochemical requirements for the budding, targeting, and fusion of ER-derived transport vesicles. J. Cell Biol. 1991, 114, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-L.; Ahluwalia, J.P.; Stamnes, M. Selective effects of calcium chelators on anterograde and retrograde protein transport in the cell. J. Biol. Chem. 2002, 277, 35682–35687. [Google Scholar] [CrossRef] [PubMed]
- Parkinson, K.; Baines, A.E.; Keller, T.; Gruenheit, N.; Bragg, L.; North, R.A.; Thompson, C.R.L. Calcium-dependent regulation of Rab activation and vesicle fusion by an intracellular P2X ion channel. Nat. Cell Biol. 2013, 16, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Miklavc, P.; Mair, N.; Wittekindt, O.H.; Haller, T.; Dietl, P.; Felder, E.; Timmler, M.; Frick, M. Fusion-activated Ca2+ entry via vesicular P2X4 receptors promotes fusion pore opening and exocytotic content release in pneumocytes. Proc. Natl. Acad. Sci. USA 2011, 108, 14503–14508. [Google Scholar] [CrossRef] [PubMed]
- Fountain, S.J.; Burnstock, G. An evolutionary history of P2X receptors. Purinergic Signal. 2009, 5, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Dark, A.; Demidchik, V.; Richards, S.L.; Shabala, S.; Davies, J.M. Release of extracellular purines from plant roots and effect on ion fluxes. Plant Signal. Behav. 2011, 6, 1855–1857. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Nichols, C.; Oliynyk, M.; Dark, A.; Glover, B.J.; Davies, J.M. Is ATP a signaling agent in plants? Plant Physiol. 2003, 133, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Demidchik, V.; Shang, Z.; Shin, R.; Thompson, E.; Rubio, L.; Laohavisit, A.; Mortimer, J.C.; Chivasa, S.; Slabas, A.R.; Glover, B.J.; et al. Plant extracellular ATP signalling by plasma membrane NADPH oxidase and Ca2+ channels. Plant J. Cell Mol. Biol. 2009, 58, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Jeter, C.R.; Tang, W.; Henaff, E.; Butterfield, T.; Roux, S.J. Evidence of a novel cell signaling role for extracellular adenosine triphosphates and diphosphates in Arabidopsis. Plant Cell 2004, 16, 2652–2664. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, K.; Choi, J.; Cao, Y.; Stacey, G. Extracellular ATP acts as a damage-associated molecular pattern (DAMP) signal in plants. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.B.; Morgan, R.O.; Fernandez, M.-P.; Salmi, M.L.; Roux, S.J. Breakthroughs spotlighting roles for extracellular nucleotides and apyrases in stress responses and growth and development. Plant Sci. 2014, 225, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Tanaka, K.; Cao, Y.; Qi, Y.; Qiu, J.; Liang, Y.; Lee, S.Y.; Stacey, G. Identification of a plant receptor for extracellular ATP. Science 2014, 343, 290–294. [Google Scholar] [CrossRef] [PubMed]
- Rubio, L.; Laohavisit, A.; Mortimer, J.; Dark, A.; Davies, J. Salt stress signalling involves ATP release and Arabidopsis annexin 1. Comp. Biochem. Physiol. Part Mol. Physiol. 2009, 153, S192–S194. [Google Scholar] [CrossRef]
- Yamasaki, A.; Tani, K.; Yamamoto, A.; Kitamura, N.; Komada, M. The Ca2+-binding protein ALG-2 is recruited to endoplasmic reticulum exit sites by Sec31A and stabilizes the localization of Sec31A. Mol. Biol. Cell 2006, 17, 4876–4887. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Ballew, N.; Barlowe, C. Initial docking of ER-derived vesicles requires Uso1p and Ypt1p but is independent of SNARE proteins. EMBO J. 1998, 17, 2156–2165. [Google Scholar] [CrossRef] [PubMed]
- Munro, S. The golgin coiled-coil proteins of the Golgi apparatus. Cold Spring Harb. Perspect. Biol. 2011, 3, a005256. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, I.; Barlowe, C. Overexpression of Sly41 suppresses COPII vesicle-tethering deficiencies by elevating intracellular calcium levels. Mol. Biol. Cell 2016, 27, 1635–1649. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, H.D.; Puzicha, M.; Gallwitz, D. Study of a temperature-sensitive mutant of the ras-related YPT1 gene product in yeast suggests a role in the regulation of intracellular calcium. Cell 1988, 53, 635–647. [Google Scholar] [CrossRef]
- Tester, M.; Zorec, R. Cytoplasmic calcium stimulates exocytosis in a plant secretory cell. Biophys. J. 1992, 63, 864–867. [Google Scholar] [CrossRef]
- Yaghmur, A.; Sartori, B.; Rappolt, M. The role of calcium in membrane condensation and spontaneous curvature variations in model lipidic systems. Phys. Chem. Chem. Phys. PCCP 2011, 13, 3115–3125. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Kanadome, T.; Sugiura, H.; Yokoyama, T.; Yamamuro, M.; Moss, S.E.; Maki, M. A new role for annexin A11 in the early secretory pathway via stabilizing Sec31A protein at the endoplasmic reticulum exit sites (ERES). J. Biol. Chem. 2015, 290, 4981–4993. [Google Scholar] [CrossRef] [PubMed]
- Cardenas, L.; Lovy-Wheeler, A.; Kunkel, J.G.; Hepler, P.K. Pollen tube growth oscillations and intracellular calcium levels are reversibly modulated by actin polymerization. Plant Physiol. 2008, 146, 1611–1621. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.-H.; Acharya, B.; Liu, W.; Zhang, W. Interaction between calcium and actin in guard cell and pollen signaling networks. Plants 2013, 2, 615–634. [Google Scholar] [CrossRef] [PubMed]
- Snowman, B.N.; Kovar, D.R.; Shevchenko, G.; Franklin-Tong, V.E.; Staiger, C.J. Signal-mediated depolymerization of actin in pollen during the self-incompatibility response. Plant Cell 2002, 14, 2613–2626. [Google Scholar] [CrossRef] [PubMed]
- Steinhorst, L.; Kudla, J. Calcium—A central regulator of pollen germination and tube growth. Biochim. Biophys. Acta BBA Mol. Cell Res. 2013, 1833, 1573–1581. [Google Scholar] [CrossRef] [PubMed]
- Ahluwalia, J.P.; Topp, J.D.; Weirather, K.; Zimmerman, M.; Stamnes, M. A role for calcium in stabilizing transport vesicle coats. J. Biol. Chem. 2001, 276, 34148–34155. [Google Scholar] [CrossRef] [PubMed]
- Bentley, M.; Nycz, D.C.; Joglekar, A.; Fertschai, I.; Malli, R.; Graier, W.F.; Hay, J.C. Vesicular calcium regulates coat retention, fusogenicity, and size of Pre-Golgi intermediates. Mol. Biol. Cell 2010, 21, 1033–1046. [Google Scholar] [CrossRef] [PubMed]
- Samaj, J.; Baluska, F.; Voigt, B.; Schlicht, M.; Volkmann, D.; Menzel, D. Endocytosis, actin cytoskeleton, and signaling. Plant Physiol. 2004, 135, 1150–1161. [Google Scholar] [CrossRef] [PubMed]
- Sticher, L.; Penel, C.; Greppin, H. Calcium requirement for the secretion of peroxidases by plant cell suspensions. J. Cell Sci. 1981, 48, 345–353. [Google Scholar] [PubMed]
