Nutritional and Acquired Deficiencies in Inositol Bioavailability. Correlations with Metabolic Disorders
Abstract
:1. Introduction. Health Protection from Whole-Grain Cereals: Key Components beyond Fiber
2. Inositol and Phytic Acid
3. Inositol Deficiency
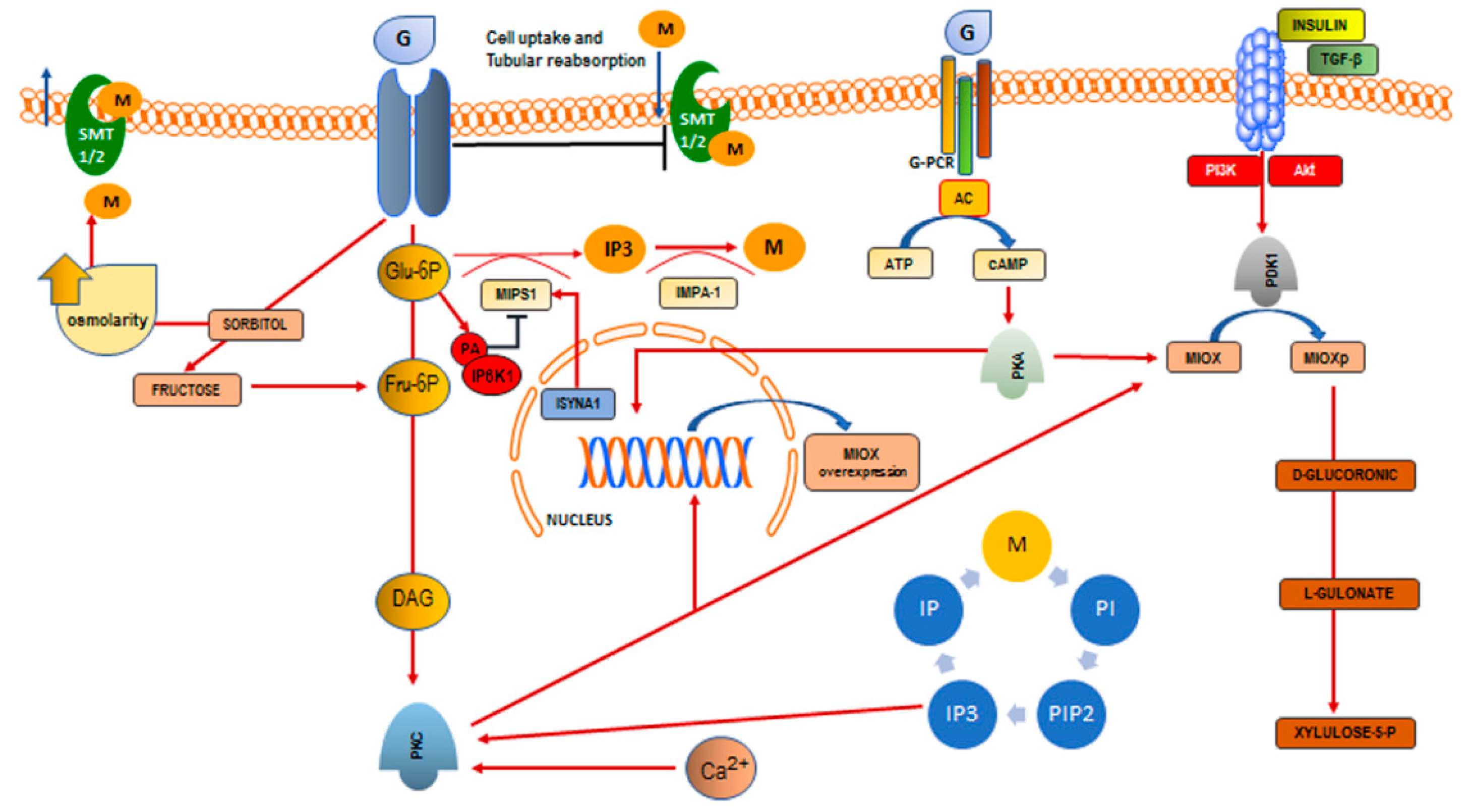
3.1. Reduced Supply from Food Sources
3.2. Impairment of Inositol Biosynthesis
3.3. Reduced Absorption Due to Glucose-Dependent Inhibition of Inositol Uptake
3.4. Increased Renal Excretion and Catabolism
4. Hypothesis for Future Research
Author Contributions
Conflicts of Interest
Abbreviations
| AC | adenylate cyclase |
| AP1 | activator protein 1 |
| ChREBP | carbohydrate responsive element-binding protein (ChREBP) |
| DAG | diacylglycerol |
| d-Chiro-Ins | d-Chiro-Inositol |
| G-6P | glucose-6-phosphate |
| G-PCR | G-protein coupled receptor |
| GSK3 | glycogen synthase kinase 3 |
| HMIT1 | H+/myoinositiol co-transporter |
| IMPA-1/IMPase | inositol monophosphatase-1 |
| INO1 | Inositol-3-phosphate synthase |
| Ins3P/IP3 | inositol-3-phosphate |
| InsP6 | Inositol-hexakisphosphate or phytic acid |
| IP | inositol phosphate |
| IP6K1 | inositol hexakisphosphate kinase |
| IP7 | pyrophosphate |
| ISYNA1 | Inositol-3-Phosphate Synthase 1 |
| Mfn2 | Mitofusin-2 |
| MIOX | myo-inositol oxygenase |
| MIPS1 | myo-inositol-1-phosphate synthase 1 |
| myo-Ins | myo-Inositol |
| NFAT-5 | nuclear factor of activated T-cells 5 |
| Nrf-2 | nuclear factor (erythroid-derived 2)-like 2 |
| PA | phosphatidic acid |
| PCOS | polycystic ovary syndrome |
| PDK1 | pyruvate dehydrogenase kinase 1 |
| PI | phosphatidylinositol |
| Pink 1 | PTEN-induced putative kinase 1 |
| PIP2 | phosphatidyl-inositol-4,5-biphosphate |
| PKA | protein kinase A |
| PKC | protein kinase C |
| SGLT1/2 | sodium glucose transporter |
| SMIT1/2 | sodium/inositol symporter |
References
- De Munter, J.S.; Hu, F.B.; Spiegelman, D.; Franz, M.; van Dam, R.M. Whole grain, bran, and germ intake and risk of type 2 diabetes: A prospective cohort study and systematic review. PLoS Med. 2007, 4, e261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esmaillzadeh, A.; Mirmiran, P.; Azizi, F. Wholegrain consumption and the metabolic syndrome: A favorable association in Tehranian adults. Eur. J. Clin. Nutr. 2005, 59, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Mellen, P.B.; Walsh, T.F.; Herrington, D.M. Whole grain intake and cardiovascular disease: A meta-analysis. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Chatenoud, L.; Tavani, A.; La Vecchia, C.; Jacobs, D.R.; Negri, E.; Levi, F.; Franceschi, S. Whole grain food intake and cancer risk. Int. J. Cancer 1998, 77, 24–28. [Google Scholar] [CrossRef]
- Jacobs, D.R.; Andersen, L.F.; Blomhoff, R. Wholegrain consumption is associated with a reduced risk of noncardiovascular, noncancer death attributed to inflammatory diseases in the Iowa Women’s Health Study. Am. J. Clin. Nutr. 2007, 85, 1606–1614. [Google Scholar] [PubMed]
- Chatenoud, L.; La Vecchia, C.; Franceschi, S.; Tavani, A.; Jacobs, D.R., Jr.; Parpinel, M.T.; Soler, M.; Negri, E. Refined-cereal intake and risk of selected cancers in Italy. Am. J. Clin. Nutr. 1999, 70, 1107–1110. [Google Scholar] [PubMed]
- Fardet, A. New hypotheses for the health-protective mechanisms of whole-grain cereals: What is beyond fibre? Nutr. Res. Rev. 2010, 23, 65–134. [Google Scholar] [CrossRef] [PubMed]
- Csermely, P.; Korcsmáros, T.; Kiss, H.J.; London, G.; Nussinov, R. Structure and dynamics of molecular networks: A novel paradigm of drug discovery A comprehensive review. Pharmacol. Ther. 2013, 138, 333–408. [Google Scholar] [CrossRef] [PubMed]
- Cohen LA: Dietary fiber and breast cancer. Anticancer Res. 1999, 19, 3685–3688.
- Graf, E.; Eaton, J.W. Dietary suppression of colonic cancer: Fiber or phytate? Cancer 1985, 56, 717–718. [Google Scholar] [CrossRef]
- Nakahara, N.; Okazaki, Y.; Katayama, T. Hypertrophic effect of dietary phytate on cecum in rats. Trace Nutr. Res. 2000, 17, 47–52. [Google Scholar]
- Okazaki, Y.; Katayama, T. Dietary phytic acid modulates characteristics of the colonic luminal environment and reduces serum levels of proinflammatory cytokines in rats fed a high-fat diet. Nutr. Res. 2014, 34, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Tantivejkul, K.; Vucenik, I.; Shamsuddin, A.M. Inositol hexaphosphate (IP6) inhibits key events of cancer metastasis: I. In vitro studies of adhesion, migration and invasion of MDA-MB 231 human breast cancer cells. Anticancer Res. 2003, 23, 3671–3679. [Google Scholar] [PubMed]
