1. Introduction
Secondary hyperparathyroidism (sHPT) develops during the progression of chronic kidney disease (CKD) and affects morbidity and mortality in these patients [
1]. This has already been evaluated in patients with a reduced glomerular filtration rate (GFR) as with patients with CKD stages II and III and aggravating in patients with no or only partial residual function on renal replacement therapy [
2]. Vitamin D receptors (VDRs) are targets for pharmacological therapy of sHPT. The vitamin D receptor belongs to the superordinate family of steroid and nuclear hormone receptors [
3]. As a nuclear transcription factor, it elicits complex physiological regulation in most tissues and cells following binding of its ligand 1,25-dihydroxyvitamin D. VDR is not only involved in the metabolism of calcium and phosphate, but also crucially implicated in events such cell differentiation, proliferation, apoptosis and different immune responses [
3]. Importantly, ligand-bound VDR forms heterodimer with 9-
cis-retinoid X receptor (RXR) couples to a vitamin d responsive element (VDRE) and with the aid of distinct co-regulatory molecules activating and/or repressing vitamin D target genes [
4]. There are two principal functional domains of VDR, a conserved NH
2-terminal DNA binding domain (DBD) and the more variable COOH-terminal ligand binding domain (LBD). DBD, which is a cysteine-rich zinc finger region, and LBD are connected through a hinge region, probably stabilizing the whole complex [
3].
Substitution with active vitamin D compounds might lead to a slowing of sHPT progression [
5]. For this purpose, vitamin D receptor activators (VDRAs) were synthesized; however, these agents were nonselective.
In an attempt to identify a VDRA that would be more specific and selective, paricalcitol was developed by chemical modification of the side chain and the A-ring of the molecule, resulting in a selective VDRA [
6]. Paricalcitol (19-Nor-1alpha,25-dihydroxyvitamin D2) differs from other VDRA such as calcitriol not only in the side chain structure, but in contrast to other compounds, also in an A-ring configuration. In contrast to previous nonselective VDRAs, paricalcitol yields a reduced intestinal calcium absorption via reduced calbindin induction [
7], maintaining the parathyroid hormone (PTH) suppression of VDRAs through a distinct cellular downstream signaling pattern, a finding that is also noted in dialysis patients [
8]. Of note, after binding to the vitamin D receptor (VDR), paricalcitol recruits different co-regulators in comparison to other VDRAs, impacting gene expression and regulation differently [
9,
10]. Wu-Wong et al. have shown that paricalcitol regulates different genes in vascular smooth muscle cells (VSMCs) compared with calcitriol and reduces the expression of several genes that are virtually upregulated in the uremic state [
11,
12]. In addition, in patients with moderate CKD without diabetes, paricalcitol is able to ameliorate the decline in endothelial function [
13]. Those results seem to suggest a specific therapeutic role of paricalcitol that is more distinct, acting at different facets of sHPT. Registration trials have documented efficacy of intravenous [
14] and oral [
15] paricalcitol in dialysis patients, as well as in non-dialysis patients with CKD stages 3 and 4 [
16]. After approval of intravenous paricalcitol in the year 2004 in Germany, the oral compound has been approved for treatment of sHPT in 2008.
Although data from several studies have shown the efficacy of paricalcitol in the treatment of patients with sHPT, most results documented treatment with intravenous (IV) paricalcitol, and had a short follow-up time, with the exception of the study by Lindberg et al. [
17]. However, the routine use of paricalcitol (i.e., clinical and laboratory efficiency, safety, patient compliance) in daily practice should be studied more closely to increase knowledge in this scope of application. Other medications related and prescribed for sHPT may influence the effects of paricalcitol; moreover, country-specific issues in the treatment of CKD together with sHPT should be considered in this context.
Observational studies are an indispensable tool to confirm data obtained from randomized controlled trials (RCTs) [
18]. To this end, an observational study including patients from German and Austrian dialysis centers was designed to obtain information regarding paricalcitol treatment of sHPT in daily routine for an extended period of time (12 months). Specifically, this study encompasses predialysis and dialysis patients and oral and IV paricalcitol dosing within the dialysis patient group. Of note, patients irrespective of their native/active Vitamin D pretreatment mode, or other medications, were included in the observation period and were thus analyzed.