- Holdaway-Clarke, T.L.; Hepler, P.K. Control of pollen tube growth: Role of ion gradients and fluxes: Tansley review. New Phytol. 2003, 159, 539–563. [Google Scholar] [CrossRef]
- Stael, S.; Wurzinger, B.; Mair, A.; Mehlmer, N.; Vothknecht, U.C.; Teige, M. Plant organellar calcium signalling: An emerging field. J. Exp. Bot. 2012, 63, 1525–1542. [Google Scholar] [CrossRef] [PubMed]
- Caohuy, H.; Srivastava, M.; Pollard, H.B. Membrane fusion protein synexin (annexin VII) as a Ca2+/GTP sensor in exocytotic secretion. Proc. Natl. Acad. Sci. USA 1996, 93, 10797–10802. [Google Scholar] [CrossRef] [PubMed]
- Chander, A.; Sen, N.; Spitzer, A.R. Synexin and GTP increase surfactant secretion in permeabilized alveolar type II cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 280, L991–L998. [Google Scholar] [PubMed]
- Lafont, F.; Lecat, S.; Verkade, P.; Simons, K. Annexin XIIIb associates with lipid microdomains to function in apical delivery. J. Cell Biol. 1998, 142, 1413–1427. [Google Scholar] [CrossRef] [PubMed]
- Gruenberg, J.; Emans, N. Annexins in membrane traffic. Trends Cell Biol. 1993, 3, 224–227. [Google Scholar] [CrossRef]
- Grieve, A.G.; Moss, S.E.; Hayes, M.J. Annexin A2 at the interface of actin and membrane dynamics: A focus on its roles in endocytosis and cell polarization. Int. J. Cell Biol. 2012, 2012, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zobiack, N.; Rescher, U.; Ludwig, C.; Zeuschner, D.; Gerke, V. The annexin 2/S100A10 complex controls the distribution of transferrin receptor-containing recycling endosomes. Mol. Biol. Cell 2003, 14, 4896–4908. [Google Scholar] [CrossRef] [PubMed]
- Futter, C.E.; Felder, S.; Schlessinger, J.; Ullrich, A.; Hopkins, C.R. Annexin I is phosphorylated in the multivesicular body during the processing of the epidermal growth factor receptor. J. Cell Biol. 1993, 120, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Mayran, N.; Parton, R.G.; Gruenberg, J. Annexin II regulates multivesicular endosome biogenesis in the degradation pathway of animal cells. EMBO J. 2003, 22, 3242–3253. [Google Scholar] [CrossRef] [PubMed]
- Gerke, V.; Carl, E.; Creutz, C.E.; Moss, S.E. Annexins: Linking Ca2+ signalling to membrane dynamics. Nat. Rev. Mol. Cell Biol. 2005, 6, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Monastyrskaya, K.; Babiychuk, E.B.; Draeger, A. The annexins: Spatial and temporal coordination of signaling events during cellular stress. Cell. Mol. Life Sci. 2009, 66, 2623–2642. [Google Scholar] [CrossRef] [PubMed]
- Chasserot-Golaz, S.; Vitale, N.; Umbrecht-Jenck, E.; Knight, D.; Gerke, V.; Bader, M.-F. Annexin 2 promotes the formation of lipid microdomains required for calcium-regulated exocytosis of dense-core vesicles. Mol. Biol. Cell 2005, 16, 1108–1119. [Google Scholar] [CrossRef] [PubMed]
- Drucker, P.; Pejic, M.; Galla, H.-J.; Gerke, V. Lipid segregation and membrane budding induced by the peripheral membrane binding protein Annexin A2. J. Biol. Chem. 2013, 288, 24764–24776. [Google Scholar] [CrossRef] [PubMed]
- Roth, D.; Morgan, A.; Burgoyne, R.D. Identification of a key domain in annexin and 14-3-3 proteins that stimulate calcium-dependent exocytosis in permeabilized adrenal chromaffin cells. FEBS Lett. 1993, 320, 207–210. [Google Scholar] [CrossRef]
- Burgoyne, R.D.; Morgan, A. Calcium sensors in regulated exocytosis. Cell Calcium 1998, 24, 367–376. [Google Scholar] [CrossRef]
- Caohuy, H.; Pollard, H.B. Annexin 7: A non-SNARE proteolytic substrate for botulinum toxin type C in secreting chromaffin cells. Ann. N. Y. Acad. Sci. 2002, 971, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Futter, C.E.; White, I.J. Annexins and endocytosis. Traffic Cph. Den. 2007, 8, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Sun, H.; Tanowitz, M.; Liang, X.-H.; Crooke, S.T. Annexin A2 facilitates endocytic trafficking of antisense oligonucleotides. Nucleic Acids Res. 2016, 44, 7314–7330. [Google Scholar] [CrossRef] [PubMed]
- Eden, E.R.; Sanchez-Heras, E.; Tsapara, A.; Sobota, A.; Levine, T.P.; Futter, C.E. Annexin A1 tethers membrane contact sites that mediate ER to endosome cholesterol transport. Dev. Cell 2016, 37, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Klokk, T.I.; Kavaliauskiene, S.; Sandvig, K. Cross-linking of glycosphingolipids at the plasma membrane: Consequences for intracellular signaling and traffic. Cell. Mol. Life Sci. 2016, 73, 1301–1316. [Google Scholar] [CrossRef] [PubMed]
- Tcatchoff, L.; Andersson, S.; Utskarpen, A.; Klokk, T.I.; Skånland, S.S.; Pust, S.; Gerke, V.; Sandvig, K. Annexin A1 and A2:rRoles in retrograde trafficking of shiga toxin. PLoS ONE 2012, 7, e40429. [Google Scholar] [CrossRef] [PubMed]
- Valapala, M.; Vishwanatha, J.K. Lipid raft endocytosis and exosomal transport facilitate extracellular trafficking of annexin A2. J. Biol. Chem. 2011, 286, 30911–30925. [Google Scholar] [CrossRef] [PubMed]
- White, I.J.; Bailey, L.M.; Aghakhani, M.R.; Moss, S.E.; Futter, C.E. EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J. 2006, 25, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, L.S.; Berón, W.; Sarrouf, M.N.; Colombo, M.I.; Creutz, C.; Stahl, P.D. Calcium-dependent fusion among endosomes. J. Biol. Chem. 1994, 269, 30927–30934. [Google Scholar] [PubMed]
- Potez, S.; Luginbuhl, M.; Monastyrskaya, K.; Hostettler, A.; Draeger, A.; Babiychuk, E.B. Tailored protection against plasmalemmal injury by annexins with different Ca2+ sensitivities. J. Biol. Chem. 2011, 286, 17982–17991. [Google Scholar] [CrossRef] [PubMed]
- McNeil, A.K.; Rescher, U.; Gerke, V.; McNeil, P.L. Requirement for annexin A1 in plasma membrane repair. J. Biol. Chem. 2006, 281, 35202–35207. [Google Scholar] [CrossRef] [PubMed]
- Babiychuk, E.B.; Monastyrskaya, K.; Potez, S.; Draeger, A. Intracellular Ca2+ operates a switch between repair and lysis of streptolysin O-perforated cells. Cell Death Differ. 2009, 16, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Idone, V.; Tam, C.; Goss, J.W.; Toomre, D.; Pypaert, M.; Andrews, N.W. Repair of injured plasma membrane by rapid Ca2+-dependent endocytosis. J. Cell Biol. 2008, 180, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Babiychuk, E.B.; Monastyrskaya, K.; Potez, S.; Draeger, A. Blebbing confers resistance against cell lysis. Cell Death Differ. 2011, 18, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Blazek, A.D.; Paleo, B.J.; Weisleder, N. Plasmam membrane repair: A central process for maintaining cellular homeostasis. Physiology 2015, 30, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Bouter, A.; Gounou, C.; Bérat, R.; Tan, S.; Gallois, B.; Granier, T.; d’Estaintot, B.L.; Pöschl, E.; Brachvogel, B.; Brisson, A.R. Annexin-A5 assembled into two-dimensional arrays promotes cell membrane repair. Nat. Commun. 2011, 2, 270. [Google Scholar] [CrossRef] [PubMed]
