PARP1 in Carcinomas and PARP1 Inhibitors as Antineoplastic Drugs
Abstract
:1. Introduction
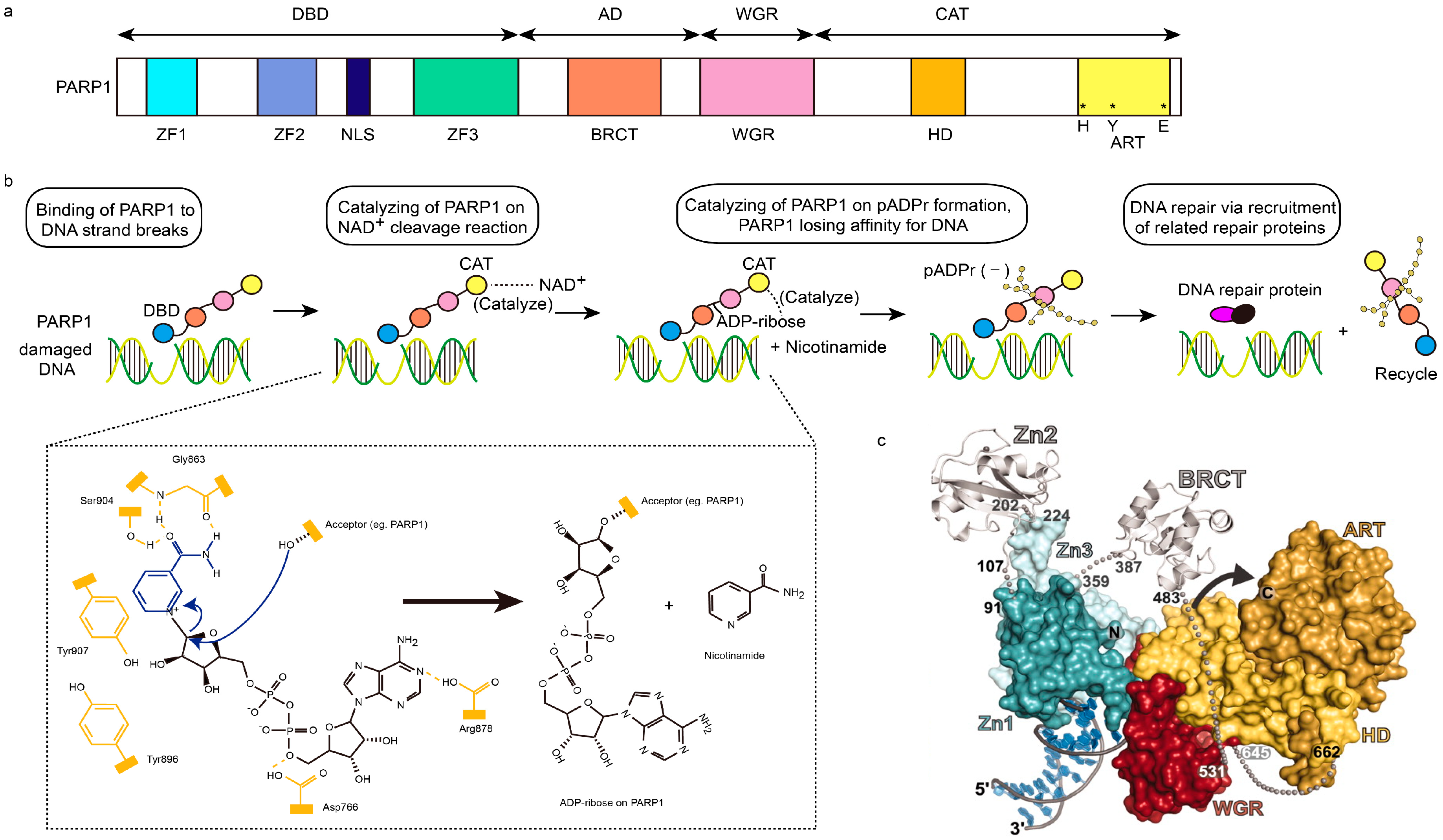
2. PARP1 Structure and Expression in Carcinomas
3. PARP1 Function and Mechanism in Carcinomas
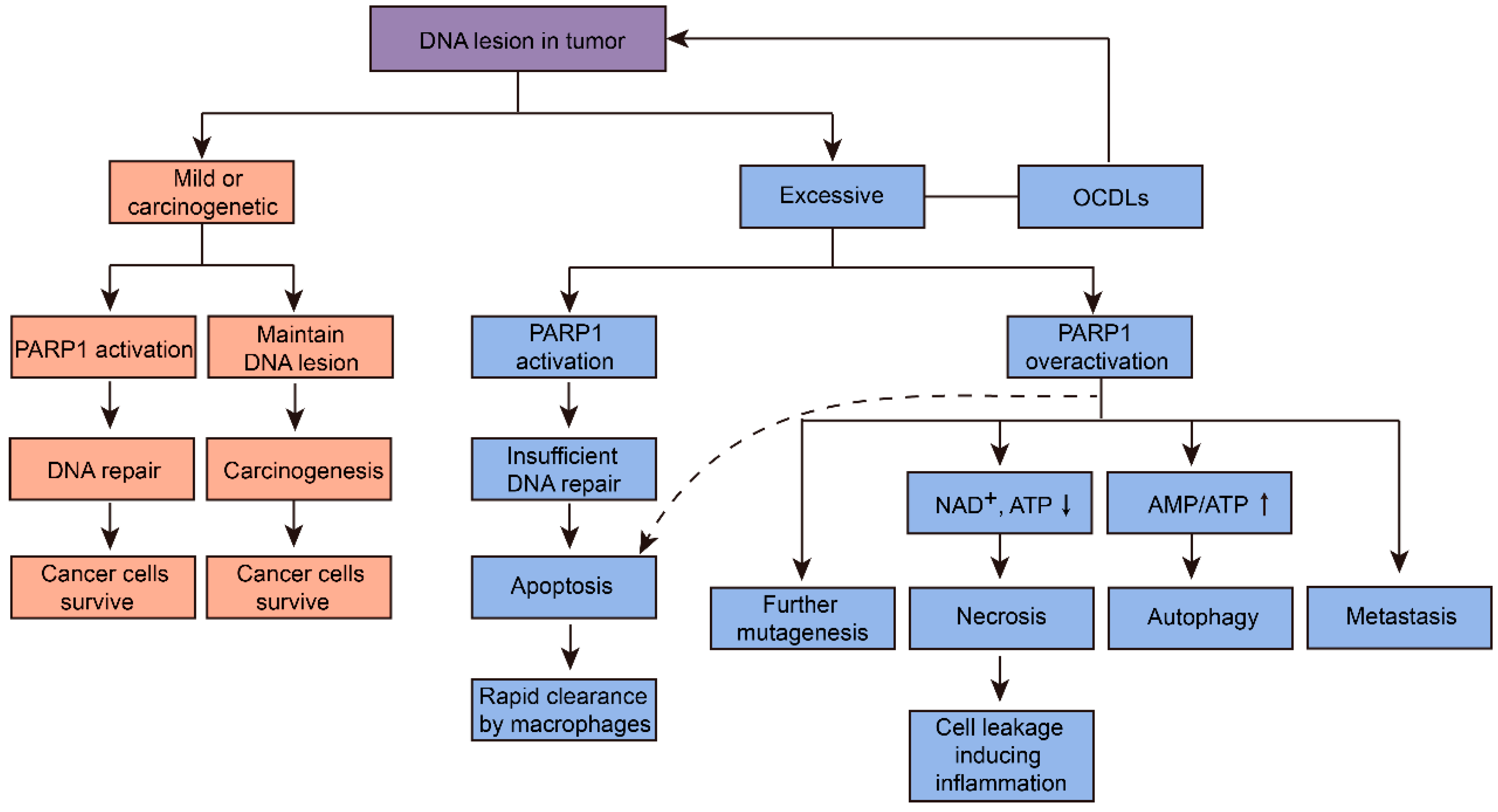
3.1. Multifaceted Function on DNA Repair
3.2. PARP1 in Gene Transcription
3.3. PARP1 in Tumor-Promoting Inflammation
3.4. PARP1 in Cell Cycling Regulation
3.5. PARP1 in Metastasis and Angiogenesis
4. PARP1 Inhibitors against Carcinomas
5. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kaufman, B.; Shapira-Frommer, R.; Schmutzler, R.K.; Audeh, M.W.; Friedlander, M.; Balmana, J.; Mitchell, G.; Fried, G.; Stemmer, S.M.; Hubert, A.; et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol 2015, 33, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Solimando, D.A., Jr.; Waddell, J.A. Drug Monographs: Olaratumab and Rucaparib. Hosp. Pharm. 2017, 52, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Kummar, S.; Chen, A.; Ji, J.; Zhang, Y.; Reid, J.M.; Ames, M.; Jia, L.; Weil, M.; Speranza, G.; Murgo, A.J.; et al. Phase I study of PARP inhibitor ABT-888 in combination with topotecan in adults with refractory solid tumors and lymphomas. Cancer Res. 2011, 71, 5626–5634. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, P.; Szabó, C. Poly(ADP-ribose) polymerase and the therapeutic effects of its inhibitors. Nat. Rev. Drug Discov. 2005, 4, 421–440. [Google Scholar] [CrossRef] [PubMed]
- Weaver, A.N.; Yang, E.S. Beyond DNA Repair: Additional Functions of PARP-1 in Cancer. Front. Oncol. 2013, 3, 290. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, L.A. Advances in Prostate Cancer. In The Role of PARP Activation in Prostate Cancer; Hamilton, G., Ed.; InTech: Lexington, KY, USA, 2013. [Google Scholar]
- Wielgos, M.; Rajbhandari, R.; Cooper, T.S.; Wei, S.; Nozell, S.; Yang, E.S. Let-7 Status is crucial for PARP1 expression in HER2-over-expressing breast tumors. Mol. Cancer Res. 2017, 15, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.S.; Lindahl, T. Role of poly(ADP-ribose) formation in DNA repair. Nature 1992, 356, 356–358. [Google Scholar] [CrossRef] [PubMed]
- Beck, C.; Robert, I.; Reina-San-Martin, B.; Schreiber, V.; Dantzer, F. Poly(ADP-ribose) polymerases in double-strand break repair: focus on PARP1, PARP2 and PARP3. Exp. Cell Res. 2014, 329, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Bryant, H.E.; Schultz, N.; Thomas, H.D.; Parker, K.M.; Flower, D.; Lopez, E.; Kyle, S.; Meuth, M.; Curtin, N.J.; Helleday, T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005, 434, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Farmer, H.; McCabe, N.; Lord, C.J.; Tutt, A.N.J.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Ashworth, A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J. Clin. Oncol. 2008, 26, 3785–3790. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.A. PARP inhibitors in ovarian cancer. Ann. Oncol. 2016, 27, i40–i44. [Google Scholar] [CrossRef] [PubMed]
- Swindall, A.F.; Stanley, J.A.; Yang, E.S. PARP-1: Friend or Foe of DNA Damage and Repair in Tumorigenesis? Cancers 2013, 5, 943–958. [Google Scholar] [CrossRef] [PubMed]
- Brenner, J.C.; Feng, F.Y.; Han, S.; Patel, S.; Goyal, S.V.; Bou-Maroun, L.M.; Liu, M.; Lonigro, R.; Prensner, J.R.; Tomlins, S.A.; et al. PARP-1 inhibition as a targeted strategy to treat Ewing’s sarcoma. Cancer Res. 2012, 72, 1608–1613. [Google Scholar] [CrossRef] [PubMed]
- Nowsheen, S.; Cooper, T.; Bonner, J.A.; LoBuglio, A.F.; Yang, E.S. HER2 overexpression renders human breast cancers sensitive to PARP inhibition independently of any defect in homologous recombination DNA repair. Cancer Res. 2012, 72, 4796–4806. [Google Scholar] [CrossRef] [PubMed]