1.1. Patients
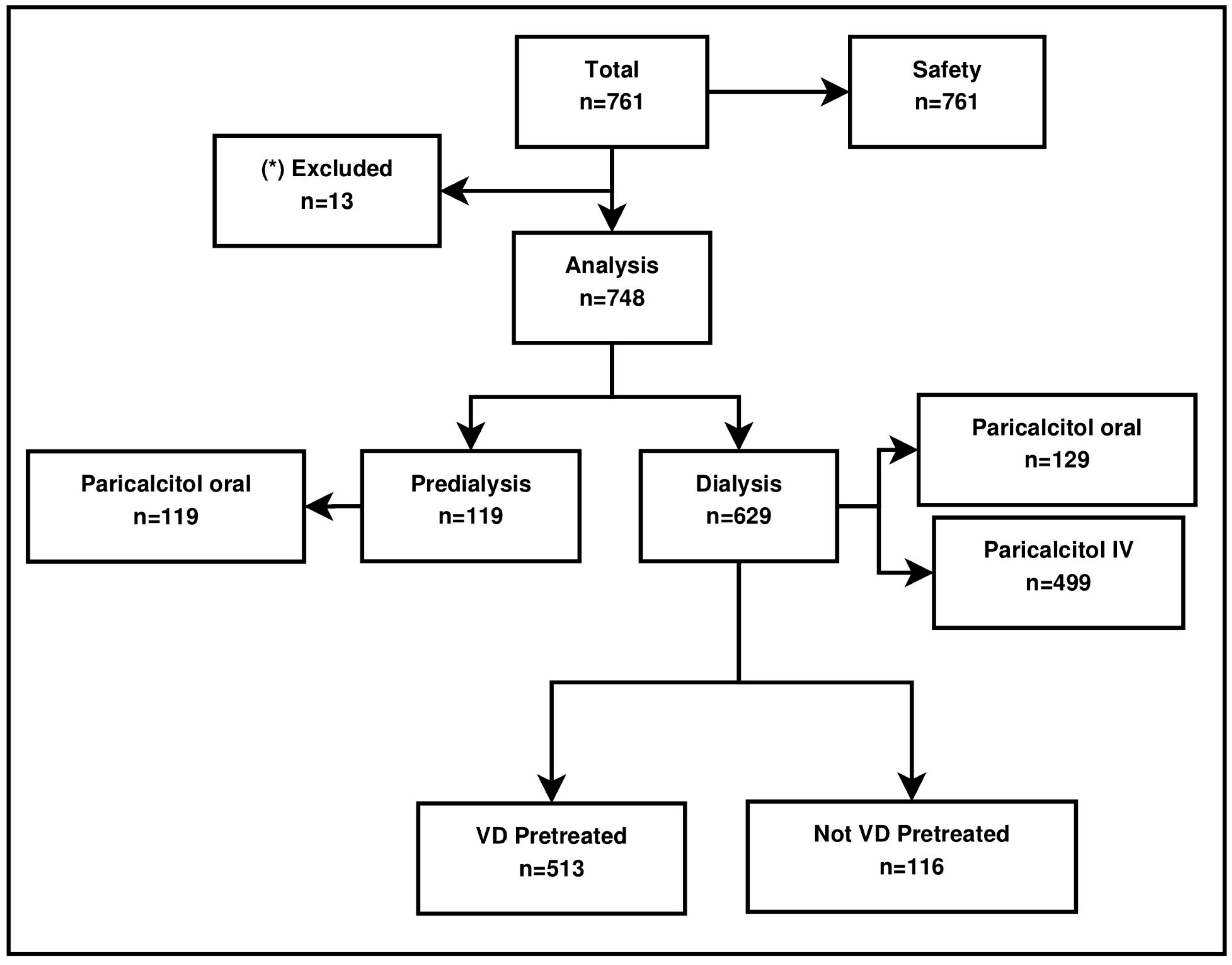
The study was conducted in sites specializing in the treatment of CKD and in the offices of community-based specialists/nephrologists. Sites taking part in the study were expected to document data on ≥5 patients. For this study, 761 patients were documented by 90 physicians/clinical study sites throughout Germany (79 sites) and Austria (11 sites). The first patient was treated on 24 March 2008 and the last visit was performed on 11 December 2013. The decision to participate in this study was independent of the prescription of therapies (besides paricalcitol prescription). All adult hemodialysis (HD) and peritoneal dialysis (PD) patients as well as predialysis patients with CKD stages 3 to 5 were eligible for study inclusion. The dose and route of application (paricalcitol capsules or the IV drug formulation) were at the physician’s discretion.
