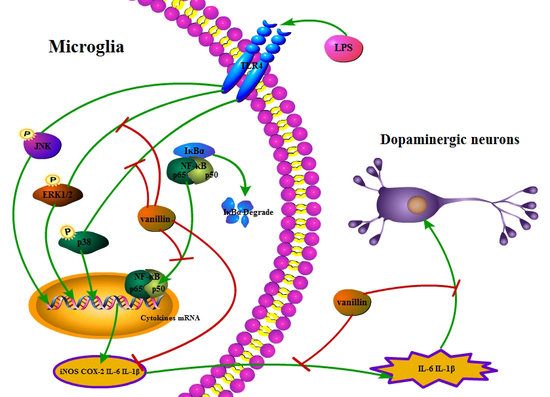
Vanillin Protects Dopaminergic Neurons against Inflammation-Mediated Cell Death by Inhibiting ERK1/2, P38 and the NF-κB Signaling Pathway
Abstract
:1. Introduction
2. Results
2.1. Vanillin Treatment Improves Motor Dysfunction Induced by LPS Intranigral Injection
2.2. Vanillin Adminastration Increases the Survival Rate of Dopaminergic Neurons in the SN
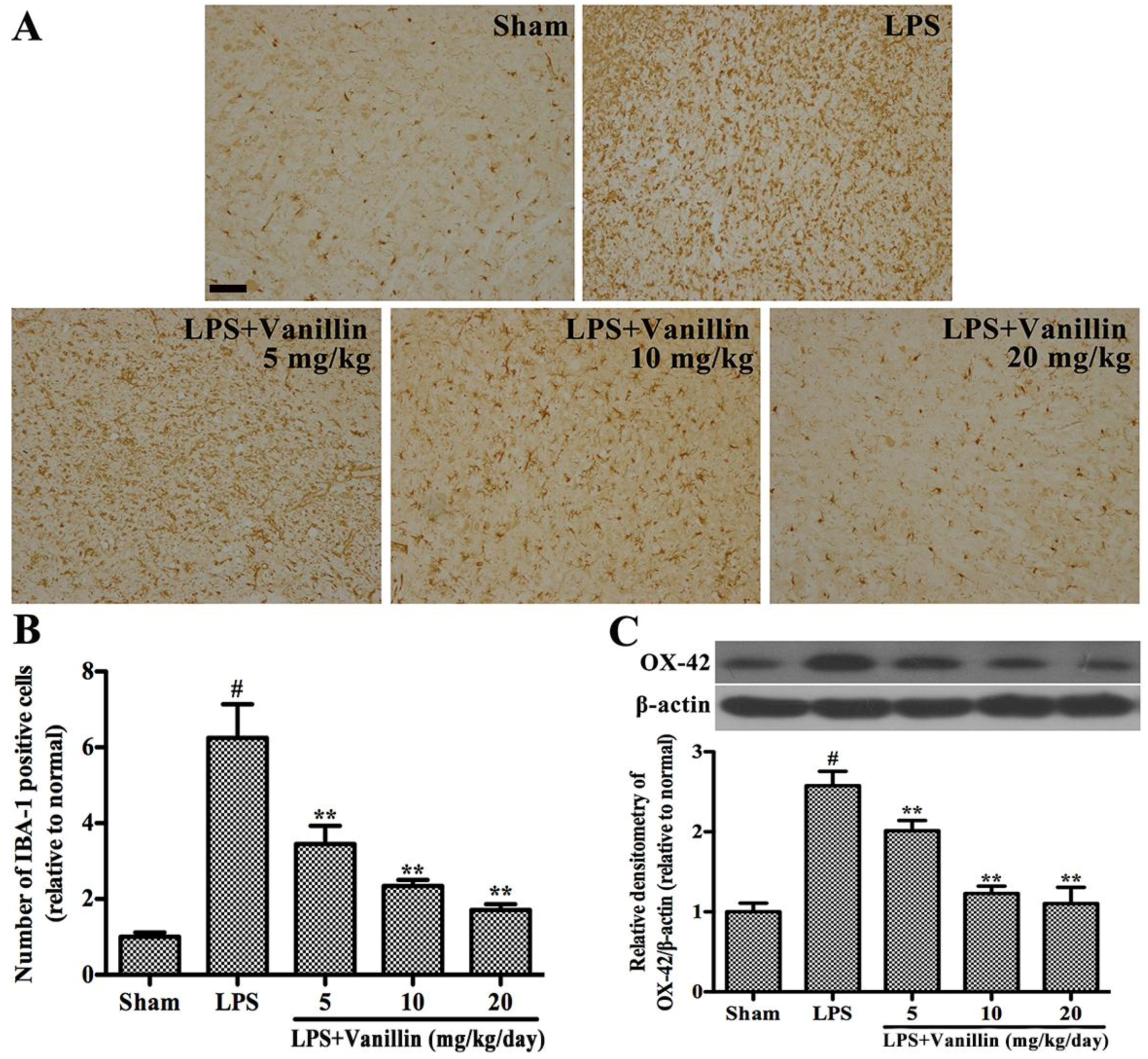
2.3. Vanillin Inhibits LPS-Induced Activation of Microglia in the SN
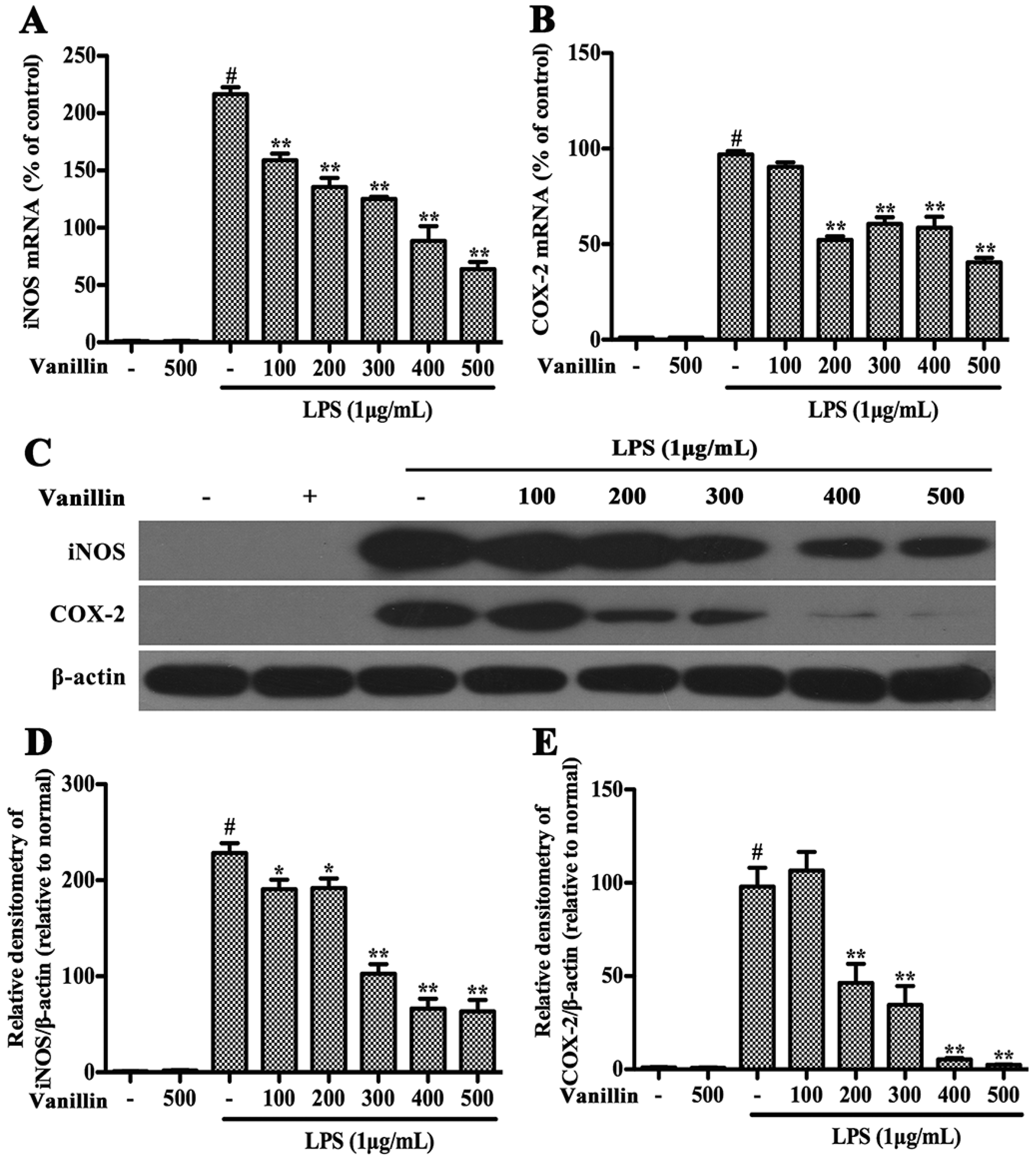
2.4. Vanillin Inhibits Inflammatory Responses Induced by LPS in BV-2 Cells
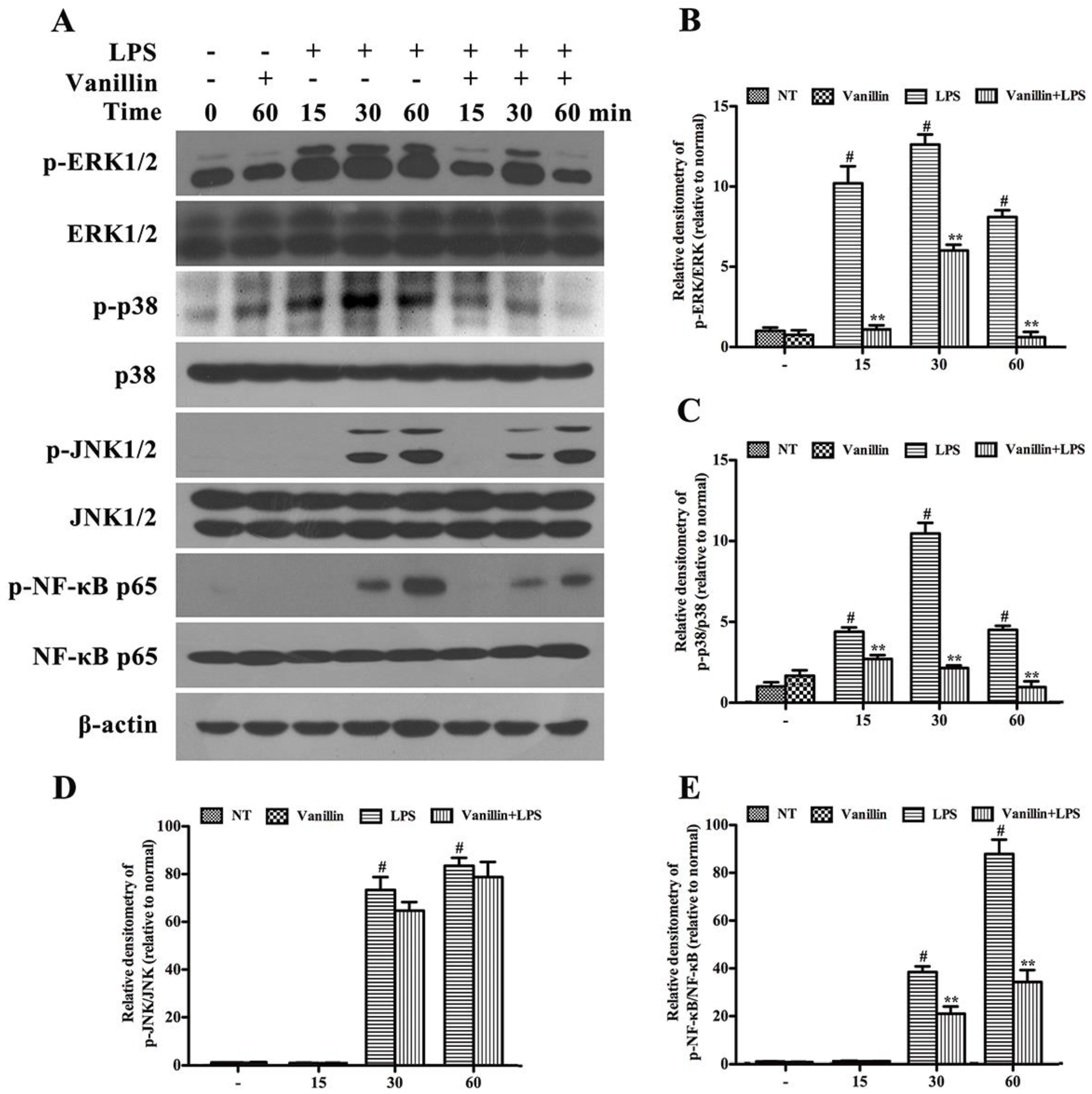
2.5. Vanillin Inhibits Activation of ERK1/2, p38 and NF-κB Induced by LPS in BV-2 Cells
3. Discussion
4. Materials and Methods
4.1. Animals and Surgery
4.2. Administration of Vanillin
4.3. Apomorphine-Induced Rotational Behavior
4.4. Cell Treatments
4.5. RNA Extraction and Quantitative Real-Time PCR
4.6. ELISA
4.7. Immunohistological Analysis
4.8. Western Blot Analysis
4.9. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PD | Parkinson’s disease |
| LPS | lipopolysaccharide |
| SN | substantia nigra |
| SNpc | substantia nigra pars compacta |
| TH | tyrosine hydroxylase |
| IBA-1 | ionized calcium-binding adaptor molecule-1 |
References
- Dorsey, E.R.; Constantinescu, R.; Thompson, J.P.; Biglan, K.M.; Holloway, R.G.; Kieburtz, K.; Marshall, F.J.; Ravina, B.M.; Schifitto, G.; Siderowf, A.; et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology 2007, 68, 384–386. [Google Scholar] [CrossRef] [PubMed]
- Athauda, D.; Foltynie, T. Challenges in detecting disease modification in Parkinson’s disease clinical trials. Parkinsonism Relat. Disord. 2016, 32, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Sun, Q.; Chen, S. Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog. Neurobiol. 