- Swaggart, K.A.; Demonbreun, A.R.; Vo, A.H.; Swanson, K.E.; Kim, E.Y.; Fahrenbach, J.P.; Holley-Cuthrell, J.; Eskin, A.; Chen, Z.; Squire, K.; et al. Annexin A6 modifies muscular dystrophy by mediating sarcolemmal repair. Proc. Natl. Acad. Sci. USA 2014, 111, 6004–6009. [Google Scholar] [CrossRef] [PubMed]
- Jami, S.K.; Clark, G.B.; Ayele, B.T.; Ashe, P.; Kirti, P.B. Genome-wide comparative analysis of annexin superfamily in plants. PLoS ONE 2012, 7, e47801. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.B.; Sessions, A.; Eastburn, D.J.; Roux, S.J. Differential expression of members of the annexin multigene family in Arabidopsis. Plant Physiol. 2001, 126, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
- Jami, S.K.; Clark, G.B.; Ayele, B.T.; Roux, S.J.; Kirti, P.B. Identification and characterization of annexin gene family in rice. Plant Cell Rep. 2012, 31, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.M.; Wei, X.K.; Liao, W.X.; Huang, L.H.; Zhang, H.; Liang, S.C.; Peng, H. Molecular analysis of the annexin gene family in soybean. Biol. Plant. 2013, 57, 655–662. [Google Scholar] [CrossRef]
- Lu, Y.; Ouyang, B.; Zhang, J.; Wang, T.; Lu, C.; Han, Q.; Zhao, S.; Ye, Z.; Li, H. Genomic organization, phylogenetic comparison and expression profiles of annexin gene family in tomato (Solanum lycopersicum). Gene 2012, 499, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.B.; Dauwalder, M.; Roux, S.J. Purification and immunolocalization of an annexin-like protein in pea seedlings. Planta 1992, 187. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.B.; Dauwalder, M.; Roux, S.J. Immunological and biochemical evidence for nuclear localization of annexin in peas. Plant Physiol. Biochem. 1998, 36, 621–627. [Google Scholar] [CrossRef]
- Seals, D.F.; Parrish, M.L.; Randall, S.K. A 42-kilodalton annexin-like protein is associated with plant vacuoles. Plant Physiol. 1994, 106, 1403–1412. [Google Scholar] [CrossRef] [PubMed]
- Blackbourn, H.; Barker, P.; Huskisson, N.; Battey, N. Properties and partial protein-sequence of plant annexins. Plant Physiol. 1992, 99, 864–871. [Google Scholar] [CrossRef] [PubMed]
- Clark, G.B.; Lee, D.; Dauwalder, M.; Roux, S.J. Immunolocalization and histochemical evidence for the association of two different Arabidopsis annexins with secretion during early seedling growth and development. Planta 2005, 220, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Blackbourn, H.D.; Battey, N.H. Annexin-mediated secretory vesicle aggregation in plants. Physiol. Plant. 1993, 89, 27–32. [Google Scholar] [CrossRef]
- Carroll, A.D.; Moyen, C.; Van Kesteren, P.; Tooke, F.; Battey, N.H.; Brownlee, C. Ca2+, annexins, and GTP modulate exocytosis from maize root cap protoplasts. Plant Cell 1998, 10, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Dalal, A.; Vishwakarma, A.; Singh, N.K.; Gudla, T.; Bhattacharyya, M.K.; Padmasree, K.; Viehhauser, A.; Dietz, K.-J.; Kirti, P.B. Attenuation of hydrogen peroxide-mediated oxidative stress by Brassica juncea annexin-3 counteracts thiol-specific antioxidant (TSA1) deficiency in Saccharomyces cerevisiae. FEBS Lett. 2014, 588, 584–593. [Google Scholar] [CrossRef] [PubMed]
- Divya, K.; Jami, S.K.; Kirti, P.B. Constitutive expression of mustard annexin, AnnBj1 enhances abiotic stress tolerance and fiber quality in cotton under stress. Plant Mol. Biol. 2010, 73, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Gidrol, X.; Sabelli, P.A.; Fern, Y.S.; Kush, A.K. Annexin-like protein from Arabidopsis thaliana rescues delta oxyR mutant of Escherichia coli from H2O2 stress. Proc. Natl. Acad. Sci. USA 1996, 93, 11268–11273. [Google Scholar] [CrossRef] [PubMed]
- Jänicke, R.U.; Porter, A.G.; Kush, A. A novel Arabidopsis thaliana protein protects tumor cells from tumor necrosis factor-induced apoptosis. Biochim. Biophys. Acta 1998, 1402, 70–78. [Google Scholar] [CrossRef]
- Kush, A.; Sabapathy, K. Oxy5, a novel protein from Arabidopsis thaliana, protects mammalian cells from oxidative stress. Int. J. Biochem. Cell Biol. 2001, 33, 591–602. [Google Scholar] [CrossRef]
- Reddy Sareddy, G. Novel antiproliferative and antioxidant role of BjAnn1, a mustard annexin protein in human glioblastoma cell lines. J. Cancer Sci. Ther. 2013, 5. [Google Scholar] [CrossRef]
- Madureira, P.; Waisman, D. Annexin A2: The importance of being redox sSensitive. Int. J. Mol. Sci. 2013, 14, 3568–3594. [Google Scholar] [CrossRef] [PubMed]
- Madureira, P.A.; Hill, R.; Miller, V.A.; Giacomantonio, C.; Lee, P.W.; Waisman, D.M. Annexin A2 is a novel cellular redox regulatory protein involved in tumorigenesis. Oncotarget 2011, 2, 1075–1093. [Google Scholar] [CrossRef] [PubMed]
- Davies, J. Annexin-mediated calcium signalling in plants. Plants 2014, 3, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhao, Y.; Xia, Q.; Zheng, L.; Liu, L.; Zhao, B.; Shi, J. Nuclear translocation of annexin 1 following oxygen-glucose deprivation-reperfusion induces apoptosis by regulating Bid expression via p53 binding. Cell Death Dis. 2016, 7, e2356. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wang, J.; Liu, L.; Li, X.; Liu, S.; Xia, Q.; Shi, J. Annexin A1 translocates to nucleus and promotes the expression of pro-inflammatory cytokines in a PKC-dependent manner after OGD/R. Sci. Rep. 2016, 6, 27028. [Google Scholar] [CrossRef] [PubMed]
- Calvert, C.M. Tomato annexins p34 and p35 bind to F-actin and display nucleotide phosphodiesterase activity inhibited by phospholipid binding. Plant Cell 1996, 8, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Hoshino, D.; Hayashi, A.; Temmei, Y.; Kanzawa, N.; Tsuchiya, T. Biochemical and immunohistochemical characterization of Mimosa annexin. Planta 2004, 219. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-B.; Dammer, E.B.; Ren, R.-J.; Wang, G. The endosomal-lysosomal system: From acidification and cargo sorting to neurodegeneration. Transl. Neurodegener. 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Konopka-Postupolska, D. Annexins: Putative linkers in dynamic membrane-cytoskeleton interactions in plant cells. Protoplasma 2007, 230, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Himschoot, E.; Beeckman, T.; Friml, J.; Vanneste, S. Calcium is an organizer of cell polarity in plants. Biochim. Biophys. Acta BBA Mol. Cell Res. 2015, 1853, 2168–2172. [Google Scholar] [CrossRef] [PubMed]