Patients aged >18 years with a prescription for paricalcitol according to the summary of product information (SmPC) and no pretreatment with paricalcitol within the past 6 months were included in the study. It was suggested that patients with an intact PTH (iPTH) value of >1000 pg/mL not be included in the study; however, this suggestion was not mandatory.
The study was approved by the ethical committee of the medical faculty, Johann Wolfgang Goethe-Universität Frankfurt (reference number 332/08, 10 November 2008) and was registered under
ClinicalTrials.gov.
1.2. Study Design and Aims
The study population exclusively consisted of patients with CKD (predialysis CKD stages 3–5 and dialysis) and diagnosis of secondary hyperparathyroidism. A sample size of 748 patients was available for analysis (in total, 761 patients comprised the safety population).
Paricalcitol was provided to patients on an on-label basis in an everyday setting. An observation period of 12 months was chosen to observe real-world treatment of patients with oral and IV paricalcitol. The study profile is detailed in
Figure 1; the vast majority of the patients received paricalcitol intravenously.
As specific primary study objectives, the proportion of patients attaining an iPTH within the Kidney Disease Outcomes Quality Initiative (KDOQI) [
19] target range (CKD stage 3, 35–70 pg/mL; CKD stage 4, 70–110 pg/mL, CKD stage 5, 150–300 pg/mL) in the study period was evaluated, including the time when KDOQI target levels were first achieved and whether these target levels were sustained. However, it should be noted that during the study enrollment, the Kidney Disease Improving Global Outcomes (KDIGO) guidelines [