2016, 147, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Zhang, S.Z.; Tang, M.; Zhang, X.H.; Zhou, Z.; Yin, Y.Q.; Zhou, Q.B.; Huang, Y.Y.; Liu, Y.J.; Wawrousek, E.; et al. Suppression of neuroinflammation by astrocytic dopamine D2 receptors via αB-crystallin. Nature 2013, 494, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Vijitruth, R.; Liu, M.; Choi, D.Y.; Nguyen, X.V.; Hunter, R.L.; Bing, G. Cyclooxygenase-2 mediates microglial activation and secondary dopaminergic cell death in the mouse MPTP model of Parkinson’s disease. J. Neuroinflamm. 2006, 3, 6. [Google Scholar] [CrossRef] [PubMed]
- Tansey, M.G.; Goldberg, M.S. Neuroinflammation in Parkinson’s disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 2010, 37, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Appel, S.H. Inflammation in Parkinson’s disease: Cause or consequence? Mov. Disord. 2012, 27, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Sochocka, M.; Diniz, B.S.; Leszek, J. Inflammatory response in the CNS: Friend or foe? Mol. Neurobiol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, A.; Patnala, R.; Jadhav, S.P.; Ling, E.A.; Dheen, S.T. MicroRNAs: Key players in microglia and astrocyte mediated inflammation in CNS pathologies. Curr. Med. Chem. 2016, 23, 3528–3546. [Google Scholar] [CrossRef] [PubMed]
- De Virgilio, A.; Greco, A.; Fabbrini, G.; Inghilleri, M.; Rizzo, M.I.; Gallo, A.; Conte, M.; Rosato, C.; Appiani, M.C.; de Vincentiis, M. Parkinson’s disease: Autoimmunity and neuroinflammation. Autoimmun. Rev. 2016, 15, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Tufekci, K.U.; Meuwissen, R.; Genc, S.; Genc, K. Inflammation in Parkinson’s disease. Adv. Protein Chem. Struct. 2012, 88, 69–132. [Google Scholar]
- Rao, S.R.; Ravishankar, G.A. Vanilla flavour: Production by conventional and biotechnological routes. J. Sci. Food Agric. 2000, 80, 289–304. [Google Scholar]
- Cerrutti, P.; Alzamora, S.M. Inhibitory effects of vanillin on some food spoilage yeasts in laboratory media and fruit purees. Int. J. Food Microbial. 1996, 29, 379–386. [Google Scholar] [CrossRef]