- Mangano, S.; Juárez, S.P. D.; Estevez, J.M. ROS regulation of polar growth in plant cells. Plant Physiol. 2016, 171, 1593–1605. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Carbonell, E.; Takahashi, D.; Lüthje, S.; González-Reyes, J.A.; Mongrand, S.; Contreras-Moreira, B.; Abadía, A.; Uemura, M.; Abadía, J.; López-Millán, A.F. A shotgun proteomic approach reveals that fe deficiency causes marked changes in the protein profiles of plasma membrane and detergent-resistant microdomain preparations from β vulgaris roots. J. Proteome Res. 2016, 15, 2510–2524. [Google Scholar] [CrossRef] [PubMed]
- Lichocka, M.; Voigt, B.; Baluska, F.; Konopka-Postupolska, D. Annexin 1 interacts with specialized fluctuating membrane microdomains enriched in sterols. In Proceedings of the 8th Conference on Annexins, Maastricht, The Netherlands, 8–11 September 2015. [Google Scholar]
- Luczak, M.; Krzeszowiec-Jeleń, W.; Konopka-Postupolska, D.; Wojtaszek, P. Collagenase as a useful tool for the analysis of plant cellular peripheries. Phytochemistry 2015, 112, 195–209. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, X.; Wang, H.; Li, B.; Clark, G.; Guo, Y.; Roux, S.; Sun, D.; Tang, W. Proteomic study of microsomal proteins reveals a key role for Arabidopsis annexin 1 in mediating heat stress-induced increase in intracellular calcium levels. Mol. Cell. Proteom. 2015, 14, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, E.J.; Yang, E.J.; Lee, J.E.; Park, A.R.; Song, W.H.; Park, O.K. Proteomic identification of annexins, calcium-dependent membrane binding proteins that mediate osmotic stress and abscisic acid signal transduction in Arabidopsis. Plant Cell 2004, 16, 1378–1391. [Google Scholar] [CrossRef] [PubMed]
- Carter, C.; Pan, S.; Zouhar, J.; Avila, E.L.; Girke, T.; Raikhel, N.V. The vegetative vacuole proteome of Arabidopsis thaliana reveals predicted and unexpected proteins. Plant Cell 2004, 16, 3285–3303. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, M.; Uemura, T.; Ebine, K.; Nishimori, Y.; Ueda, T.; Nakano, A.; Sato, M.H.; Fukao, Y. Interactomics of Qa-SNARE in Arabidopsis thaliana. Plant Cell Physiol. 2014, 55, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Scheuring, D.; Viotti, C.; Kruger, F.; Kunzl, F.; Sturm, S.; Bubeck, J.; Hillmer, S.; Frigerio, L.; Robinson, D.G.; Pimpl, P.; et al. Multivesicular bodies mature from the trans-Golgi network/early endosome in Arabidopsis. Plant Cell Online 2011, 23, 3463–3481. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Wu, X.; Yuan, S.; Qian, D.; Nan, Q.; An, L.; Xiang, Y. Annexin5 plays a vital role in Arabidopsis pollen development via Ca2+-dependent membrane trafficking. PLoS ONE 2014, 9, e102407. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Yuan, S.; Wei, G.; Qian, D.; Wu, X.; Jia, H.; Gui, M.; Liu, W.; An, L.; Xiang, Y. Annexin5 Is essential for pollen development in Arabidopsis. Mol. Plant 2014, 7, 751–754. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Kong, L.; Hao, H.; Wang, X.; Lin, J.; Samaj, J.; Baluska, F. Effects of brefeldin A on pollen germination and tube growth. Antagonistic effects on endocytosis and secretion. Plant Physiol. 2005, 139, 1692–1703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Qu, X.; Bao, C.; Khurana, P.; Wang, Q.; Xie, Y.; Zheng, Y.; Chen, N.; Blanchoin, L.; Staiger, C.J.; et al. Arabidopsis VILLIN5, an ctin filament bundling and severing protein, is necessary for normal pollen tube growth. Plant Cell 2010, 22, 2749–2767. [Google Scholar] [CrossRef] [PubMed]
- Andrawis, A.; Solomon, M.; Delmer, D.P. Cotton fiber annexins: A potential role in the regulation of callose synthase. Plant J. 1993, 3, 763–772. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; He, Y.; Tu, L.; Wang, M.; Li, Y.; Ruan, Y.-L.; Zhang, X. Down-regulating annexin gene GhAnn2 inhibits cotton fiber elongation and decreases Ca2+ influx at the cell apex. Plant Mol. Biol. 2014, 85, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.-Y.; Zhao, P.-M.; Han, L.-B.; Jiao, G.-L.; Zheng, Y.-Y.; Huang, S.-J.; Xia, G.-X. Overexpression of a profilin (GhPFN2) promotes the progression of developmental phases in cotton fibers. Plant Cell Physiol. 2010, 51, 1276–1290. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Jin, X.; Wang, L.; Li, S.; Wu, S.; Cheng, C.; Zhang, T.; Guo, W. A cotton annexin affects fiber elongation and secondary cell wall biosynthesis associated with Ca2+ influx, ROS homeostasis, and actin filament reorganization. Plant Physiol. 2016, 171, 1750–1770. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, S.R.; Rothman, J.E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu. Rev. Biochem. 1987, 56, 829–852. [Google Scholar] [CrossRef] [PubMed]
- Phillipson, B.A.; Pimpl, P.; daSilva, L.L.; Crofts, A.J.; Taylor, J.P.; Movafeghi, A.; Robinson, D.G.; Denecke, J. Secretory bulk flow of soluble proteins is efficient and COPII dependent. Plant Cell 2001, 13, 2005–2020. [Google Scholar] [CrossRef] [PubMed]
- Barlowe, C.; Helenius, A. Cargo capture and bulk flow in the early secretory pathway. Annu. Rev. Cell Dev. Biol. 2016, 32, 197–222. [Google Scholar] [CrossRef] [PubMed]
- Thor, F.; Gautschi, M.; Geiger, R.; Helenius, A. Bulk flow revisited: Transport of a soluble protein in the secretory pathway. Traffic 2009, 10, 1819–1830. [Google Scholar] [CrossRef] [PubMed]
- Vernoud, V.; Horton, A.C.; Yang, Z.; Nielsen, E. Analysis of the small GTPase gene superfamily of Arabidopsis. Plant Physiol. 2003, 131, 1191–1208. [Google Scholar] [CrossRef] [PubMed]
- Cevher-Keskin, B. ARF1 and SAR1 GTPases in endomembrane trafficking in plants. Int. J. Mol. Sci. 2013, 14, 18181–18199. [Google Scholar] [CrossRef] [PubMed]
- Gillingham, A.K.; Munro, S. The small G proteins of the ARF family and their regulators. Annu. Rev. Cell Dev. Biol. 2007, 23, 579–611. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, E.; Cheung, A.Y.; Ueda, T. The regulatory RAB and ARF GTPases for vesicular trafficking. Plant Physiol. 2008, 147, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Zerial, M.; McBride, H. Rab proteins as membrane organizers. Nat. Rev. Mol. Cell Biol. 2001, 2, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Grosshans, B.L.; Ortiz, D.; Novick, P. Rabs and their effectors: Achieving specificity in membrane traffic. Proc. Natl. Acad. Sci. USA 2006, 103, 11821–11827. [Google Scholar] [CrossRef] [PubMed]