5] were issued, thereby expanding the PTH range (150–300 pg/mL) to “approximately two to nine times the upper normal limit for the assay”. For this study, conventional PTH assays/second-generation immunometric PTH assays were employed. Further laboratory blood parameters assessed included calcium, phosphate, hemoglobin, blood urea nitrogen, C-reactive protein, creatinine, albumin, and alkaline phosphatase.
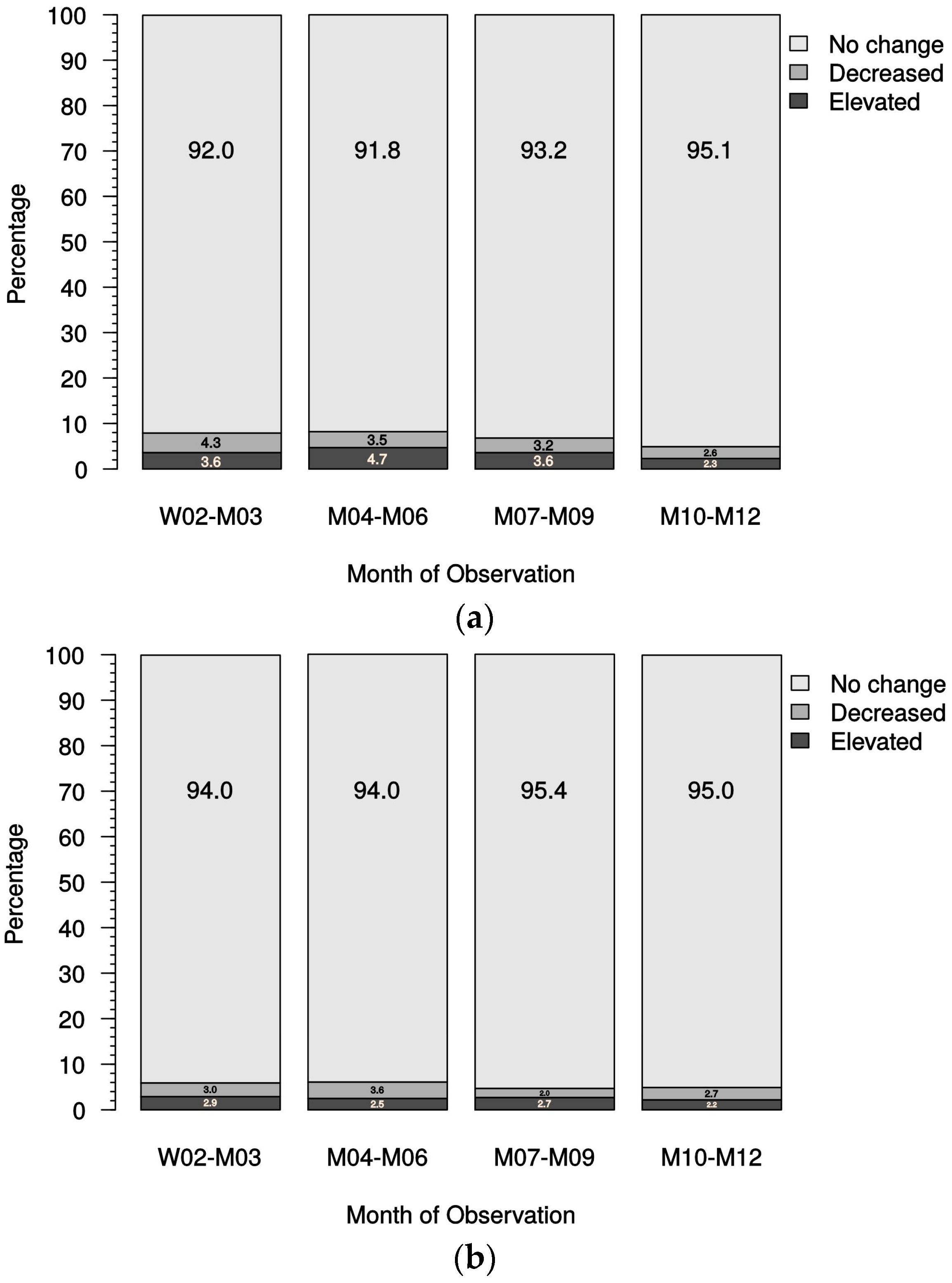
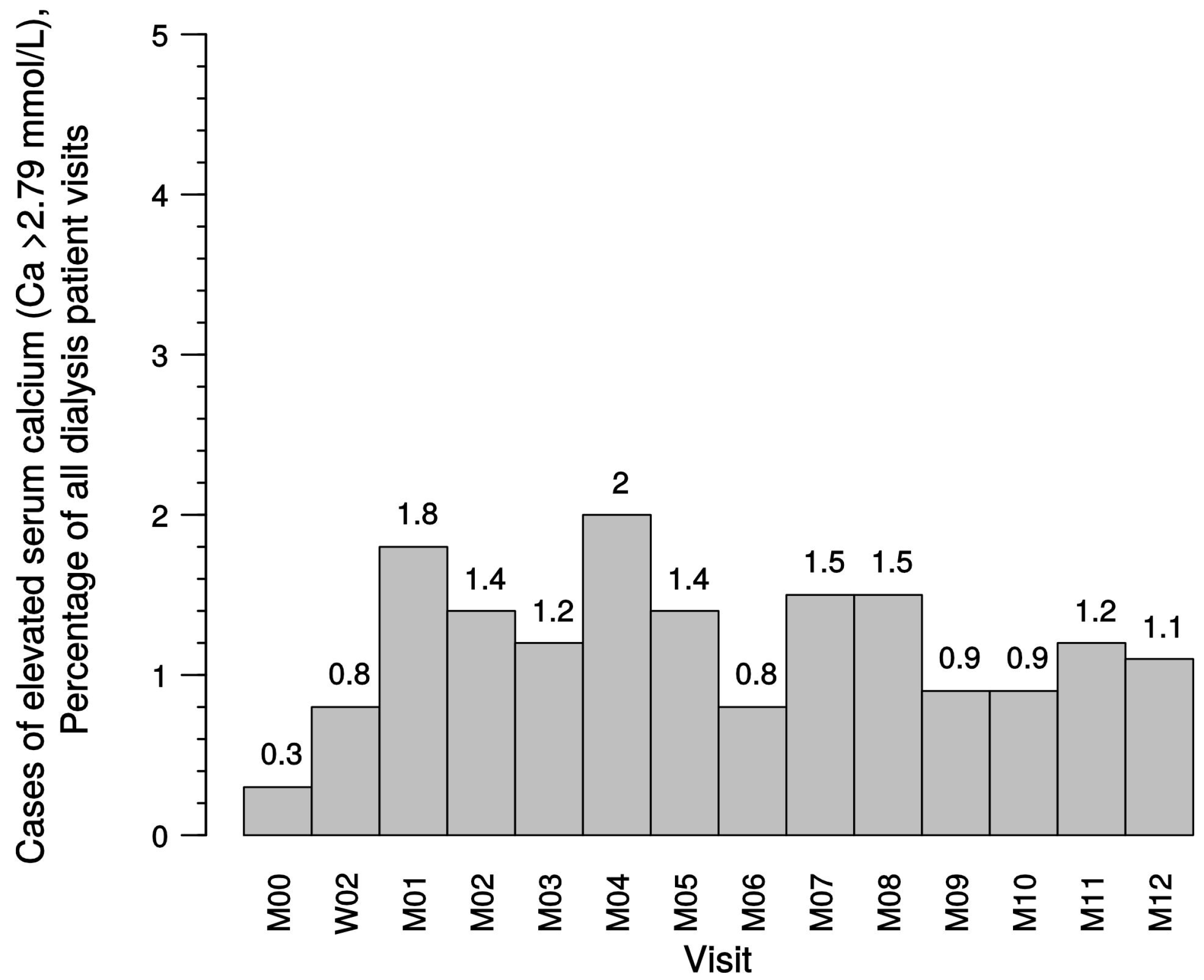
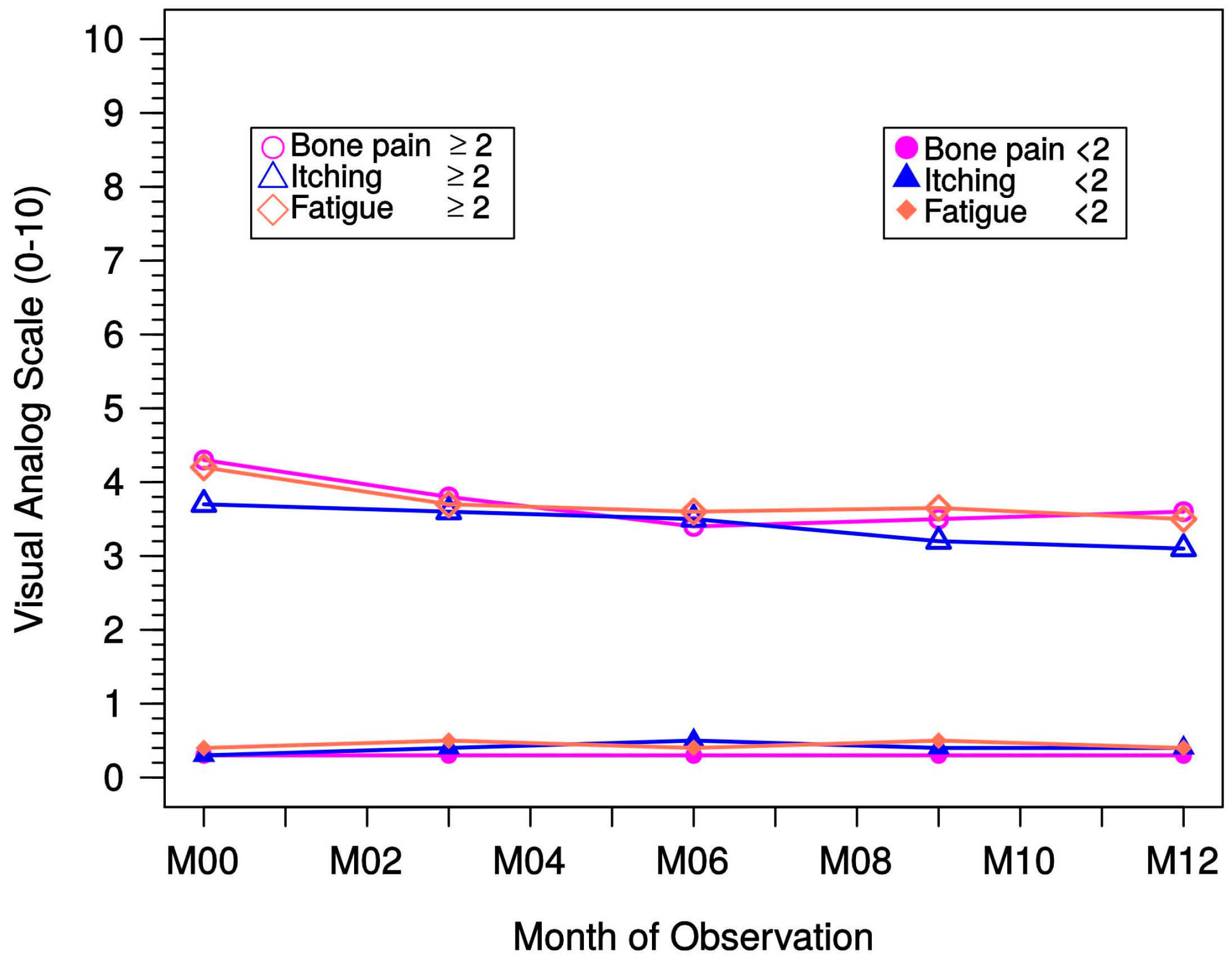
Secondary objectives of the study were the analysis of episodes with elevated serum calcium and serum phosphate levels in different modality groups. Hypercalcemic values were defined as >2.79 mmol/L. In addition, data regarding the clinical consequences of sHPT (e.g., itching, bone pain, fatigue) were obtained using a visual analog scale (VAS). Furthermore, general safety analyses were conducted.