- Tai, A.; Sawano, T.; Yazama, F.; Ito, H. Evaluation of antioxidant activity of vanillin by using multiple antioxidant assays. Bba Gen Subjects 2011, 1810, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Dhanalakshmi, C.; Manivasagam, T.; Nataraj, J.; Justin Thenmozhi, A.; Essa, M.M. Neurosupportive role of vanillin, a natural phenolic compound, on rotenone induced neurotoxicity in SH-SY5Y neuroblastoma cells. Evid. Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef] [PubMed]
- Dhanalakshmi, C.; Janakiraman, U.; Manivasagam, T.; Justin Thenmozhi, A.; Essa, M.M.; Kalandar, A.; Khan, M.A.; Guillemin, G.J. Vanillin attenuated behavioural impairments, neurochemical deficts, oxidative stress and apoptosis against rotenone induced rat model of Parkinson’s disease. Neurochem. Res. 2016, 41, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Makni, M.; Chtourou, Y.; Fetoui, H.; Garoui, M.; Boudawara, T.; Zeghal, N. Evaluation of the antioxidant, anti-inflammatory and hepatoprotective properties of vanillin in carbon tetrachloride-treated rats. Eur. J. Pharmacol. 2011, 668, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Murakami, Y.; Hirata, A.; Ito, S.; Shoji, M.; Tanaka, S.; Yasui, T.; Machino, M.; Fujisawa, S. Re-evaluation of cyclooxygenase-2-inhibiting activity of vanillin and guaiacol in macrophages stimulated with lipopolysaccharide. Anticancer Res. 2007, 27, 801–807. [Google Scholar] [PubMed]
- Wu, S.L.; Chen, J.C.; Li, C.C.; Lo, H.Y.; Ho, T.Y.; Hsiang, C.Y. Vanillin improves and prevents trinitrobenzene sulfonic acid-induced colitis in mice. J. Pharmacol. Exp. Ther. 2009, 330, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.P.; Wang, J.F.; Xue, W.J.; Liu, H.M.; Liu, B.R.; Zeng, Y.L.; Li, S.N.; Huang, B.X.; Lv, Q.K.; Wang, W.; et al. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J. Neuroinflamm. 2015, 12, 9. [Google Scholar] [CrossRef] [PubMed]