- Malyuchenko, N.V.; Kotova, E.Y.; Kulaeva, O.I.; Kirpichnikov, M.P.; Studitskiy, V.M. PARP1 Inhibitors: antitumor drug design. Acta Naturae. 2015, 7, 27–37. [Google Scholar] [PubMed]
- Rouleau, M.; Patel, A.; Hendzel, M.J.; Kaufmann, S.H.; Poirier, G.G. PARP inhibition: PARP1 and beyond. Nat. Rev. Cancer 2010, 10, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Langelier, M.F.; Planck, J.L.; Roy, S.; Pascal, J.M. Structural basis for DNA damage-dependent poly(ADP-ribosyl)ation by human PARP-1. Science 2012, 336, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.A.E.; Timinszky, G.; Arribas-Bosacoma, R.; Kozlowski, M.; Hassa, P.O.; Hassler, M.; Ladurner, A.G.; Pearl, L.H.; Oliver, A.W. The zinc-finger domains of PARP1 cooperate to recognize DNA strand breaks. Nat. Struct. Mol. Biol. 2012, 19, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Gao, P.; Hoffman, D.W.; Liu, H.-W. Domain C of Human Poly(ADP-ribose) Polymerase-1 Is Important for Enzyme Activity and Contains a Novel Zinc-Ribbon Motif. Biochemistry 2008, 47, 5804–5813. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, P.A.; Cuneo, M.J.; Mueller, G.A.; DeRose, E.F.; Gabel, S.A.; London, R.E. Structural studies of the PARP-1 BRCT domain. BMC Struct. Biol. 2011, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Ray Chaudhuri, A.; Nussenzweig, A. The multifaceted roles of PARP1 in DNA repair and chromatin remodelling. Nat. Rev. Mol. Cell Biol. 2017, 18, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Eustermann, S.; Wu, W.-F.; Langelier, M.-F.; Yang, J.-C.; Easton, L.E.; Riccio, A.A.; Pascal, J.M.; Neuhaus, D. Structural Basis of Detection and Signaling of DNA Single-Strand Breaks by Human PARP-1. Mol. Cell 2015, 60, 742–754. [Google Scholar] [CrossRef] [PubMed]
- Ossovskaya, V.; Koo, I.C.; Kaldjian, E.P.; Alvares, C.; Sherman, B.M. Upregulation of Poly (ADP-Ribose) Polymerase-1 (PARP1) in Triple-Negative Breast Cancer and Other Primary Human Tumor Types. Genes Cancer 2010, 1, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Domagala, P.; Huzarski, T.; Lubinski, J.; Gugala, K.; Domagala, W. PARP-1 expression in breast cancer including BRCA1-associated, triple negative and basal-like tumors: possible implications for PARP-1 inhibitor therapy. Breast Cancer Res. Treat 2011, 127, 861–869. [Google Scholar] [CrossRef] [PubMed]
- Donizy, P.; Pietrzyk, G.; Halon, A.; Kozyra, C.; Gansukh, T.; Lage, H.; Surowiak, P.; Matkowski, R. Nuclear-cytoplasmic PARP-1 expression as an unfavorable prognostic marker in lymph nodenegative early breast cancer: 15-year follow-up. Oncol. Rep. 2014, 31, 1777–1787. [Google Scholar] [PubMed]
- Mazzotta, A.; Partipilo, G.; De Summa, S.; Giotta, F.; Simone, G.; Mangia, A. Nuclear PARP1 expression and its prognostic significance in breast cancer patients. Tumor Biol. 2016, 37, 6143–6153. [Google Scholar] [CrossRef] [PubMed]
- Byers, L.A.; Wang, J.; Nilsson, M.B.; Fujimoto, J.; Saintigny, P.; Yordy, J.; Giri, U.; Peyton, M.; Fan, Y.H.; Diao, L.; et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov. 2012, 2, 798–811. [Google Scholar] [CrossRef] [PubMed]
- Galia, A.; Calogero, A.E.; Condorelli, R.; Fraggetta, F.; La Corte, A.; Ridolfo, F.; Bosco, P.; Castiglione, R.; Salemi, M. PARP-1 protein expression in glioblastoma multiforme. Eur. J. Histochem. 2012, 56, e9. [Google Scholar] [CrossRef] [PubMed]
- Schiewer, M.J.; Goodwin, J.F.; Han, S.; Brenner, J.C.; Augello, M.A.; Dean, J.L.; Liu, F.; Planck, J.L.; Ravindranathan, P.; Chinnaiyan, A.M.; et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012, 2, 1134–1149. [Google Scholar] [CrossRef] [PubMed]