All data, including safety and compliance specifications, were obtained from case report forms for documentation in Germany; in Austrian sites, electronic data report forms were used. Data were obtained at baseline (month 0), after two weeks, and thereafter monthly up to 12 months for dialysis patients. Data collection from patients on predialysis was done at baseline (month 0), and then quarterly up to month 12. Data concerning adverse events (AEs) and serious adverse events (SAEs) were reported by the participating physicians regardless of type of paricalcitol medication.
1.3. Drug
Paricalcitol was administered as soft capsules (1 or 2 µg per capsule), in 1 mL-vials (5 µg) or in 2 mL-vials (10 µg) manufactured by Abbott/AbbVie under the trade name Zemplar®.
1.4. Statistical Methods
Descriptive statistical analysis was applied using SAS version 9.2 (SAS Institute, Cary, NC, USA) and R version 3.2.2 (The R Foundation, Vienna, Austria). Unavailable values were categorized as either non-evaluable or missing in the case that data were not retrievable or incomplete/fragmentary. For description of medical symptoms, both predialysis and dialysis patient groups were stratified by VAS < 2 and VAS ≥ 2. Statistical tests (Wilcoxon rank sum test, two-sided) were performed to determine the difference between parameters at month 0 and month 12 and were exploratory. All other statistical comparisons and tests were applied exclusively in an exploratory sense.
3. Discussion
A disadvantage of only considering RCTs for investigating novel drugs lays in the fact that study populations are not always representative of real-world patient populations. By contrast, in observational studies, the inclusion criteria are often broader and there are wider ranges of coexisting illnesses and disease burden, disease severity, and concomitant treatments. Results from database studies such as the present TOP study can be applied more generally to the entire CKD population and also reflect treatment preferences. The evidence contained within these databases may contribute to clinical decision-making and allows the efficacy and safety of therapy to be assessed. Specifically in nephrology, the important and complementary role of both RCTs and observational studies is appreciated [