- Ferguson, L.R.; Harris, P.J. Protection against cancer by wheat bran: Role of dietary fibre and phytochemicals. Eur. J. Cancer Prev. 1999, 8, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Pretlow, T.P.; O’Riordan, M.A.; Somich, G.A.; Amini, S.B.; Pretlow, T.G. Aberrant crypts correlate with tumor incidence in F344 rats treated with azoxymethane and phytate. Carcinogenesis 1992, 13, 1509–1512. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, M.; Dinicola, S.; Bevilacqua, A.; Cucina, A. Broad spectrum anti-cancer activity of myo-inositol and inositol hexakisphosphate. Int. J. Endocrinol. 2016, 2016, 5616807. [Google Scholar] [CrossRef] [PubMed]
- Dinicola, S.; Chiu, T.T.; Unfer, V.; Carlomagno, G.; Bizzarri, M. The rationale of the myo-inositol and d-chiro-inositol combined treatment for polycystic ovary syndrome. J. Clin. Pharmacol. 2014, 54, 1079–1092. [Google Scholar] [CrossRef] [PubMed]
- Facchinetti, F.; Bizzarri, M.; Benvenga, S.; D’Anna, R.; Lanzone, A.; Soulage, C.; Di Renzo, G.C.; Hod, M.; Cavalli, P.; Chiu, T.T.; et al. Results from the international consensus conference on myo-inositol and d-chiro-inositol in obstetrics and gynecology: The link between metabolic syndrome and PCOS. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015, 195, 72–76. [Google Scholar] [CrossRef] [PubMed]
- Tsui, M.M.; York, J.D. Roles of inositol phosphates and inositol pyrophosphates in development, cell signaling and nuclear processes. Adv. Enzyme Regul. 2010, 50, 324–337. [Google Scholar] [CrossRef] [PubMed]
- Chiu, T.T.; Rogers, M.S.; Law, E.L.; Briton-Jones, C.M.; Cheung, L.P.; Haines, C.J. Follicular fluid and serum concentrations of myo-inositol in patients undergoing IVF: Relationship with oocyte quality. Hum. Reprod. 2002, 17, 1591–1596. [Google Scholar] [CrossRef] [PubMed]
- Condorelli, R.A.; La Vignera, S.; Di Bari, F.; Unfer, V.; Calogero, A.E. Effects of myo-inositol on sperm mitochondrial function in-vitro. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 129–134. [Google Scholar] [PubMed]
- Croze, M.L.; Soulage, C.O. Potential role and therapeutic interests of myoinositol in metabolic diseases. Biochimie 2013, 95, 1811–1827. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, A.; Giordano, D.; Corrado, F.; Pintaudi, B.; Interdonato, M.L.; Vieste, G.D.; Benedetto, A.D.; D’Anna, R. One-year effects of myo-inositol supplementation in postmenopausal women with metabolic syndrome. Climacteric 2012, 15, 490–495. [Google Scholar] [CrossRef] [PubMed]
- Colazingari, S.; Fiorenza, M.T.; Carlomagno, G.; Najjar, R.; Bevilacqua, A. Improvement of mouse embryo quality by myo-inositol supplementation of IVF media. J. Assist. Reprod. Genet. 2014, 31, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Greene, N.D.; Copp, A.J. Inositol prevents folate-resistant neural tube defects in the mouse. Nat. Med. 1997, 3, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Forlenza, O.V.; De-Paula, V.J.; Diniz, B.S. Neuroprotective effects of lithium: Implications for the treatment of Alzheimer’s disease and related neurodegenerative disorders. ACS Chem. Neurosci. 2014, 5, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Hallman, M.; Epstein, B.L. Role of myo-inositol in the synthesis of phosphatidylglycerol and phosphatidylinositol in the lung. Bioch. Biophys. Res. Comm. 1980, 92, 1151–1159. [Google Scholar] [CrossRef]
- Bizzarri, M.; Fuso, A.; Dinicola, S.; Cucina, A.; Bevilacqua, A. Pharmacodynamics and pharmacokinetics of inositol(s) in health and disease. Expert. Opin. Drug Metab. Toxicol. 2016, 12, 1181–1196. [Google Scholar] [CrossRef] [PubMed]
- Klein, B.P. Handbook of Vitamins: Nutritional, Biochemical, and Clinical Aspects; Machlin, L.J., Ed.; Marcel Dekker, Inc.: New York, NY, USA, 1984; Volume 74, pp. 1024–1025. [Google Scholar]
- Oberleas, D. Phytates. In Toxicants Occurring Naturally in Foods, 2nd ed.; National Academy of Sciences Eds.: Washington, DC, USA, 1973; pp. 363–371. [Google Scholar]
- Schlemmer, U.; Frølich, W.; Prieto, R.M.; Grases, F. Phytate in foods and significance for humans: Food sources, intake, processing, bioavailability, protective role and analysis. Mol. Nutr. Food Res. 2009, 53 (Suppl. 2), S330–S375. [Google Scholar] [CrossRef] [PubMed]
- Holub, B.J. The nutritional significance, metabolism, and function of myo-inositol and phosphatidylinositol in health and disease. Adv. Nutr. Res. 1982, 4, 107–141. [Google Scholar] [PubMed]
- Eagle, H.; Oyama, V.I.; Levy, M.; Freeman, A. Myo-inositol as an essential growth factor for normal and malignant human cells in tissue culture. Science 1956, 123, 845–847. [Google Scholar] [CrossRef] [PubMed]