- Nosho, K.; Yamamoto, H.; Mikami, M.; Taniguchi, H.; Takahashi, T.; Adachi, Y.; Imamura, A.; Imai, K.; Shinomura, Y. Overexpression of poly(ADP-ribose) polymerase-1 (PARP-1) in the early stage of colorectal carcinogenesis. Eur. J. Cancer 2006, 42, 2374–2381. [Google Scholar] [CrossRef] [PubMed]
- Barton, V.N.; Donson, A.M.; Kleinschmidt-DeMasters, B.K.; Gore, L.; Liu, A.K.; Foreman, N.K. PARP1 Expression in Pediatric Central Nervous System Tumors. Pediatric. Blood Cancer 2009, 53, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Mego, M.; Cierna, Z.; Svetlovska, D.; Macak, D.; Machalekova, K.; Miskovska, V.; Chovanec, M.; Usakova, V.; Obertova, J.; Babal, P.; Mardiak, J. PARP expression in germ cell tumours. J Clin Pathol 2013, 66, 607–612. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Yu, X. Functions of PARylation in DNA Damage Repair Pathways. Genomics Proteomics Bioinformatics 2016, 14, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Gibson, B.A.; Zhang, Y.; Jiang, H.; Hussey, K.M.; Shrimp, J.H.; Lin, H.; Schwede, F.; Yu, Y.; Kraus, W.L. Chemical genetic discovery of PARP targets reveals a role for PARP-1 in transcription elongation. Science 2016, 353, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Kraus, W.L. Transcriptional control by PARP-1: chromatin modulation, enhancer-binding, coregulation, and insulation. Curr. Opin. Cell Biol. 2008, 20, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Quiles-Perez, R.; Muñoz-Gámez, J.A.; Ruiz-Extremera, A.; O’Valle, F.; Sanjuán-Nuñez, A.; Martín-Álvarez, A.B.; Martín-Oliva, D.; Caballero, T.; Muñoz de Rueda, P.; León, J.; et al. Inhibition of poly adenosine diphosphate-ribose polymerase decreases hepatocellular carcinoma growth by modulation of tumor-related gene expression. Hepatology 2010, 51, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Martin-Oliva, D.; Aguilar-Quesada, R.; O’Valle, F.; Muñoz-Gámez, F.A.; Martínez-Romero, R.; García del Moral, R.; Ruiz de Almodóvar, J.M.; Villuendas, R.; Piris, M.A.; Oliver, F.J. Inhibition of poly(ADP-ribose) polymerase modulates tumor-related gene expression, including hypoxia-inducible factor-1 activation, during skin carcinogenesis. Cancer Res. 2006, 66, 5744–5756. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Xu, M.; Makowski, M.M.; Zhang, T.; Law, M.H.; Kovacs, M.A.; Granzhan, A.; Kim, W.J.; Parikh, H.; Gartside, M.; et al. A common intronic variant of PARP1 confers melanoma risk and mediates melanocyte growth via regulation of MITF. Nature Genetics 2017, 49, 1326–1335. [Google Scholar] [CrossRef] [PubMed]
- Nirodi, C.; NagDas, S.; Gygi, S.P.; Olson, G.; Aebersold, R.; Richmond, A. A role for poly(ADP-ribose) polymerase in the transcriptional regulation of the melanoma growth stimulatory activity (CXCL1) gene expression. J. Biol. Chem. 2001, 276, 9366–9374. [Google Scholar] [CrossRef] [PubMed]
- Schiewer, M.J.; Knudsen, K.E. Transcriptional roles of PARP1 in cancer. Mol Cancer Res. 2014, 12, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- Wieler, S.; Gagné, J.-P.; Vaziri, H.; Poirier, G.G.; Benchimol, S. Poly(ADP-ribose) polymerase-1 is a positive regulator of the p53-mediated G1 arrest response following ionizing radiation. J. Biol. Chem. 2003, 278, 18914–18921. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.Y.; de Bono, J.S.; Rubin, M.A.; Knudsen, K.E. Chromatin to Clinic: The Molecular Rationale for PARP1 Inhibitor Function. Mol. Cell 2015, 58, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Tulin, A.; Spradling, A. Chromatin Loosening by Poly(ADP)-Ribose Polymerase (PARP) at Drosophila Puff Loci. Science reports 2003, 299, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Bouchard, V.J.; Rouleau, M.; Poirier, G.G. PARP-1, a determinant of cell survival in response to DNA damage. Exp. Hematol. 2003, 31, 446–454. [Google Scholar] [CrossRef]