22].
The present observational study gives insights into the routine management of sHPT in patients with advanced CKD, including dialysis, with a focus on paricalcitol therapy during a 12-month period. Importantly, this study included patients treated with both oral and IV paricalcitol treatment in dialysis patients and oral treatment in predialysis patients, extending data from shorter study periods focusing on treatment with IV paricalcitol [
15,
23].
Because of the non-interventional nature of this study, no site monitoring was conducted. Because of this, the data quality, including feedback on queries, is not as robust and complete as in an interventional study.
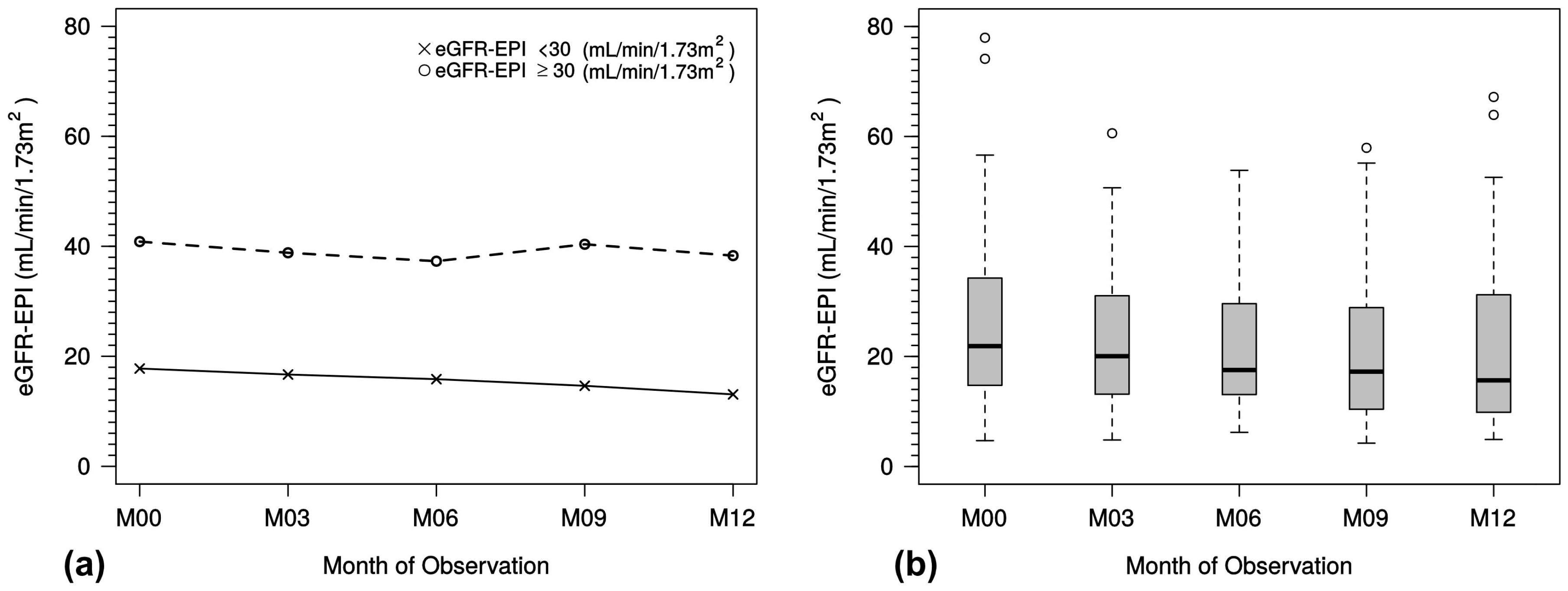
In our study, we also analyzed patients from the predialysis stage (CKD stage 3, CKD stage 4 and CKD stage 5 non-dialysis). Data from the latter group are of high relevance; these patients represent a very inhomogeneous group—with timely unforeseeable deterioration of kidney function with regard to end-stage kidney disease. Some of these patients might rapidly progress to end-stage kidney disease and dialysis, while others will not, making valid interpretation of pharmacotherapeutic effects difficult.
Stable function was observed in patients with an eGFR ≥ 30 mL/min/1.73 m2; patients with an eGFR < 30 mL/min/1.73 m² showed a further slight decline within the study period. There was no obvious change in iPTH in either group (data not shown). Whether oral paricalcitol therapy helps stabilize iPTH values in patients in the predialysis phases of CKD cannot be confirmed by this observation.
Recent data from Coyne et al. [
24] indicated favorable efficacy in iPTH suppression comparing paricalcitol (−52%) and calcitriol (−46%), with low incidences of hypercalcemia, in patients with stages 3 and 4 CKD. In contrast to our observational study, dosing was mainly based on iPTH, calcium, and phosphorus levels.
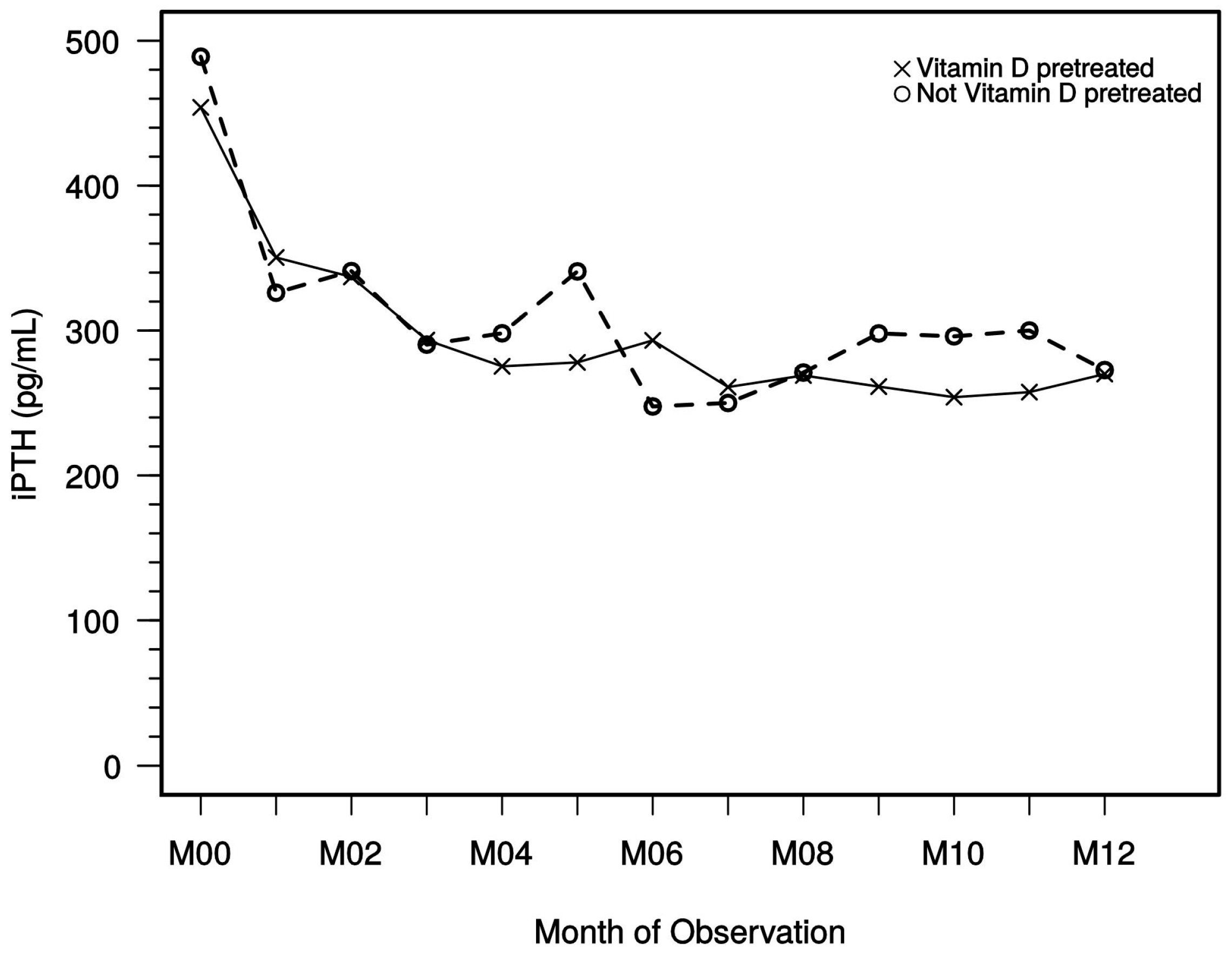
The impact of previous and concomitant vitamin D therapy (active/native) on iPTH levels has also been evaluated. Interestingly, our study data did not show relevant differences between the dialysis patients with previous vitamin D therapy and those without. Moreover, no obvious influence of previous vitamin D therapy was observed with regard to calcium and/or phosphate levels during the study period.