- Bonifacino, J.S.; Glick, B.S. The mechanisms of vesicle budding and fusion. Cell 2004, 116, 153–166. [Google Scholar] [CrossRef]
- Jahn, R.; Scheller, R.H. SNAREs—Engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 2006, 7, 631–643. [Google Scholar] [CrossRef] [PubMed]
- Bethani, I.; Lang, T.; Geumann, U.; Sieber, J.J.; Jahn, R.; Rizzoli, S.O. The specificity of SNARE pairing in biological membranes is mediated by both proof-reading and spatial segregation. EMBO J. 2007, 26, 3981–3992. [Google Scholar] [CrossRef] [PubMed]
- Sanderfoot, A.A.; Assaad, F.F.; Raikhel, N.V. The Arabidopsis genome. An abundance of soluble N-ethylmaleimide-sensitive factor adaptor protein receptors. Plant Physiol. 2000, 124, 1558–1569. [Google Scholar] [CrossRef] [PubMed]
- Turpin, E.; Russo-Marie, F.; Dubois, T.; de Paillerets, C.; Alfsen, A.; Bomsel, M. In adrenocortical tissue, annexins II and VI are attached to clathrin coated vesicles in a calcium-independent manner. Biochim. Biophys. Acta 1998, 1402, 115–130. [Google Scholar] [CrossRef]
- Creutz, C.E.; Snyder, S.L. Interactions of annexins with the mu subunits of the clathrin assembly proteins. Biochemistry 2005, 44, 13795–13806. [Google Scholar] [CrossRef] [PubMed]
- Houy, S.; Croisé, P.; Gubar, O.; Chasserot-Golaz, S.; Tryoen-Tóth, P.; Bailly, Y.; Ory, S.; Bader, M.-F.; Gasman, S. Exocytosis and endocytosis in neuroendocrine cells: Inseparable membranes! Front. Endocrinol. 2013, 4, 135. [Google Scholar] [CrossRef] [PubMed]
- Czech, M.P. Dynamics of phosphoinositides in membrane retrieval and insertion. Annu. Rev. Physiol. 2003, 65, 791–815. [Google Scholar] [CrossRef] [PubMed]
- Martin, T.F.J. PI(4,5)P2-binding effector proteins for vesicle exocytosis. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2015, 1851, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Posor, Y.; Eichhorn-Gruenig, M.; Puchkov, D.; Schöneberg, J.; Ullrich, A.; Lampe, A.; Müller, R.; Zarbakhsh, S.; Gulluni, F.; Hirsch, E.; et al. Spatiotemporal control of endocytosis by phosphatidylinositol-3,4-bisphosphate. Nature 2013, 499, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Posor, Y.; Eichhorn-Grünig, M.; Haucke, V. Phosphoinositides in endocytosis. Biochim. Biophys. Acta BBA Mol. Cell Biol. Lipids 2015, 1851, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Lemmon, M.A. Membrane recognition by phospholipid-binding domains. Nat. Rev. Mol. Cell Biol. 2008, 9, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Takenawa, T. Phosphoinositide-binding interface proteins involved in shaping cell membranes. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2010, 86, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Takenawa, T.; Itoh, T. Membrane targeting and remodeling through phosphoinositide-binding domains. IUBMB Life 2006, 58, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Ungewickell, E.J.; Hinrichsen, L. Endocytosis: Clathrin-mediated membrane budding. Curr. Opin. Cell Biol. 2007, 19, 417–425. [Google Scholar] [CrossRef] [PubMed]
- Kooijman, E.; Testerink, C. Phosphatidic acid: An electrostatic/hydrogenbond switch? In Lipid Signaling in Plants; Munnik Teun: Berlin/Heidelberg, Germany, 2010; pp. 203–222. [Google Scholar]
- Lundbaek, J.A.; Collingwood, S.A.; Ingólfsson, H.I.; Kapoor, R.; Andersen, O.S. Lipid bilayer regulation of membrane protein function: Gramicidin channels as molecular force probes. J. R. Soc. Interface 2010, 7, 373–395. [Google Scholar] [CrossRef] [PubMed]
- Poccia, D.; Larijani, B. Phosphatidylinositol metabolism and membrane fusion. Biochem. J. 2009, 418, 233–246. [Google Scholar] [CrossRef] [PubMed]
- Chernomordik, L.V.; Zimmerberg, J. Bending membranes to the task: Structural intermediates in bilayer fusion. Curr. Opin. Struct. Biol. 1995, 5, 541–547. [Google Scholar] [CrossRef]
- James, D.J.; Khodthong, C.; Kowalchyk, J.A.; Martin, T.F.J. Phosphatidylinositol 4,5-bisphosphate regulates SNARE-dependent membrane fusion. J. Cell Biol. 2008, 182, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Balla, T. Phosphoinositides: Tiny lipids with giant impact on cell regulation. Physiol. Rev. 2013, 93, 1019–1137. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, J.E.M.; Thole, J.M.; Goedhart, J.; Nielsen, E.; Munnik, T.; Gadella, T.W.J. Imaging phosphatidylinositol 4-phosphate dynamics in living plant cells. Plant J. Cell Mol. Biol. 2009, 57, 356–372. [Google Scholar] [CrossRef] [PubMed]
- Vermeer, J.E.M.; van Leeuwen, W.; Tobeña-Santamaria, R.; Laxalt, A.M.; Jones, D.R.; Divecha, N.; Gadella, T.W.J.; Munnik, T. Visualization of PtdIns3P dynamics in living plant cells. Plant J. Cell Mol. Biol. 2006, 47, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, W.; Vermeer, J.E.M.; Gadella, T.W.J.; Munnik, T. Visualization of phosphatidylinositol 4,5-bisphosphate in the plasma membrane of suspension-cultured tobacco BY-2 cells and whole Arabidopsis seedlings. Plant J. Cell Mol. Biol. 2007, 52, 1014–1026. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, I. Phosphoinositide signaling in plant development. Dev. Camb. Engl. 2016, 143, 2044–2055. [Google Scholar] [CrossRef] [PubMed]
- Heilmann, M.; Heilmann, I. Plant phosphoinositides—Complex networks controlling growth and adaptation. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2015, 1851, 759–769. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Ufer, G.; Bartels, D. Lipid signalling in plant responses to abiotic stress: Lipid signalling in plant responses to abiotic stress. Plant Cell Environ. 2016, 39, 1029–1048. [Google Scholar] [CrossRef] [PubMed]
- Goebeler, V.; Ruhe, D.; Gerke, V.; Rescher, U. Annexin A8 displays unique phospholipid and F-actin binding properties. FEBS Lett. 2006, 580, 2430–2434. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.J.; Merrifield, C.J.; Shao, D.; Ayala-Sanmartin, J.; Schorey, C.D.; Levine, T.P.; Proust, J.; Curran, J.; Bailly, M.; Moss, S.E. Annexin 2 binding to phosphatidylinositol 4,5-bisphosphate on endocytic vesicles is regulated by the stress response pathway. J. Biol. Chem. 2004, 279, 14157–14164. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.J.; Shao, D.-M.; Grieve, A.; Levine, T.; Bailly, M.; Moss, S.E. Annexin A2 at the interface between F-actin and membranes enriched in phosphatidylinositol 4,5-bisphosphate. Biochim. Biophys. Acta 2009, 1793, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- Rescher, U. Annexin 2 is a phosphatidylinositol (4,5)-bisphosphate binding protein recruited to actin assembly sites at cellular membranes. J. Cell Sci. 2004, 117, 3473–3480. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.M.; Chichili, G.R.; Rodgers, W. Compartmentalization of phosphatidylinositol 4,5-bisphosphate signaling evidenced using targeted phosphatases. J. Biol. Chem. 2008, 283, 29920–29928. [Google Scholar] [CrossRef] [PubMed]