- D’Amours, D.; Desnoyers, S.; D’Silva, I.; Poirier, G.G. Poly(ADP-ribosyl)ation reactions in the regulation of nuclear functions. Biochem. J. 1999, 342, 249–268. [Google Scholar] [CrossRef] [PubMed]
- Kraus, W.L.; Hottiger, M.O. PARP-1 and gene regulation: progress and puzzles. Mol. Aspects Med. 2013, 34, 1109–1123. [Google Scholar] [CrossRef] [PubMed]
- Frizzell, K.M.; Gamble, M.J.; Berrocal, J.G.; Zhang, T.; Krishnakumar, R.; Cen, Y.; Sauve, A.A.; Kraus, W.L. Global analysis of transcriptional regulation by poly(ADP-ribose) polymerase-1 and poly(ADP-ribose) glycohydrolase in MCF-7 human breast cancer cells. J. Biol. Chem. 2009, 284, 33926–33938. [Google Scholar] [CrossRef] [PubMed]
- Reale, A.; De Matteis, G.; Galleazzi, G.; Zampieri, M.; Caiafa, P. Modulation of DNMT1 activity by ADP-ribose polymers. Oncogene 2005, 24, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Hoesel, B.; Schmid, J.A. The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 2013, 12, 86. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M.; Rhinn, H.; Bloquel, C.; Coqueran, B.; Szabó, C.; Plotkine, M.; Scherman, D.; Margaill, I. Anti-inflammatory effects of PJ34, a poly(ADP-ribose) polymerase inhibitor, in transient focal cerebral ischemia in mice. Br. J. Pharmacol. 2006, 149, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Smyth, M.J.; Dunn, G.P.; Schreiber, R.D. Cancer Immunosurveillance and Immunoediting: The Roles of Immunity in Suppressing Tumor Development and Shaping Tumor Immunogenicity. Adv. Immunol. 2006, 90, 1–50. [Google Scholar] [PubMed]
- Santini, D.; Perrone, G.; Roato, I.; Godio, L.; Pantano, F.; Grasso, D.; Russo, A.; Vincenzi, B.; Fratto, M.E.; Sabbatini, R.; et al. Expression pattern of receptor activator of NFkappaB (RANK) in a series of primary solid tumors and related bone metastases. J. Cell Physiol. 2011, 226, 780–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Luo, J.L.; Karin, M. IκB kinase α kinase activity is required for self-renewal of ErbB2/Her2-transformed mammary tumor-initiating cells. Proc. Natl. Acad. Sci. USA 2007, 104, 15852–15857. [Google Scholar] [CrossRef] [PubMed]
- Dalaklioglu, S.; Sahin, P.; Ordueri, E.G.; Celik-Ozenci, C.; Tasatargil, A. Potential role of poly(ADP-ribose) polymerase (PARP) activation in methotrexate-induced nephrotoxicity and tubular apoptosis. Int. J. Toxicol. 2012, 31, 430–440. [Google Scholar] [CrossRef] [PubMed]
- Chang, P.; Jacobson, M.K.; Mitchison, T.J. Poly(ADP-ribose) is required for spindle assembly and structure. Nature 2004, 432, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Boucher, M.-J.; Morisset, J.; Vachon, P.H.; Reed, J.C.; Lainé, J.; Rivard, N. MEK/ERK Signaling Pathway Regulates the Expression of Bcl-2, Bcl-XL, and Mcl-1 and Promotes Survival of Human Pancreatic Cancer Cells. J. Cell. Biochem. 2000, 79, 355–369. [Google Scholar] [CrossRef]
- Zhang, S.; Lin, Y.; Kim, Y.S.; Hande, M.P.; Liu, Z.G.; Shen, H.M. c-Jun N-terminal kinase mediates hydrogen peroxide-induced cell death via sustained poly(ADP-ribose) polymerase-1 activation. Cell. Death. Differ. 2007, 14, 1001. [Google Scholar] [CrossRef] [PubMed]