- Henn, A.; Lund, S.; Hedtjam, M.; Porzgen, P.; Leist, M. The suitability of BV2 cells as alternative model system for primary microglia cultures or animal experiments of brain inflammation. Eur. J. Cell Biol. 2009, 26, 83–94. [Google Scholar] [CrossRef]
- Li, N.; Liu, B.W.; Ren, W.Z.; Liu, J.X.; Li, S.N.; Fu, S.P.; Zeng, Y.L.; Xu, S.Y.; Yan, X.; Gao, Y.J.; et al. GLP-2 Attenuates LPS-induced inflammation in BV-2 cells by inhibiting ERK1/2, JNK1/2 and NF-κB signaling pathways. Int. J. Mol. Sci. 2016, 17, 190. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.P.; Zou, M.; Wang, J.Y.; Zhu, J.J.; Lai, J.M.; Zhou, L.L.; Chen, S.F.; Zhang, X.; Zhu, J.H. Paroxetine ameliorates lipopolysaccharide-induced microglia activation via differential regulation of MAPK signaling. J. Neuroinflamm. 2014, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.N.; Zong, Y.; Zhong, L.M.; Li, Y.M.; Zhang, W.; Bian, L.G.; Ai, Q.L.; Liu, Y.D.; Sun, J.; Lu, D. Gastrodin inhibits expression of inducible NO synthase, cyclooxygenase-2 and proinflammatory cytokines in cultured LPS-stimulated microglia via MAPK pathways. PLoS ONE 2011, 6, e21891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Kang, M.; Zhang, T.; Li, B.; Liao, X.; Wang, R. Triptolide combined with radiotherapy for the treatment of nasopharyngeal carcinoma via NF-κB-related mechanism. Int. J. Mol. Sci. 2016, 17, 2139. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sharma, B. Pharmacological benefits of agomelatine and vanillin in experimental model of Huntington’s disease. Pharmacol. Biochem. Behav. 2014, 122, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, E.C.; Hunot, S.; Damier, P.; Faucheux, B. Glial cells and inflammation in Parkinson’s disease: A role in neurodegeneration? Ann. Neurol. 1998, 44, S115–S120. [Google Scholar] [CrossRef] [PubMed]
- Kitamura, Y.; Itano, Y.; Kubo, T.; Nomura, Y. Suppressive effect of FK-506, a novel immunosuppressant, against MPTP-induced dopamine depletion in the striatum of young C57BL/6 mice. J. Neuroimmunol. 1994, 50, 221–224. [Google Scholar] [CrossRef]