- Holub, B.J. Metabolism and function of myo-inositol and inositol phospholipids. Annu. Rev. Nutr. 1986, 6, 563–597. [Google Scholar] [CrossRef] [PubMed]
- Beemster, P.; Groenen, P.; Steegers-Theunissen, R. Involvement of inositol in reproduction. Nutr. Rev. 2002, 60, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Goodhart, R.S. Bioflavonoids. In Modern Nutrition in Health and Disease; Dietotherapy, 5th ed.; Goodhart, R.S., Shils, M.E., Eds.; Lea & Febiger: Philadelphia, PA, USA, 1973; pp. 259–267. [Google Scholar]
- Sandberg, A.S.; Andersson, H. Effect of dietary phytases on the digestion of phytate in the stomach and small intestine of humans. J. Nutr. 1988, 118, 469–473. [Google Scholar] [PubMed]
- Schlemmer, U.; Jany, K.D.; Berk, A.; Schulz, E.; Rechkemmer, G. Degradation of phytate in the gut of pigs—Pathway of gastro-intestinal inositol phosphate hydrolysis and enzymes involved. Arch. Anim. Nutr. 2001, 55, 255–280. [Google Scholar] [CrossRef]
- Wilson, M.S.; Bulley, S.J.; Pisani, F.; Irvine, R.F.; Saiardi, A. A novel method for the purification of inositol phosphates from biological samples reveals that no phytate is present in human plasma or urine. Open Biol. 2015, 5, 150014. [Google Scholar] [CrossRef] [PubMed]
- Vucenik, I. Conundrum of IP6. Open Biol. 2015, 5, 150048. [Google Scholar] [CrossRef] [PubMed]
- Nahapetian, A.; Young, V.R. Metabolism of 14C-phytate in rats: Effect of low and high dietary calcium intakes. J. Nutr. 1980, 110, 1458–1472. [Google Scholar] [PubMed]
- Grases, F.; Simonet, B.M.; March, J.G.; Prieto, R.M. Inositol hexakisphosphate in urine: The relationship between oral intake and urinary excretion. Br. J. Urol. Int. 2000, 85, 138–142. [Google Scholar] [CrossRef]
- Tur, F.; Tur, E.; Lentheric, I.; Mendoza, P.; Encabo, M.; Isern, B.; Grases, F.; Maraschiello, C.; Perelló, J. Validation of an LC–MS bioanalytical method for quantification of phytate levels in rat, dog and human plasma. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2013, 928, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Schlemmer, U.; Jany, K.D. Proceedings of the Society of Nutritional Physiology; 57th conference; Breves, G., Ed.; DLG-Verlag: Frankfurt/Main, Germany, 2003; p. 29. [Google Scholar]
- Grases, F.; Simonet, B.M.; Prieto, R.M.; March, J.G. Variation of InsP(4), InsP(5) and InsP(6) levels in tissues and biological fluids depending on dietary phytate. J. Nutr. Biochem. 2001, 12, 595–601. [Google Scholar] [CrossRef]
- Sakamoto, K.; Vucenik, I.; Shamsuddin, A.M. [3H]-Phytic acid (inositol hexaphosphate) is absorbed and distributed to various tissues in rats. J. Nutr. 1993, 123, 713–720. [Google Scholar] [PubMed]
- Grases, F.; Simonet, B.M.; Vucenik, I.; Prieto, R.M.; Costa-Bauzá, A.; March, J.G.; Shamsuddin, A.M. Absorption and excretion of orally administered inositol hexaphosphate (IP6 or phytate) in humans. Biofactors 2001, 15, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Simonet, B.M.; Prieto, R.M.; March, J.G. Phytate levels in diverse rat tissues: Influence of dietary phytate. Br. J. Nutr. 2001, 86, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Raboy, V. Seeds for a better future: Low phytate grains help to overcome malnutrition and reduce pollution. Trends Plant Sci. 2001, 6, 458–462. [Google Scholar] [CrossRef]
- Harland, B.F.; Morris, E.R. Phytate: A good or a bad food component? Nutr. Res. 1995, 15, 733–754. [Google Scholar] [CrossRef]
- Raboy, V. Response to demonizing phytate. Nat. Biotechnol. 2008, 26, 497–498. [Google Scholar] [CrossRef]
- Shamsuddin, A.M. Demonizing phytate. Nat. Biotechnol. 2008, 26, 496–497. [Google Scholar] [CrossRef] [PubMed]
- Kelsay, J.L. Effects of fiber, phytic acid and oxalic acid in the diet on mineral bioavailability. Am. J. Gastroenterol. 1987, 278, 983–986. [Google Scholar]
- Sandstrom, B.; Bugel, S.; McGaw, B.A.; Price, J.; Reid, M.D. A high oat-bran intake does not impair zinc absorption in humans when added to a low-fiber animal protein-based diet. J. Nutr. 2000, 130, 594–599. [Google Scholar] [PubMed]
- Walker, A.R.; Fox, F.W.; Irving, J.T. Studies in human mineral metabolism: 1. The effect of bread rich in phytate phosphorus on the metabolism of certain mineral salts with special reference to calcium. Biochem. J. 1948, 42, 452–462. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Simonet, B.M.; Vucenik, I.; Perelló, J.; Prieto, R.M.; Shamsuddin, A.M. Effects of exogenous inositol hexakisphosphate (InsP6) on the levels of InsP6 and of inositol trisphosphate (InsP3) in malignant cells, tissues and biological fluids. Life Sci. 2002, 71, 1535–1546. [Google Scholar] [CrossRef]