- Simbulan-Rosenthal, C.M.; Ly, D.H.; Rosenthal, D.S.; Konopka, G.; Luo, R.; Wang, Z.Q.; Schultz, P.G.; Smulson, M.E. Misregulation of gene expression in primary fibroblasts lacking poly(ADP-ribose) polymerase. Proc. Natl. Acad. Sci. USA 2000, 97, 11274–11279. [Google Scholar] [CrossRef] [PubMed]
- Lacal, P.M.; Tentori, L.; Muzi, A.; Ruffini, F.; Dorio, A.S.; Xu, W.; Arcelli, D.; Zhang, J.; Graziani, G. Pharmacological inhibition of poly(ADP-ribose) polymerase activity down-regulates the expression of syndecan-4 and Id-1 in endothelial cells. Int. J. Oncol. 2009, 34, 861–872. [Google Scholar] [PubMed]
- Rajesh, M.; Mukhopadhyay, P.; Godlewski, G.; Bátkai, S.; Haskó, G.; Liaudet, L.; Pacher, P. Poly(ADP-ribose)polymerase inhibition decreases angiogenesis. Biochem. Biophys. Res. Commun. 2006, 350, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, Y.; Lv, S.; Zhang, C.; Tian, Y. PARP-1 may be involved in angiogenesis in epithelial ovarian cancer. Oncol. Lett. 2016, 12, 4561–4567. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, M.I.; Peralta-Leal, A.; O’Valle, F.; Rodriguez-Vargas, J.M.; Gonzalez-Flores, A.; Majuelos-Melguizo, J.; López, L.; Serrano, S.; de Herreros, A.G.; Rodríguez-Manzaneque, J.-C.; et al. PARP-1 regulates metastatic melanoma through modulation of vimentin-induced malignant transformation. PLoS Genet. 2013, 9, e1003531. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.B.; Yang, A.Y.; Kim, S.C.; Lee, J.; Choi, J.K.; Choi, C.; Kim, M.Y. PARP1 enhances lung adenocarcinoma metastasis by novel mechanisms independent of DNA repair. Oncogene 2016, 35, 4569–4579. [Google Scholar] [CrossRef] [PubMed]
- Curtin, N.J.; Szabo, C. Therapeutic applications of PARP inhibitors: anticancer therapy and beyond. Mol. Aspects Med. 2013, 34, 1217–1256. [Google Scholar] [CrossRef] [PubMed]
- Murai, J.; Huang, S.Y.; Das, B.B.; Renaud, A.; Zhang, Y.; Doroshow, J.H.; Ji, J.; Takeda, S.; Pommier, Y. Trapping of PARP1 and PARP2 by Clinical PARP Inhibitors. Cancer Res. 2012, 72, 5588–5599. [Google Scholar] [CrossRef] [PubMed]
- Helleday, T. The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Mol. Oncol. 2011, 5, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Purnell, M.R.; Whish, W.J.D. Novel Inhibitors of Poly(ADP-Ribose) Synthetase. Biochem. J. 1980, 185, 775–777. [Google Scholar] [CrossRef] [PubMed]
- Papeo, G.; Forte, B.; Orsini, P.; Perrera, C.; Posteri, H.; Scolaro, A.; Montagnoli, A. Poly(ADP-ribose) polymerase inhibition in cancer therapy: Are we close to maturity? Expert. Opin. Ther. Pat. 2009, 19, 1377–1400. [Google Scholar] [CrossRef] [PubMed]
- Durrant, L.G.; Boyle, J.M. Potentiation of cell killing by inhibitors of poly (ADP-ribose) polymerase in four rodent cell lines exposed to N-methyl-N-nitrosourea or UV light. Chem. Biol. Inter. 1982, 38, 325–338. [Google Scholar] [CrossRef]
- Fong, P.C.; Boss, D.S.; Yap, T.A.; Tutt, A.; Wu, P.; Mergui-Roelvink, M.; Mortimer, P.; Swaisland, H.; Lau, A.; O’Connor, M.J.; et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 2009, 361, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.; Ison, G.; McKee, A.E.; Zhang, H.; Tang, S.; Gwise, T.; Sridhara, R.; Lee, E.; Tzou, A.; Philip, R.; et al. FDA Approval Summary: Olaparib Monotherapy in Patients with Deleterious Germline BRCA-Mutated Advanced Ovarian Cancer Treated with Three or More Lines of Chemotherapy. Clin. Cancer Res. 2015, 21, 4257–4261. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, D.; Martin, P.; Busschots, S.; Thery, J.; O’leary, J.J.; Hennessy, B.T.; Stordal, B. PARP Inhibitors as P-glyoprotein Substrates. J. Pharm. Sci. 2014, 103, 1913–1920. [Google Scholar] [CrossRef] [PubMed]