- Gokhale, N.A.; Abraham, A.; Digman, M.A.; Gratton, E.; Cho, W. Phosphoinositide specificity of and mechanism of lipid domain formation by Annexin A2-p11 heterotetramer. J. Biol. Chem. 2005, 280, 42831–42840. [Google Scholar] [CrossRef] [PubMed]
- Chrispeels, M.J. Sorting of proteins in the secretory system. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1991, 42, 21–53. [Google Scholar] [CrossRef]
- Neuhaus, J.M.; Rogers, J.C. Sorting of proteins to vacuoles in plant cells. Plant Mol. Biol. 1998, 38, 127–144. [Google Scholar] [CrossRef] [PubMed]
- Pedrazzini, E.; Komarova, N.Y.; Rentsch, D.; Vitale, A. Traffic routes and signals for the tonoplast: Protein sorting to the tonoplast. Traffic 2013, 14, 622–628. [Google Scholar] [CrossRef] [PubMed]
- Hawes, C.; Satiat-Jeunemaitre, B. The plant Golgi apparatus—Going with the flow. Biochim. Biophys. Acta 2005, 1744, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Von Blume, J.; Alleaume, A.-M.; Kienzle, C.; Carreras-Sureda, A.; Valverde, M.; Malhotra, V. Cab45 is required for Ca2+-dependent secretory cargo sorting at the trans-Golgi network. J. Cell Biol. 2012, 199, 1057–1066. [Google Scholar] [CrossRef] [PubMed]
- Curwin, A.J.; von Blume, J.; Malhotra, V. Cofilin-mediated sorting and export of specific cargo from the Golgi apparatus in yeast. Mol. Biol. Cell 2012, 23, 2327–2338. [Google Scholar] [CrossRef] [PubMed]
- Zárský, V.; Cvrcková, F.; Potocký, M.; Hála, M. Exocytosis and cell polarity in plants-exocyst and recycling domains. New Phytol. 2009, 183, 255–272. [Google Scholar] [CrossRef] [PubMed]
- Hala, M.; Cole, R.; Synek, L.; Drdova, E.; Pecenkova, T.; Nordheim, A.; Lamkemeyer, T.; Madlung, J.; Hochholdinger, F.; Fowler, J.E.; et al. An exocyst complex functions in plant cell growth in Arabidopsis and tobacco. Plant Cell Online 2008, 20, 1330–1345. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, G.; Deng, Y.; Xie, M.; Feng, Z.; Sun, M.; Zhao, Y.; Liang, L.; Ding, N.; Jia, W. Excretion and folding of plasmalemma function to accommodate alterations in guard cell volume during stomatal closure in Vicia faba L. J. Exp. Bot. 2010, 61, 3749–3758. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xie, M.; Sun, M.; Jia, W. How does plasmalemma surface area accommodate alterations in guard cell volume during stomatal closing? Plant Signal. Behav. 2010, 5, 1468–1469. [Google Scholar] [CrossRef] [PubMed]
- Shope, J.C.; Mott, K.A. Membrane trafficking and osmotically induced volume changes in guard cells. J. Exp. Bot. 2006, 57, 4123–4131. [Google Scholar] [CrossRef] [PubMed]
- Shope, J.C.; DeWald, D.B.; Mott, K.A. Changes in surface area of intact guard cells are correlated with membrane internalization. Plant Physiol. 2003, 133, 1314–1321. [Google Scholar] [CrossRef] [PubMed]
- Nakata, T.; Sobue, K.; Hirokawa, N. Conformational change and localization of calpactin I complex involved in exocytosis as revealed by quick-freeze, deep-etch electron microscopy and immunocytochemistry. J. Cell Biol. 1990, 110, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Senda, T.; Okabe, T.; Matsuda, M.; Fujita, H. Quick-freeze, deep-etch visualization of exocytosis in anterior pituitary secretory cells: Localization and possible roles of actin and annexin II. Cell Tissue Res. 1994, 277, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.M.; Geisow, M.J.; Burgoyne, R.D. A role for calpactin in calcium-dependent exocytosis in adrenal chromaffin cells. Nature 1989, 340, 313–315. [Google Scholar] [CrossRef] [PubMed]
- Sarafian, T.; Pradel, L.A.; Henry, J.P.; Aunis, D.; Bader, M.F. The participation of annexin II (calpactin I) in calcium-evoked exocytosis requires protein kinase C. J. Cell Biol. 1991, 114, 1135–1147. [Google Scholar] [CrossRef] [PubMed]
- Uemura, T.; Ueda, T.; Ohniwa, R.L.; Nakano, A.; Takeyasu, K.; Sato, M.H. Systematic analysis of SNARE molecules in Arabidopsis: Dissection of the post-Golgi network in plant cells. Cell Struct. Funct. 2004, 29, 49–65. [Google Scholar] [CrossRef] [PubMed]
- Umbrecht-Jenck, E.; Demais, V.; Calco, V.; Bailly, Y.; Bader, M.-F.; Chasserot-Golaz, S. S100A10-mediated translocation of annexin-A2 to SNARE proteins in adrenergic chromaffin cells undergoing exocytosis. Traffic Cph. Den. 2010, 11, 958–971. [Google Scholar] [CrossRef] [PubMed]
- Chander, A.; Gerelsaikhan, T.; Vasa, P.K.; Holbrook, K. Annexin A7 trafficking to alveolar type II cell surface: Possible roles for protein insertion into membranes and lamellar body secretion. Biochim. Biophys. Acta BBA Mol. Cell Res. 2013, 1833, 1244–1255. [Google Scholar] [CrossRef] [PubMed]
- Chander, A.; Gerelsaikhan, T.; Vasa, P.K. Annexin A7 interactions with SNARE protein SNAP23 are regulated by calcium and protein phosphorylation during surfactant secretion in alveolar type II cells. FASEB J. 2012, 26, S698. [Google Scholar]
- Wang, P.; Chintagari, N.R.; Gou, D.; Su, L.; Liu, L. Physical and functional interactions of SNAP-23 with annexin A2. Am. J. Respir. Cell Mol. Biol. 2007, 37, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Maxfield, F.R. Plasma membrane microdomains. Curr. Opin. Cell Biol. 2002, 14, 483–487. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Kitakura, S.; Vanneste, S.; Robert, S.; Löfke, C.; Teichmann, T.; Tanaka, H.; Friml, J. Clathrin mediates endocytosis and polar distribution of PIN auxin transporters in Arabidopsis. Plant Cell 2011, 23, 1920–1931. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Rodriguez-Furlan, C.; Wang, J.; van de Ven, W.; Gao, T.; Raikhel, N.V.; Hicks, G.R. Different endomembrane trafficking pathways establish apical and basal polarities. Plant Cell 2017, 29, 90–108. [Google Scholar] [CrossRef] [PubMed]
- Luschnig, C.; Vert, G. The dynamics of plant plasma membrane proteins: PINs and beyond. Development 2014, 141, 2924–2938. [Google Scholar] [CrossRef] [PubMed]
- Kleine-Vehn, J.; Friml, J. Polar targeting and endocytic recycling in auxin-dependent plant development. Annu. Rev. Cell Dev. Biol. 2008, 24, 447–473. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wang, H.; Zhang, Y.; Qi, B.; Xu, G.; Zheng, H. A proteomic approach to analyzing responses of Arabidopsis thaliana root cells to different gravitational conditions using an agravitropic mutant, pin2 and its wild type. Proteome Sci. 2011, 9, 72. [Google Scholar] [CrossRef] [PubMed]