- Kurkowska-Jastrzebska, I.; Wronska, A.; Kohutnicka, M.; Czlonkowski, A.; Czlonkowska, A. The inflammatory reaction following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine intoxication in mouse. Exp. Neurol. 1999, 156, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Herrera, A.J.; Castano, A.; Venero, J.L.; Cano, J.; Machado, A. The single intranigral injection of LPS as a new model for studying the selective effects of inflammatory reactions on dopaminergic system. Neurobiol. Dis. 2000, 7, 429–447. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jiang, J.W.; Wilson, B.C.; Du, L.; Yang, S.N.; Wang, J.Y.; Wu, G.C.; Cao, X.D.; Hong, J.S. Systemic infusion of naloxone reduces degeneration of rat substantia nigral dopaminergic neurons induced by intranigral injection of lipopolysaccharide. J. Pharmacol. Exp. Ther. 2000, 295, 125–132. [Google Scholar] [PubMed]
- Henn, A.; Lund, S.; Hedtjarn, M.; Schrattenholz, A.; Porzgen, P.; Leist, M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. ALTEX 2009, 26, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Tai, W.J.; Ye, X.; Bao, X.Q.; Zhao, B.Z.; Wang, X.L.; Zhang, D. Inhibition of Src tyrosine kinase activity by squamosamide derivative FLZ attenuates neuroinflammation in both in vivo and in vitro Parkinson’s disease models. Neuropharmacology 2013, 75, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Chen, J.; Ye, J.; Zhai, X.; Song, J.; Jiang, C.; Wang, J.; Zhang, H.; Jia, X.; Zhu, F. Anti-inflammatory effect of the six compounds isolated from Nauclea officinalis Pierrc ex Pitard, and molecular mechanism of strictosamide via suppressing the NF-κB and MAPK signaling pathway in LPS-induced RAW 264.7 macrophages. J. Ethnopharmacol. 2016, 196, 66–74. [Google Scholar] [CrossRef] [PubMed]




| Gene | Sequences | Length (bp) |
|---|---|---|