- Gilabert, M.; Launay, S.; Ginestier, C.; Bertucci, F.; Audebert, S.; Pophillat, M.; Toiron, Y.; Baudelet, E.; Finetti, P.; Noguchi, T.; Sobol, H.; et al. Poly(ADP-Ribose) Polymerase 1 (PARP1) Overexpression in Human Breast Cancer Stem Cells and Resistance to Olaparib. PLoS ONE 2014, 9, e104302. [Google Scholar] [CrossRef] [PubMed]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lupo, B.; Trusolino, L. Inhibition of poly(ADP-ribosyl)ation in cancer: old and new paradigms revisited. Biochim. Biophys. Acta. 2014, 1846, 201–215. [Google Scholar] [CrossRef] [PubMed]
- Dockery, L.E.; Gunderson, C.C.; Moore, K.N. Rucaparib: the past, present, and future of a newly approved PARP inhibitor for ovarian cancer. Onco Targets Ther. 2017, 10, 3029–3037. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Sun, J.; Maloney, L.; Goble, S.; Oza, A.; Coleman, R.; Scott, C.; Robillard, L.; Mann, E.; Isaacson, J.; et al. Quantification of genomic loss of heterozygosity enables prospective selection of ovarian cancer patients who may derive benefit from the PARP inhibitor rucaparib. Europ. Cancer Congr. 2015, 51, S531. [Google Scholar] [CrossRef]
- Ihnen, M.; zu Eulenburg, C.; Kolarova, T.; Qi, J.W.; Manivong, K.; Chalukya, M.; Dering, J.; Anderson, L.; Ginther, C.; Meuter, A.; et al. Therapeutic potential of the poly(ADP-ribose) polymerase inhibitor rucaparib for the treatment of sporadic human ovarian cancer. Mol. Cancer Ther. 2013, 12, 1002–1015. [Google Scholar] [CrossRef] [PubMed]
- Soumerai, J.D.; Zelenetz, A.D.; Moskowitz, C.H.; Palomba, M.L.; Hamlin, P.A., Jr.; Noy, A.; Straus, D.J.; Moskowitz, A.J.; Younes, A.; Matasar, M.J.; et al. The PARP Inhibitor Veliparib Can Be Safely Added to Bendamustine and Rituximab and Has Preliminary Evidence of Activity in B-Cell Lymphoma. Clin. Cancer Res. 2017, 23, 4119–4126. [Google Scholar] [CrossRef] [PubMed]
- Somlo, G.; Frankel, P.H.; Arun, B.K.; Ma, C.X.; Garcia, A.A.; Cigler, T.; Cream, L.V.; Harvey, H.A.; Sparano, J.A.; Nanda, R.; et al. Efficacy of the PARP Inhibitor Veliparib with Carboplatin or as a Single Agent in Patients with Germline BRCA1- or BRCA2-Associated Metastatic Breast Cancer: California Cancer Consortium Trial NCT01149083. Clin. Cancer Res. 2017, 23, 4066–4076. [Google Scholar] [CrossRef] [PubMed]
- Reiss, K.A.; Herman, J.M.; Zahurak, M.; Brade, A.; Dawson, L.A.; Scardina, A.; Joffe, C.; Petito, E.; Hacker-Prietz, A.; Kinders, R.J.; et al. A Phase I study of veliparib (ABT-888) in combination with low-dose fractionated whole abdominal radiation therapy in patients with advanced solid malignancies and peritoneal carcinomatosis. Clin. Cancer Res. 2015, 21, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Reynolds, C.P.; Kang, M.H.; Kolb, E.A.; Gorlick, R.; Carol, H.; Lock, R.B.; Keir, S.T.; Maris, J.M.; Billups, C.A.; et al. Synergistic activity of PARP inhibition by talazoparib (BMN 673) with temozolomide in pediatric cancer models in the pediatric preclinical testing program. Clin. Cancer Res. 2015, 21, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.K.; Scoggins, M.; Ramirez, D.L.; Murthy, R.K.; Whitman, G.J.; Hess, K.R.; Adrada, B.E.; Moulder, S.L.; Barcenas, C.H.; Valero, V.; et al. A pilot study of neoadjuvant talazoparib for early-stage breast cancer patients with a BRCA mutation. Ann. Oncol. 2016. [Google Scholar] [CrossRef]
- Ashleigh, H.; Tudhope, S.J.; Junge, G.; Rodrigues, N.; Patterson, M.J.; Woodhouse, L.; Lunec, J.; Hunter, J.E.; Mulligan, E.A.; Cole, M.; et al. PARP1 expression, activity and ex vivo sensitivity to the PARP inhibitor, talazoparib (BMN 673), in chronic lymphocytic leukaemia. Oncotarget 2015, 6, 43978–43991. [Google Scholar]