- Dhonukshe, P.; Baluska, F.; Schlicht, M.; Hlavacka, A.; Samaj, J.; Friml, J.; Gadella, T.W.J. Endocytosis of cell surface material mediates cell plate formation during plant cytokinesis. Dev. Cell 2006, 10, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Dettmer, J.; Hong-Hermesdorf, A.; Stierhof, Y.-D.; Schumacher, K. Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 2006, 18, 715–730. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.K.; Siu, C.L.; Hillmer, S.; Jang, S.; An, G.; Robinson, D.G.; Jiang, L. Rice SCAMP1 defines clathrin-coated, trans-Golgi-located tubular-vesicular structures as an early endosome in tobacco BY-2 cells. Plant Cell 2007, 19, 296–319. [Google Scholar] [CrossRef] [PubMed]
- Geldner, N.; Jürgens, G. Endocytosis in signalling and development. Curr. Opin. Plant Biol. 2006, 9, 589–594. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Jürgens, G. Membrane traffic and fusion at post-Golgi compartments. Front. Plant Sci. 2012, 2. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.R.G.; Nickerson, D.P.; Odorizzi, G. Molecular mechanisms of late endosome morphology, identity and sorting. Curr. Opin. Cell Biol. 2006, 18, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Perret, E.; Lakkaraju, A.; Deborde, S.; Schreiner, R.; Rodriguez-Boulan, E. Evolving endosomes: How many varieties and why? Curr. Opin. Cell Biol. 2005, 17, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Sachse, M.; Ramm, G.; Strous, G.; Klumperman, J. Endosomes: Multipurpose designs for integrating housekeeping and specialized tasks. Histochem. Cell Biol. 2002, 117, 91–104. [Google Scholar] [CrossRef] [PubMed]
- Cain, C.C.; Sipe, D.M.; Murphy, R.F. Regulation of endocytic pH by the Na+,K+-ATPase in living cells. Proc. Natl. Acad. Sci. USA 1989, 86, 544–548. [Google Scholar] [CrossRef] [PubMed]
- Irani, N.G.; Russinova, E. Receptor endocytosis and signaling in plants. Curr. Opin. Plant Biol. 2009, 12, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Mindell, J.A. Lysosomal acidification mechanisms. Annu. Rev. Physiol. 2012, 74, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Muller, J.; Mettbach, U.; Menzel, D.; Samaj, J. Molecular dissection of endosomal compartments in plants. Plant Physiol. 2007, 145, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, P.; Wan, Y.; Chen, T.; Wang, Q.; Mettbach, U.; Baluska, F.; Samaj, J.; Fang, X.; Lucas, W.J.; et al. A membrane microdomain-associated protein, Arabidopsis Flot1, is involved in a clathrin-independent endocytic pathway and is required for seedling development. Plant Cell 2012, 24, 2105–2122. [Google Scholar] [CrossRef] [PubMed]
- Viotti, C.; Bubeck, J.; Stierhof, Y.-D.; Krebs, M.; Langhans, M.; van den Berg, W.; van Dongen, W.; Richter, S.; Geldner, N.; Takano, J.; et al. Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell 2010, 22, 1344–1357. [Google Scholar] [CrossRef] [PubMed]
- Raiborg, C.; Stenmark, H. The ESCRT machinery in endosomal sorting of ubiquitylated membrane proteins. Nature 2009, 458, 445–452. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhuang, X.; 3, C.; Wang, X.; Jiang, L. The Arabidopsis endosomal sorting complex required for transport III regulates internal vesicle formation of the prevacuolar compartment and is required for plant development. Plant Physiol. 2014, 165, 1328–1343. [Google Scholar] [CrossRef] [PubMed]
- Leung, K.F.; Dacks, J.B.; Field, M.C. Evolution of the multivesicular body ESCRT machinery; retention across the eukaryotic lineage. Traffic 2008, 9, 1698–1716. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, C.; Schellmann, S.; Sabovljevic, A.; Shahriari, M.; Keshavaiah, C.; Bechtold, N.; Herzog, M.; Muller, S.; Hanisch, F.-G.; Hulskamp, M. The Arabidopsis elch mutant reveals functions of an ESCRT component in cytokinesis. Development 2006, 133, 4679–4689. [Google Scholar] [CrossRef] [PubMed]
- Winter, V.; Hauser, M.-T. Exploring the ESCRTing machinery in eukaryotes. Trends Plant Sci. 2006, 11, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Luo, M.; Zhao, Q.; Yang, R.; Cui, Y.; Zeng, Y.; Xia, J.; Jiang, L. A unique plant ESCRT component, FREE1, regulates multivesicular body protein sorting and plant growth. Curr. Biol. 2014, 24, 2556–2563. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Cui, Y.; Gao, C.; Jiang, L. Endocytic and autophagic pathways crosstalk in plants. Curr. Opin. Plant Biol. 2015, 28, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Seals, D.F.; Randall, S.K. A vacuole-associated annexin protein, VCaB42, correlates with the expansion of tobacco cells. Plant Physiol. 1997, 115, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Hicks, G.R.; Raikhel, N.V. Plant vacuole morphology and vacuolar trafficking. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Paris, N.; Stanley, C.M.; Jones, R.L.; Rogers, J.C. Plant cells contain two functionally distinct vacuolar compartments. Cell 1996, 85, 563–572. [Google Scholar] [CrossRef]
- Rogers, J.C. Multiple vacuoles in plant cells. Plant Physiol. 2008, 146, 1024–1025. [Google Scholar] [CrossRef] [PubMed]
- Frigerio, L.; Hinz, G.; Robinson, D.G. Multiple vacuoles in plant cells: Rule or exception? Traffic 2008, 9, 1564–1570. [Google Scholar] [CrossRef] [PubMed]
- Zouhar, J.; Rojo, E. Plant vacuoles: Where did they come from and where are they heading? Curr. Opin. Plant Biol. 2009, 12, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Hinz, G.; Hillmer, S.; Baumer, M.; Hohl, I. Vacuolar storage proteins and the putative vacuolar sorting receptor BP-80 exit the Golgi apparatus of developing pea cotyledons in different transport vesicles. Plant Cell 1999, 11, 1509–1524. [Google Scholar] [CrossRef] [PubMed]
- Jauh, G.-Y.; Phillips, T.E.; Rogers, J.C. Tonoplast intrinsic protein isoforms as markers for vacuolar functions. Plant Cell 1999, 11, 1867–1882. [Google Scholar] [CrossRef] [PubMed]
- Olbrich, A.; Hillmer, S.; Hinz, G.; Oliviusson, P.; Robinson, D.G. Newly formed vacuoles in root meristems of barley and pea seedlings have characteristics of both protein storage and lytic vacuoles. Plant Physiol. 2007, 145, 1383–1394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.F.; Staehelin, L.A. Functional compartmentation of the Golgi apparatus of plant cells: Immunocytochemical analysis of high-pressure frozen- and freeze-substituted sycamore maple suspension culture cells. Plant Physiol. 1992, 99, 1070–1083. [Google Scholar] [CrossRef] [PubMed]