| β-actin | (F) 5′-GTCAGGTCATCACTATCGGCAAT-3′ (R) 5′-AGAGGTCTTTACGGATGTCAACGT-3′ | 147 |
| iNOS | (F) 5′-CACCCAGAAGAGTTACAGC-3′ (R) 5′-GGAGGGAAGGGAGAATAG-3′ | 186 |
| COX-2 | (F) 5′-AGAGTCAGTTAGTGGGTAGT-3′ (R) 5′-CTTGTAGTAGGCTTAAACATAG-3′ | 170 |
| TNF-α | (F) 5′-CCACGCTCTTCTGTCTACTG-3′ (R) 5′-GCTACGGGCTTGTCACTC-3′ | 145 |
| IL-1β | (F) 5′-TGTGATGTTCCCATTAGAC-3′ (R) 5′-AATACCACTTGTTGGCTTA-3′ | 131 |
| IL-6 | (F) 5′-AGCCACTGCCTTCCCTAC-3′ (R) 5′-TTGCCATTGCACAACTCTT-3′ | 156 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Liu, D.-F.; Zhang, X.-Y.; Liu, D.; Xu, S.-Y.; Chen, G.-X.; Huang, B.-X.; Ren, W.-Z.; Wang, W.; Fu, S.-P.; et al. Vanillin Protects Dopaminergic Neurons against Inflammation-Mediated Cell Death by Inhibiting ERK1/2, P38 and the NF-κB Signaling Pathway. Int. J. Mol. Sci. 2017, 18, 389. https://doi.org/10.3390/ijms18020389
Yan X, Liu D-F, Zhang X-Y, Liu D, Xu S-Y, Chen G-X, Huang B-X, Ren W-Z, Wang W, Fu S-P, et al. Vanillin Protects Dopaminergic Neurons against Inflammation-Mediated Cell Death by Inhibiting ERK1/2, P38 and the NF-κB Signaling Pathway. International Journal of Molecular Sciences. 2017; 18(2):389. https://doi.org/10.3390/ijms18020389
Chicago/Turabian StyleYan, Xuan, Dian-Feng Liu, Xiang-Yang Zhang, Dong Liu, Shi-Yao Xu, Guang-Xin Chen, Bing-Xu Huang, Wen-Zhi Ren, Wei Wang, Shou-Peng Fu, and et al. 2017. "Vanillin Protects Dopaminergic Neurons against Inflammation-Mediated Cell Death by Inhibiting ERK1/2, P38 and the NF-κB Signaling Pathway" International Journal of Molecular Sciences 18, no. 2: 389. https://doi.org/10.3390/ijms18020389
APA StyleYan, X., Liu, D.-F., Zhang, X.-Y., Liu, D., Xu, S.-Y., Chen, G.-X., Huang, B.-X., Ren, W.-Z., Wang, W., Fu, S.-P., & Liu, J.-X. (2017). Vanillin Protects Dopaminergic Neurons against Inflammation-Mediated Cell Death by Inhibiting ERK1/2, P38 and the NF-κB Signaling Pathway. International Journal of Molecular Sciences, 18(2), 389. https://doi.org/10.3390/ijms18020389