- Engert, F.; Kovac, M.; Baumhoer, D.; Nathrath, M.; Fulda, S. Osteosarcoma cells with genetic signatures of BRCAness are susceptible to the PARP inhibitor talazoparib alone or in combination with chemotherapeutics. Oncotarget 2017, 8, 48794–48806. [Google Scholar] [CrossRef] [PubMed]
- Murai, J.; Huang, S.Y.; Renaud, A.; Zhang, Y.; Ji, J.; Takeda, S.; Morris, J.; Teicher, B.; Doroshow, J.H.; Pommier, Y. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol. Cancer Ther. 2014, 13, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhou, N.; Cai, P.; Bao, J. In Silico Screening Identifies a Novel Potential PARP1 Inhibitor Targeting Synthetic Lethality in Cancer Treatment. Int. J. Mol. Sci. 2016, 17, 258. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, S.; Wang, X.; Wang, P.; Zheng, Y.; Yao, D.; Guo, M.; Zhang, L.; Ouyang, L. Crystal structure-based discovery of a novel synthesized PARP1 inhibitor (OL-1) with apoptosis-inducing mechanisms in triple-negative breast cancer. Sci. Rep. 2016, 6, 3. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; Kaye, S.B.; Yap, T.A. PARP inhibitors: The race is on. Br. J. Cancer 2016, 114, 713–715. [Google Scholar] [CrossRef] [PubMed]
- Konstantinopoulos, P.A.; Spentzos, D.; Karlan, B.Y.; Taniguchi, T.; Fountzilas, E.; Francoeur, N.; Levine, D.A.; Cannistra, S.A. Gene expression profile of BRCAness that correlates with responsiveness to chemotherapy and with outcome in patients with epithelial ovarian cancer. J. Clin. Oncol. 2010, 28, 3555–3561. [Google Scholar] [CrossRef] [PubMed]
- Ekblad, T.; Schüler, H. Sirtuins are Unaffected by PARP Inhibitors Containing Planar Nicotinamide Bioisosteres. Chem. Biol. Drug Des. 2016, 87, 478–482. [Google Scholar] [CrossRef] [PubMed]
- Zaremba, T.; Curtin, N.J. PARP Inhibitor Development for Systemic Cancer Targeting. AntiCancer Agents Med. Chem. 2007, 7, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Baptista, S.J.; Silva, M.M.C.; Moroni, E.; Meli, M.; Colombo, G.; Dinis, T.C.P.; Salvador, J.A.R. Novel PARP-1 Inhibitor Scaffolds Disclosed by a Dynamic Structure-Based Pharmacophore Approach. PLoS ONE 2017, 12, e0170846. [Google Scholar] [CrossRef] [PubMed]
- Elmongy, E.I.; Khedr, M.A.; Taleb, N.A.; Awad, H.M.; Abbas, S.E.S. Design, Synthesis, and Biological Evaluation of Some Cyclohepta[b]Thiophene and Substituted Pentahydrocycloheptathieno[2,3-d]Pyrimidine Derivatives. J. Heter. Chem. 2017, 54, 1084–1093. [Google Scholar] [CrossRef]
- Zhu, Q.; Wang, X.; Chu, Z.; He, G.; Dong, G.; Xu, Y. Design, synthesis and biological evaluation of novel imidazo[4,5-c]pyridinecarboxamide derivatives as PARP-1 inhibitors. Bioorg. Med. Chem. Lett. 2013, 23, 1993–1996. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L.M. Profile of veliparib and its potential in the treatment of solid tumors. Onco. Targets Ther. 2015, 8, 1931–1939. [Google Scholar] [CrossRef] [PubMed]
- Gavande, N.S.; VanderVere-Carozza, P.S.; Hinshaw, H.D.; Jalal, S.I.; Sears, C.R.; Pawelczak, K.S.; Turchi, J.J. DNA repair targeted therapy: The past or future of cancer treatment? Pharmacol. Ther. 2016, 160, 65–83. [Google Scholar] [CrossRef] [PubMed]
- Steffen, J.D.; Tholey, R.M.; Langelier, M.F.; Planck, J.L.; Schiewer, M.J.; Lal, S.; Bildzukewicz, N.A.; Yeo, C.J.; Knudsen, K.E.; Brody, J.R.; et al. Targeting PARP-1 allosteric regulation offers therapeutic potential against cancer. Cancer Res. 2014, 74, 31–37. [Google Scholar] [CrossRef] [PubMed]

| Cancer Types | Expression of PARP1 |
|---|---|
| Breast Cancer | Up-regulated |
| Ovarian Cancer | Up-regulated |
| Uterine Cancer | Up-regulated |
| Lung Cancer | Up-regulated |
| Skin Cancer | Up-regulated |