- Xiang, L.; Etxeberria, E.; van den Ende, W. Vacuolar protein sorting mechanisms in plants. FEBS J. 2013, 280, 979–993. [Google Scholar] [CrossRef] [PubMed]
- Jurgens, G. Membrane trafficking in plants. Annu. Rev. Cell Dev. Biol. 2004, 20, 481–504. [Google Scholar] [CrossRef] [PubMed]
- Jolliffe, N.A.; Craddock, C.P.; Frigerio, L. Pathways for protein transport to seed storage vacuoles. Biochem. Soc. Trans. 2005, 33, 1016–1018. [Google Scholar] [CrossRef] [PubMed]
- De Marcos Lousa, C.; Gershlick, D.C.; Denecke, J. Mechanisms and concepts paving the way towards a complete transport cycle of plant vacuolar sorting receptors. Plant Cell 2012, 24, 1714–1732. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Jang, M.; Song, K.; Kang, H.; Lee, M.H.; Lee, D.W.; Zouhar, J.; Rojo, E.; Sohn, E.J.; Hwang, I. Functional identification of sorting receptors involved in trafficking of soluble lytic vacuolar proteins in vegetative cells of Arabidopsis. Plant Physiol. 2013, 161, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Oufattole, M.; Rogers, J.C. Golgi-mediated vacuolar sorting in plant cells: RMR proteins are sorting receptors for the protein aggregation/membrane internalization pathway. Plant Sci. 2007, 172, 728–745. [Google Scholar] [CrossRef]
- Wang, H.; Rogers, J.C.; Jiang, L. Plant RMR proteins: Unique vacuolar sorting receptors that couple ligand sorting with membrane internalization: Plant RMR proteins. FEBS J. 2011, 278, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Bottanelli, F.; Foresti, O.; Hanton, S.; Denecke, J. Vacuolar transport in tobacco leaf epidermis cells involves a single route for soluble cargo and multiple routes for membrane cargo. Plant Cell 2011, 23, 3007–3025. [Google Scholar] [CrossRef] [PubMed]
- Wolfenstetter, S.; Wirsching, P.; Dotzauer, D.; Schneider, S.; Sauer, N. Routes to the tonoplast: The sorting of tonoplast transporters in Arabidopsis mesophyll protoplasts. Plant Cell 2012, 24, 215–232. [Google Scholar] [CrossRef] [PubMed]
- Herman, E.; Schmidt, M. Endoplasmic reticulum to vacuole trafficking of endoplasmic reticulum bodies provides an alternate pathway for protein transfer to the vacuole. Plant Physiol. 2004, 136, 3440–3446. [Google Scholar] [CrossRef] [PubMed]
- Michaeli, S.; Avin-Wittenberg, T.; Galili, G. Involvement of autophagy in the direct ER to vacuole protein trafficking route in plants. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Happel, N.; Höning, S.; Neuhaus, J.-M.; Paris, N.; Robinson, D.G.; Holstein, S.E.H. Arabidopsis mu A-adaptin interacts with the tyrosine motif of the vacuolar sorting receptor VSR-PS1. Plant J. Cell Mol. Biol. 2004, 37, 678–693. [Google Scholar] [CrossRef]
- Niemes, S.; Langhans, M.; Viotti, C.; Scheuring, D.; San Wan Yan, M.; Jiang, L.; Hillmer, S.; Robinson, D.G.; Pimpl, P. Retromer recycles vacuolar sorting receptors from the trans-Golgi network. Plant J. 2010, 61, 107–121. [Google Scholar] [CrossRef] [PubMed]
- Ebine, K.; Okatani, Y.; Uemura, T.; Goh, T.; Shoda, K.; Niihama, M.; Morita, M.T.; Spitzer, C.; Otegui, M.S.; Nakano, A.; et al. A SNARE complex unique to seed plants is required for protein storage vacuole biogenesis and seed development of Arabidopsis thaliana. Plant Cell Online 2008, 20, 3006–3021. [Google Scholar] [CrossRef] [PubMed]
- Sanderfoot, A.A.; Kovaleva, V.; Bassham, D.C.; Raikhel, N.V. Interactions between syntaxins identify at least five SNARE complexes within the Golgi/prevacuolar system of the Arabidopsis cell. Mol. Biol. Cell 2001, 12, 3733–3743. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Williamson, J.D. Is there leaderless protein secretion in plants? Plant Signal. Behav. 2010, 5, 129–131. [Google Scholar] [CrossRef] [PubMed]
- Barnes, A.; Bale, J.; Constantinidou, C.; Ashton, P.; Jones, A.; Pritchard, J. Determining protein identity from sieve element sap in Ricinus communis L. by quadrupole time of flight (Q-TOF) mass spectrometry. J. Exp. Bot. 2004, 55, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Giavalisco, P.; Kapitza, K.; Kolasa, A.; Buhtz, A.; Kehr, J. Towards the proteome of Brassica napus phloem sap. Proteomics 2006, 6, 896–909. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.-K.; Yokoyama, R.; Nishitani, K. A proteomic approach to apoplastic proteins involved in cell wall regeneration in protoplasts of Arabidopsis suspension-cultured cells. Plant Cell Physiol. 2005, 46, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Oh, I.S.; Park, A.R.; Bae, M.S.; Kwon, S.J.; Kim, Y.S.; Lee, J.E.; Kang, N.Y.; Lee, S.; Cheong, H.; Park, O.K. Secretome analysis reveals an Arabidopsis lipase involved in defense against Alternaria brassicicola. Plant Cell 2005, 17, 2832–2847. [Google Scholar] [CrossRef] [PubMed]
- Regente, M.; Pinedo, M.; Elizalde, M.; de la Canal, L. Apoplastic exosome-like vesicles: A new way of protein secretion in plants? Plant Signal. Behav. 2012, 7, 544–546. [Google Scholar] [CrossRef] [PubMed]
- Rutter, B.D.; Innes, R.W. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Blackburn, K.; Lin, Y.; Goshe, M.B.; Williamson, J.D. Absolute protein quantification by LC/MSE for global analysis of salicylic acid-induced plant protein secretion responses. J. Proteome Res. 2009, 8, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Slabas, A.R.; Ndimba, B.; Simon, W.J.; Chivasa, S. Proteomic analysis of the Arabidopsis cell wall reveals unexpected proteins with new cellular locations. Biochem. Soc. Trans. 2004, 32, 524–528. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konopka-Postupolska, D.; Clark, G. Annexins as Overlooked Regulators of Membrane Trafficking in Plant Cells. Int. J. Mol. Sci. 2017, 18, 863. https://doi.org/10.3390/ijms18040863
Konopka-Postupolska D, Clark G. Annexins as Overlooked Regulators of Membrane Trafficking in Plant Cells. International Journal of Molecular Sciences. 2017; 18(4):863. https://doi.org/10.3390/ijms18040863
Chicago/Turabian StyleKonopka-Postupolska, Dorota, and Greg Clark. 2017. "Annexins as Overlooked Regulators of Membrane Trafficking in Plant Cells" International Journal of Molecular Sciences 18, no. 4: 863. https://doi.org/10.3390/ijms18040863
APA StyleKonopka-Postupolska, D., & Clark, G. (2017). Annexins as Overlooked Regulators of Membrane Trafficking in Plant Cells. International Journal of Molecular Sciences, 18(4), 863. https://doi.org/10.3390/ijms18040863