- Grases, F.; March, J.G.; Prieto, R.M.; Simonet, B.M.; Costa-Bauzá, A.; García-Raja, A.; Conte, A. Urinary phytate in calcium oxalate stone formers and healthy people—Dietary effects on phytate excretion. Scand. J. Urol. Nephrol. 2000, 34, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Curhan, G.C.; Willett, W.C.; Knight, E.L.; Stampfer, M.J. Dietary Factors and the Risk of Incident Kidney Stones in Younger Women. Arch. Intern. Med. 2004, 164, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Grases, F.; Garcia-Gonzalez, R.; Torres, J.J.; Llobera, A. Effects of phytic acid on renal stone formation in rats. Scand. J. Urol. Nephrol. 1998, 32, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Zirwas, M.J.; Molenda, M.A. Dietary Nickel as a Cause of Systemic Contact Dermatitis. J. Clin. Aesthet. Dermatol. 2009, 2, 39–43. [Google Scholar] [PubMed]
- Jabri, E.; Carr, M.B.; Hausinger, R.P.; Karplus, P.A. The Crystall Structure of Urease from Klebsiella aerogenes. Science 1995, 268, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Iemma, F.; Cirillo, G.; Spizzilli, U.G.; Puoci, F.; Parisi, O.I.; Picci, N. Removal of metal ions from aqueous solution by chelating polymeric microspheres bearing phytic acid derivatives. Eur. Polym. J. 2008, 44, 1183–1190. [Google Scholar] [CrossRef]
- Caspary, W.F.; Crane, R.K. Active transport of myo-inositol and its relation to the sugar transport system in hamster small intestine. Biochim. Biophys. Acta 1970, 203, 308–316. [Google Scholar] [CrossRef]
- Carlomagno, G.; de Grazia, S.; Unfer, V.; Manna, F. Myo-inositol in a new pharmaceutical form: A step forward to a broader clinical use. Expert. Opin. Drug Deliv. 2012, 9, 267–271. [Google Scholar] [CrossRef] [PubMed]
- Berridge, M.J.; Downes, C.P.; Hanley, M.R. Neural and developmental actions of lithium: A unifying hypothesis. Cell 1989, 59, 411–419. [Google Scholar] [CrossRef]
- Holub, B.J. The nutritional importance of inositol and the phosphoinositides. N. Engl. J. Med. 1992, 326, 1285–1286. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.H.; Hegsted, D.M. Myo-inositol deficiency in gerbils: Changes in phospholipid composition of intestinal microsomes. J. Nutr. 1980, 110, 1217–1223. [Google Scholar] [PubMed]
- De Grazia, S.; Carlomagno, G.; Unfer, V.; Cavalli, P. Myo-inositol soft gel capsules may prevent the risk of coffee-induced neural tube defects. Expert. Opin. Drug Deliv. 2012, 9, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Khokhar, S.; Fenwick, G.R. Phytate content of Indian foods and intakes by vegetarian Indians of Hisar Region, Haryana State. J. Agric. Food Chem. 1994, 42, 2440–2444. [Google Scholar] [CrossRef]
- Murphy, S.P.; Calloway, D.H.; Beaton, G.H. Schoolchildren have similar predicted prevalences of inadequate intakes as toddlers in village populations in Egypt, Kenya, and Mexico. Eur. J. Clin. Nutr. 1995, 49, 647–657. [Google Scholar] [PubMed]
- Carnovale, E.; Lombardi-Boccia, G.; Lugaro, E. Phytate and zinc content of italian diets. Hum. Nutr. Appl. Nutr. 1987, 41, 180–186. [Google Scholar] [PubMed]
- Ruggeri, S.; De Santis, N.; Carnovale, E. Intake and sources of phytic acid in Itanlian diets. In Bioactive Substances in Food of Plant Origin; Kozlowska, H., Fornal, J., Zdunczyk, Z., Eds.; Centre for Agrotechnology and Veterinary Sciences: Olsztyn, Poland, 1994; pp. 355–359. [Google Scholar]
- Arsenault, J.E.; Brown, K.H. Zinc intake of US preschool children exceeds new dietary reference intakes. Am. J. Clin. Nutr. 2003, 78, 1011–1017. [Google Scholar] [PubMed]
- Gibson, R.S.; Vanderkooy, P.D.S.; Thompson, L. Dietary phytate, calcium zinc millimolar ratios and zinc nutriture in some Ontario preschool-children. Biol. Trace Elem. Res. 1991, 30, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.; Morris, E.R.; Hill, A.D.; Smith, J.C., Jr.; Harland, B.F. Selected Mineral Intakes of Adult African-Americans in the Washington, DC Area. J. Food Compos. Anal. 1997, 10, 334–342. [Google Scholar] [CrossRef]
- Bindra, G.S.; Gibson, R.S.; Thompson, L.U. [Phytate][calcium][zinc] ratios in asian immigrant lacto-ovo vegetarian diets and their relationship to zinc nutriture. Nutr. Res. 1986, 6, 475–483. [Google Scholar] [CrossRef]
- Wong, Y.H.; Kalmbach, S.J.; Hartman, B.K.; Sherman, W.R. Immunohistochemical staining and enzyme activity measurements show myo-inositol-1-phosphate synthase to be localized in the vasculature of brain. J. Neurochem. 1987, 48, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- Guan, G.; Dai, P.; Shechter, I. cDNA cloning and gene expression analysis of human myo-inositol 1-phosphate synthase. Arch. Biochem. Biophys. 2003, 417, 251–259. [Google Scholar] [CrossRef]