| Non-Hodgkin’s Lymphoma | Up-regulated |
| Glioblastoma Multiforme | Up-regulated |
| Prostate Cancer | Up-regulated |
| Ewing’s Sarcoma | Up-regulated |
| Colorectal Cancer | Up-regulated |
| Pediatric Central Nervous System Cancer | Up-regulated |
| Testicular Germ Cell Tumor | Up-regulated |
| Drug | Structure | Active Structural Group | Biophysical Parameters (PARP1) | Well Studied Cancer Types | Typical Clinical Trials No. (The Latest Stage) | Phase | REF |
|---|---|---|---|---|---|---|---|
| Olaparib (AZD2281) |  | Amide motif enclosed in cyclic ring | IC50 = 5 nM | Ovarian carcinoma | NCT02476968 (Phase 4) | 1–4 | [1,73] |
| Breast carcinoma | NCT02032823 (Phase 3) | 1–3 | |||||
| Prostate carcinoma | NCT02987543 (Phase 3) | 1–3 | |||||
| Pancreatic carcinoma | NCT02184195 (Phase 3) | 1–3 | |||||
| Ewing’s sarcoma | NCT01583543 (Phase 2) | 1–2 | |||||
| Gastric carcinoma | NCT01924533 (Phase 3) | 1–3 | |||||
| Lung carcinoma | NCT03009682 (Phase 2) | 1–2 | |||||
| Germ Cell Tumor | NCT02533765 (Phase 2) | 1–2 | |||||
| Colorectal carcinoma | NCT00912743 (Phase 2) | 1–2 | |||||
| Unknown solid tumors | NCT03233204 (Phase 2) | 1–2 | |||||
| Niraparib (MK-4827) |  | Conventional embedded primary amide | IC50 = 3.2 nM | Ovarian carcinoma | NCT01847274 (Phase 3) | 1–3 | [76,93] |
| Breast carcinoma | NCT01905592 (Phase 3) | 1–3 | |||||
| Endometrial carcinoma | NCT03016338 (Phase 2) | 1–2 | |||||
| Uveal melanoma | NCT03207347 (Phase 2) | 1–2 | |||||
| Fallopian tube carcinoma | NCT02657889 (Phase 2) | 1–2 | |||||
| Peritoneal carcinoma | NCT02657889 (Phase 2) | 1–2 | |||||
| Rucaparib (AG-014669, PF-01367338) |  | Amide motif enclosed in cyclic ring | IC50 = 1.4 nM | Ovarian carcinoma | NCT02855944 (Phase 3) | 1–3 | [78,80,96] |
| Fallopian tube carcinoma | NCT02855944 (Phase 3) | 1–3 | |||||
| Peritoneal carcinoma | NCT01968213 (Phase 3) | 1–3 | |||||
| Prostate carcinoma | NCT02975934 (Phase 3) | 1–3 | |||||
| Pancreatic carcinoma | NCT02042378 (Phase 2) | 1–2 | |||||
| Breast carcinoma | NCT02505048 (Phase 2) | 1–2 | |||||
| Veliparib (ABT-888) |  | Conventional embedded primary amide | Ki = 5.2 nM | Breast carcinoma | NCT02032277 (Phase 3) | 1–3 | [82,97,98] |
| Ovarian carcinoma | NCT02470585 (Phase 3) | 1–3 | |||||
| Non-small cell lung carcinoma | NCT02106546 (Phase 3) | 1–3 | |||||
| Solid tumors | NCT01193140 (Phase 2) | 1–2 | |||||
| Testicular carcinoma | NCT02860819 (Phase 2) | 1–2 | |||||
| Talazoparib (BMN-673) |  | Amide motif enclosed in cyclic ring | IC50 = 1.2 nM | Breast carcinoma | NCT01945775 (Phase 3) | 1–3 | [85,86,99,100] |
| Prostate carcinoma | NCT03148795 (Phase 2) | 1–2 | |||||
| Ovarian carcinoma | NCT02326844 (Phase 2) | 1–2 | |||||
| Endometrial carcinoma | NCT02127151 (Phase 2) | 1–2 | |||||
| Acute Myeloid Leukemia | NCT02878785 (Phase 2) | 1–2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Liang, C.; Li, F.; Guan, D.; Wu, X.; Fu, X.; Lu, A.; Zhang, G. PARP1 in Carcinomas and PARP1 Inhibitors as Antineoplastic Drugs. Int. J. Mol. Sci. 2017, 18, 2111. https://doi.org/10.3390/ijms18102111
Wang L, Liang C, Li F, Guan D, Wu X, Fu X, Lu A, Zhang G. PARP1 in Carcinomas and PARP1 Inhibitors as Antineoplastic Drugs. International Journal of Molecular Sciences. 2017; 18(10):2111. https://doi.org/10.3390/ijms18102111
Chicago/Turabian StyleWang, Luyao, Chao Liang, Fangfei Li, Daogang Guan, Xiaoqiu Wu, Xuekun Fu, Aiping Lu, and Ge Zhang. 2017. "PARP1 in Carcinomas and PARP1 Inhibitors as Antineoplastic Drugs" International Journal of Molecular Sciences 18, no. 10: 2111. https://doi.org/10.3390/ijms18102111