- Loewus, M.W.; Loewus, F.A.; Brillinger, G.U.; Otsuka, H.; Floss, H.G. Stereochemistry of the myo-inositol-1-phosphate synthase reaction. J. Biol. Chem. 1980, 255, 11710–11712. [Google Scholar] [PubMed]
- Culbertson, M.R.; Donahue, T.F.; Henry, S.A. Control of inositol biosynthesis in Saccharomyces cerevisiae: Properties of a repressible enzyme system in extracts of wild-type (Ino+) cells. J. Bacteriol. 1976, 126, 232–242. [Google Scholar] [PubMed]
- Murray, M.; Greenberg, M.L. Expression of yeast INM1 encoding inositol monophosphatase is regulated by inositol, carbon source and growth stage and is decreased by lithium and valproate. Mol. Microbiol. 2000, 36, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, J.P.; Henry, S.A. Expression of the Saccharomyces cerevisiae inositol-1-phosphate synthase (INO1) gene is regulated by factors that affect phospholipid synthesis. Mol. Cell Biol. 1986, 6, 3320–3328. [Google Scholar] [CrossRef] [PubMed]
- Ju, S.; Greenberg, M.L. 1d-myo-inositol 3-phosphate synthase: Conservation, regulation, and putative target of mood stabilizers. Clin. Neurosci. Res. 2004, 4, 181–187. [Google Scholar] [CrossRef]
- Seelan, R.S.; Lakshmanan, J.; Casanova, M.F.; Parthasarathy, R.N. Identification of myo-inositol-3-phosphate synthase isoforms: Characterization, expression, and putative role of a 16-kDa γc isoform. J. Biol. Chem. 2009, 284, 9443–9457. [Google Scholar] [CrossRef] [PubMed]
- Seelan, R.S.; Pisano, M.M.; Greene, R.M.; Casanova, M.F.; Parthasarathy, R.N. Differential methylation of the gene encoding myo-inositol 3-phosphate synthase (Isyna1) in rat tissues. Epigenomics 2011, 3, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Loewen, C.J.; Gaspar, M.L.; Jesch, S.A.; Delon, C.; Ktistakis, N.T.; Henry, S.A.; Levine, T.P. Phospholipid metabolism regulated by a transcription factor sensing phosphatidic acid. Science 2004, 304, 1644–1647. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Bandara, W.M.; Greenberg, M.L. Regulation of inositol metabolism is fine-tuned by inositol pyrophosphates in Saccharomyces cerevisiae. J. Biol. Chem. 2013, 288, 24898–24908. [Google Scholar] [CrossRef] [PubMed]
- Draskovic, P.; Saiardi, A.; Bhandari, R.; Burton, A.; Ilc, G.; Kovacevic, M.; Snyder, S.H.; Podobnik, M. Inositol hexakisphosphate kinase products contain diphosphate and triphosphate groups. Chem. Biol. 2008, 15, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Ye, C.; Greenberg, M.L. Inositol Hexakisphosphate Kinase 1 (IP6K1) Regulates Inositol Synthesis in Mammalian Cells. J. Biol. Chem. 2016, 291, 10437–10444. [Google Scholar] [CrossRef] [PubMed]
- Foster, D.A.; Salloum, D.; Menon, D.; Frias, M.A. Phospholipase D and the Maintenance of Phosphatidic Acid Levels for Regulation of Mammalian Target of Rapamycin (mTOR). J. Biol. Chem. 2014, 289, 22583–22588. [Google Scholar] [CrossRef] [PubMed]
- Zoncu, R.; Efeyan, A.; Sabatini, D.M. mTOR: From growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011, 12, 21–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whiting, P.H.; Palmano, K.P.; Hawthorne, J.N. Enzymes of myo-inositol and inositol lipid metabolism in rats with streptozotocin-induced diabetes. Biochem. J. 1979, 179, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, M.; Dinicola, S.; Manetti, C. Systems Biology approach to metabolomics in cancer studies. In Systems Biology in Cancer Research and Drug Discovery; Azmi, A.S., Ed.; Springer: Berlin, Germany, 2012; pp. 3–37. [Google Scholar]
- Yu, W.; Daniel, J.; Mehta, D.; Maddipati, K.R.; Greenberg, M.L. MCK1 is a novel regulator of myo-inositol phosphate synthase (MIPS) that is required for inhibition of inositol synthesis by the mood stabilizer valproate. PLoS ONE 2017, 12, e0182534. [Google Scholar] [CrossRef] [PubMed]
- Azab, A.N.; He, Q.; Ju, S.; Li, G.; Greenberg, M.L. Glycogen synthase kinase-3 is required for optimal de novo synthesis of inositol. Mol. Microbiol. 2007, 63, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, R.; Eisenberg, F., Jr. Selective hormonal control of myoinositol biosynthesis in reproductive organs and liver of the male rat. Proc. Natl. Acad. Sci. USA 1981, 78, 4863–4866. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Gonzalez, R.; Petersen, D.N.; Tkalcevic, G.; Thompson, D.D.; Brown, T.A. Estrogen-induced genes in the uterus of ovariectomized rats and their regulation by droloxifene and tamoxifen. J. Steroid. Biochem. Mol. Biol. 1998, 64, 13–24. [Google Scholar] [CrossRef]
- Yoon, J.H.; Thompson, L.U.; Jenkins, D.J.A. The effect of phytic acid on in vitro rate of starch digestibility and blood glucose response. Am. J. Clin. Nutr. 1983, 38, 835–842. [Google Scholar] [PubMed]
- Thompson, L.U.; Button, C.L.; Jenkins, D.J.A. Phytic acid and calcium affect the in vitro rate of navy bean starch digestion and blood glucose response in humans. Am. J. Clin. Nutr. 1987, 46, 467–473. [Google Scholar] [PubMed]
- Reyes-Moreno, C.; Paredes-López, O. Hard-to-cook phenomenon in common beans—A review. Crit. Rev. Food Sci. Nutr. 1993, 33, 227–286. [Google Scholar] [CrossRef] [PubMed]
- Chukwuma, C.I.; Ibrahim, M.A.; Islam, M.S. Myo-inositol inhibits intestinal glucose absorption and promotes muscle glucose uptake: A dual approach study. J. Physiol. Biochem. 2016, 72, 791–801. [Google Scholar] [CrossRef] [PubMed]
- Croze, M.L.; Géloën, A.; Soulage, C.O. Abnormalities in myo-inositol metabolism associated with type 2 diabetes in mice fed a high-fat diet: Benefits of a dietary myo-inositol supplementation. Br. J. Nutr. 2015, 113, 1862–1875. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S. Inositol transport proteins. FEBS Lett. 2015, 589, 1049–1058. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, F.; Coady, M.J.; Lapointe, J.Y. Determination of transport stoichiometry for two cation-coupled myo-inositol cotransporters: SMIT2 and HMIT. J. Physiol. 2005, 563, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Preston, A.S.; Yamauchi, A.; Kwon, H.M.; Handler, J.S. Activators of protein kinase A and of protein kinase C inhibit MDCK cell myo-inositol and betaine uptake. J. Am. Soc. Nephrol. 1995, 6, 1559–1564. [Google Scholar] [PubMed]
- Kollros, P.E.; Goldstein, G.W.; Betz, A.L. Myo-inositol transport into endothelial cells derived from nervous system microvessels. Brain Res. 1990, 511, 259–264. [Google Scholar] [CrossRef]
- Greene, D.A.; Lattimer, S.A. Sodium- and energy-dependent uptake of myo-inositol by rabbit peripheral nerve. Competitive inhibition by glucose and lack of an insulin effect. J. Clin. Investig. 1982, 70, 1009–1018. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.P.; Feldman, E.L.; Nakamura, J.; Kato, K.; Lien, M.; Stevens, M.J.; Greene, D.A. Ambient glucose and aldose reductase-induced myo-inositol depletion modulate basal and carbachol-stimulated inositol phospholipid metabolism and diacylglycerol accumulation in human retinal pigment epithelial cell culture. Proc. Natl. Acad. Sci. USA 1993, 90, 9712–9716. [Google Scholar] [CrossRef] [PubMed]
- Cammarata, P.R.; Chen, H.Q.; Yang, J.; Yorio, T. Modulation of myo-[3H]inositol uptake by glucose and sorbitol in cultured bovine lens epithelial cells. II. Characterization of high- and low-affinity myo-inositol transport sites. Ophthalmol. Vis. Sci. 1992, 33, 3572–3580. [Google Scholar]
- Greene, D.A.; Lattimer, S.A.; Sima, A.A. Sorbitol, phosphoinositides, and sodium-potassium-ATPase in the pathogenesis of diabetic complications. N. Engl. J. Med. 1987, 316, 599–606. [Google Scholar] [CrossRef] [PubMed]
- Haneda, M.; Kikkawa, R.; Arimura, T.; Ebata, K.; Togawa, M.; Maeda, S.; Sawada, T.; Horide, N.; Shigeta, Y. Glucose inhibits myo-inositol uptake and reduces myo-inositol content in cultured rat glomerular mesangial cells. Metabolism 1990, 39, 40–45. [Google Scholar] [CrossRef]
- Nishimura, C.; Lou, M.F.; Kinoshita, J.H. Depletion of myo-inositol and amino acids in galactosemic neuropathy. J. Neurochem. 1987, 49, 290–295. [Google Scholar] [CrossRef] [PubMed]
- Tomlinson, D.R.; Mayer, J.H. Reversal of deficits in axonal transport and nerve conduction velocity by treatment of streptozotocin diabetic rats with myo-lnositol. Exp. Neurol. 1985, 89, 420–427. [Google Scholar] [CrossRef]
- Mayer, J.H.; Tomlinson, D.R. The influence of aldose reductase inhibition and nerve myo-inositol on axonal transport and nerve conduction velocity in rats with experimental diabetes. J. Physiol. (Lond) 1983, 340, 25–26. [Google Scholar]
- Kuruvilla, R.; Eichberg, J. Depletion of phospholipid arachidonoyl-containing molecular species in a human Schwann cell line grown in elevated glucose and their restoration by an aldose reductase inhibitor. J. Neurochem. 1998, 71, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Inoguchi, T.; Kern, T.S.; Engerman, R.L.; Oates, P.J.; King, G.L. Characterization of the mechanism for the chronic activation of diacylglycerol-protein kinase C pathway in diabetes and hypergalactosemia. Diabetes 1994, 43, 1122–1129. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.P.; Porcellati, F.; Kato, K.; Stevens, M.J.; Sherman, W.R.; Greene, D.A. Effects of glucose on sorbitol pathway activation, cellular redox, and metabolism of myo-inositol, phosphoinositide, and diacylglycerol in cultured human retinal pigment epithelial cells. J. Clin. Investig. 1994, 93, 2718–2724. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.A.; Lattimer, S.A.; Sima, A.A. Are disturbances of sorbitol, phosphoinositide, and Na+-K+-ATPase regulation involved in pathogenesis of diabetic neuropathy? Diabetes 1988, 37, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Winegrad, A.I. Banting lecture 1986. Does a common mechanism induce the diverse complications of diabetes? Diabetes 1987, 36, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Asplin, I.; Galasko, G.; Larner, J. Chiro-inositol deficiency and insulin resistance: A comparison of the chiro-inositol- and the myo-inositolcontaining insulin mediators isolated from urine, hemodialysate, and muscle of control and type II diabetic subjects. Proc. Natl. Acad. Sci. USA 1993, 90, 5924–5928. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.W.; Faulds, D.; Goa, K.L. Epalrestat. A review of its pharmacology, and therapeutic potential in late-onset complications of diabetes mellitus. Drugs Aging 1993, 3, 532–555. [Google Scholar] [CrossRef] [PubMed]
- Hamada, Y.; Araki, N.; Koh, N.; Nakamura, J.; Horiuchi, S.; Hotta, N. Rapid formation of advanced glycation end products by intermediate metabolites of glycolytic pathway and polyol pathway. Biochem. Biophys. Res. Commun. 1996, 228, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Croze, M.L.; Vella, R.E.; Pillon, N.J.; Soula, H.A.; Hadji, L.; Guichardant, M.; Soulage, C.O. Chronic treatment with myo-inositol reduces white adipose tissue accretion and improves insulin sensitivity in female mice. J. Nutr. Biochem. 2013, 24, 457–466. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, M.; Dinicola, S.; Cucina, A. Modulation of both Insulin Resistance and Cancer Growth by Inositol. Curr. Pharm. Des. 2017, 23. [Google Scholar] [CrossRef]
- Yang, Q.; Dixit, B.; Wada, J.; Tian, Y.; Wallner, E.I.; Srivastva, S.K.; Kanwar, Y.S. Identification of a renal-specific oxido-reductase in newborn diabetic mice. Proc. Natl. Acad. Sci. USA 2000, 97, 9896–9901. [Google Scholar] [CrossRef] [PubMed]
- Hankes, L.V.; Politzer, W.M.; Touster, O.; Anderson, L. Myo-inositol catabolism in human pentosurics: The predominant role of the glucuronate-xylulosepentose phosphate pathway. Ann. N. Y. Acad. Sci. 1969, 165, 564–576. [Google Scholar] [PubMed]
- Pitkänen, E. Changes in serum and urinary myo-inositol levels in chronic glomerulonephritis. Clin. Chim. Acta 1976, 71, 461–468. [Google Scholar] [CrossRef]
- Kawa, J.M.; Przybylski, R.; Taylor, C.G. Urinary chiro-inositol and myoinositol excretion is elevated in the diabetic db/db mouse and streptozotocin diabetic rat. Exp. Biol. Med. 2003, 228, 907–914. [Google Scholar] [CrossRef]
- Suzuki, S.; Taneda, Y.; Hirai, S.; Abe, S.; Saski, A.; Suzuki, K.; Toyota, T. Molecular mechanism of insulin resistance in spontaneous diabetic GK (Goto- Kakizaki) rats. In New Directions in Research and Clinical Works for Obesity and Diabetes Mellitus; Saskamoto, N., Angel, A., Hotta, N., Eds.; Excerpta Medica: New York, NY, USA, 1991; pp. 197–203. [Google Scholar]
- Portha, B.; Serradas, P.; Bailbé, D.; Suzuki, K.-I.; Goto, Y.; Giroix, M.-H. Beta-cell insensitivity to glucose in the GK rat, a spontaneous nonobese model for type II diabetes. Diabetes 1991, 40, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Kunjara, S.; Wang, D.Y.; Greenbaum, A.L.; McLean, P.; Kurtz, A.; Rademacher, T.W. Inositol phosphoglycans in diabetes and obesity: Urinary levels of IPG A-Type and IPG-P Type, and relationship to pathophysiological change. Mol. Genet. Metab. 1999, 68, 488–502. [Google Scholar] [CrossRef] [PubMed]
- Shaskin, P.N.; Shashkina, E.F.; Fernqvist-Forbes, E.; Zhou, Y.-P.; Grill, V.; Katz, A. Insulin mediators in man: Effects of glucose ingestion and insulin resistance. Diabetologia 1997, 40, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Ostlund, R.E., Jr.; McGill, J.B.; Herskowitz, I.; Kipnis, D.M.; Santiago, J.V.; Sherman, W. D-chiro-inositol metabolism in diabetes mellitus. Proc. Natl. Acad. Sci. USA 1993, 90, 9988–9992. [Google Scholar] [CrossRef] [PubMed]
- Kennington, A.S.; Hill, C.R.; Craig, J.; Bogardus, C.; Raz, I.; Ortmeyer, H.K.; Hansen, B.C.; Romero, G.; Larner, J. Low urinary chiro-inositol excretion in non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 1990, 323, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Kawasaki, H.; Satoh, Y.; Ohtomo, M.; Hirai, M.; Hirai, A.; Hirai, S.; Onoda, M.; Matsumoto, M.; Hinokio, Y.; et al. Urinary chiro-inositol excretion is an index marker of insulin sensitivity in Japanese Type II Diabetes. Diabetes Care 1994, 17, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Lahjouji, K.; Aouameur, R.; Bissonnette, P.; Coady, M.J.; Bichet, D.G.; Lapointe, J.Y. Expression and functionality of the Na+/myo-inositol cotransporter SMIT2 in rabbit kidney. Biochim. Biophys. Acta 2007, 1768, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Choong, B.; Phillips, A.R.; Loomes, K.M. The diabetic rat kidney mediates inosituria and selective urinary partitioning of d-chiroinositol. Exp. Biol. Med. 2015, 240, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Raccah, D.; Coste, T.; Cameron, N.; Dufayet, D.; Vague, P.; Hohman, T. Effect of the aldose reductase inhibitor tolrestat on nerve conduction velocity, Na/K ATPase activity, and polyols in red blood cells, sciatic nerve, kidney cortex, and kidney medulla of diabetic rats. J. Diabetes Compl. 1998, 12, 154–162. [Google Scholar] [CrossRef]
- Chang, H.H.; Chao, H.N.; Walker, C.S.; Choong, S.Y.; Phillips, A.; Loomes, K.M. Renal depletion of myo-inositol is associated with its increased degradation in animal models of metabolic disease. Am. J. Physiol. Renal. Physiol. 2015, 309, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Xie, P.; Akagi, S.; Yang, Q.; Sun, L.; Wada, J.; Thakur, A.; Danesh, F.R.; Chugh, S.S.; Kanwar, Y.S. Modulation of renal-specific oxidoreductase/ myo-inositol oxygenase by high-glucose ambience. Proc. Natl. Acad. Sci. USA 2005, 102, 17952–17957. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, K.S.; Arner, R.J.; Vunta, H.; Reddy, C.C. Up-regulation of human myo-inositol oxygenase by hyperosmotic stress in renal proximal tubular epithelial cells. J. Biol. Chem. 2005, 280, 19895–19901. [Google Scholar] [CrossRef] [PubMed]
- Vallon, V.; Thomson, S. Renal function in diabetic disease models: The tubular system in the pathophysiology of the diabetic kidney. Annu. Rev. Physiol. 2012, 74, 351–375. [Google Scholar] [CrossRef] [PubMed]
- Nayak, B.; Kondeti, V.K.; Xie, P.; Lin, S.; Viswakarma, N.; Raparia, K.; Kanwar, Y.S. Transcriptional and post-translational modulation of myo-inositol oxygenase by high glucose and related pathobiological stresses. J. Biol. Chem. 2011, 286, 27594–27611. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, N.; Obayashi, H.; Fujii, M.; Fukui, M.; Yoshimori, K.; Ogata, M.; Hasegawa, G.; Shigeta, H.; Kitagawa, Y.; Yoshikawa, T.; et al. Induction of aldose reductase in cultured human microvascular endothelial cells by advanced glycation end products. Free Radic. Biol. Med. 2000, 29, 17–25. [Google Scholar] [CrossRef]
- Zhan, M.; Usman, I.M.; Sun, L.; Kanwar, Y.S. Disruption of renal tubular mitochondrial quality control by myo-inositol oxygenase in diabetic kidney disease. J. Am. Soc. Nephrol. 2015, 26, 1304–1321. [Google Scholar] [CrossRef] [PubMed]
- Pothiwala, P.; Jain, S.K.; Yaturu, S. Metabolic syndrome and cancer. Metab. Syndr. Relat. Disord. 2009, 7, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Burkitt, D.P. Epidemiology of cancer of the colon and rectum. Cancer 1971, 28, 3–13. [Google Scholar] [CrossRef]
- Stemmermann, G.N. Patterns of disease among Japanese living in Hawaii. Arch. Environ. Health 1970, 20, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Deja, S.; Dawiskiba, T.; Balcerzak, W.; Orczyk-Pawiłowicz, M.; Głód, M.; Pawełka, D.; Młynarz, P. Follicular adenomas exhibit a unique metabolic profile. ¹H NMR studies of thyroid lesions. PLoS ONE 2013, 8, e84637. [Google Scholar] [CrossRef] [PubMed]
- Beckonert, O.; Monnerjahn, J.; Bonk, U.; Leibfritz, D. Visualizing metabolic changes in breast-cancer tissue using 1H-NMR spectroscopy and self-organizing maps. NMR Biomed. 2003, 16, 1–11. [Google Scholar] [CrossRef] [PubMed]
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinicola, S.; Minini, M.; Unfer, V.; Verna, R.; Cucina, A.; Bizzarri, M. Nutritional and Acquired Deficiencies in Inositol Bioavailability. Correlations with Metabolic Disorders. Int. J. Mol. Sci. 2017, 18, 2187. https://doi.org/10.3390/ijms18102187
Dinicola S, Minini M, Unfer V, Verna R, Cucina A, Bizzarri M. Nutritional and Acquired Deficiencies in Inositol Bioavailability. Correlations with Metabolic Disorders. International Journal of Molecular Sciences. 2017; 18(10):2187. https://doi.org/10.3390/ijms18102187
Chicago/Turabian StyleDinicola, Simona, Mirko Minini, Vittorio Unfer, Roberto Verna, Alessandra Cucina, and Mariano Bizzarri. 2017. "Nutritional and Acquired Deficiencies in Inositol Bioavailability. Correlations with Metabolic Disorders" International Journal of Molecular Sciences 18, no. 10: 2187. https://doi.org/10.3390/ijms18102187