The TOP study was conducted at a time when treatment guidelines for sHPT treatment were transitioning from KDOQI in 2003 [
19] to KDIGO in 2009 [
5]. Since the aforementioned guidelines allowed higher maximum permissible iPTH values, this may have had an influence on the therapy provided to patients, essentially in the prescription practice of paricalcitol, but also of native vitamin D, phosphate binders, and other sHPT-related therapies. It is conceivable that the dosage of paricalcitol and/or native vitamin D might have been tapered in some cases in patients with low iPTH levels, according to KDOQI, had been achieved. However, there was no effect on the overall efficacy in lasting PTH reduction.
Of note, the clinical practice of administering native vitamin D in patients with stage 3 or 4 CKD 3/4 stage and maintaining this treatment in combination with active vitamin D in patients with stage 5 CKD undergoing dialysis is reflected in this study. We did not find an increased risk for hypercalcemia or prolonged hypercalcemia in patients receiving paricalcitol-containing therapy. This is in accordance with the findings of the IMPACT study [
25], which also reported only a low incidence of mild hypercalcemia in patients in the paricalcitol treatment arms.
Neither the optimal dosing nor the most beneficial 25-OH vitamin D serum levels are known in patients with CKD, nor are there currently consented repletion strategies. In this regard, KDIGO guidelines from 2009 suggested the therapeutic correction of 25-OH D deficiency and insufficiency for patients with CKD but not on dialysis, but did not provide guidance for dialysis patients [
5]. In clinical practice, however, many physicians will tend to supplement vitamin D. The risk of hypercalcemic episodes or values will be dependent on the level of combined (native/active) dosage and the application interval. Here, paricalcitol is offering some biochemical advantages, as shown in a recent clinical investigation pointing to decreased intestinal calcium absorption with paricalcitol compared with calcitriol [
8].
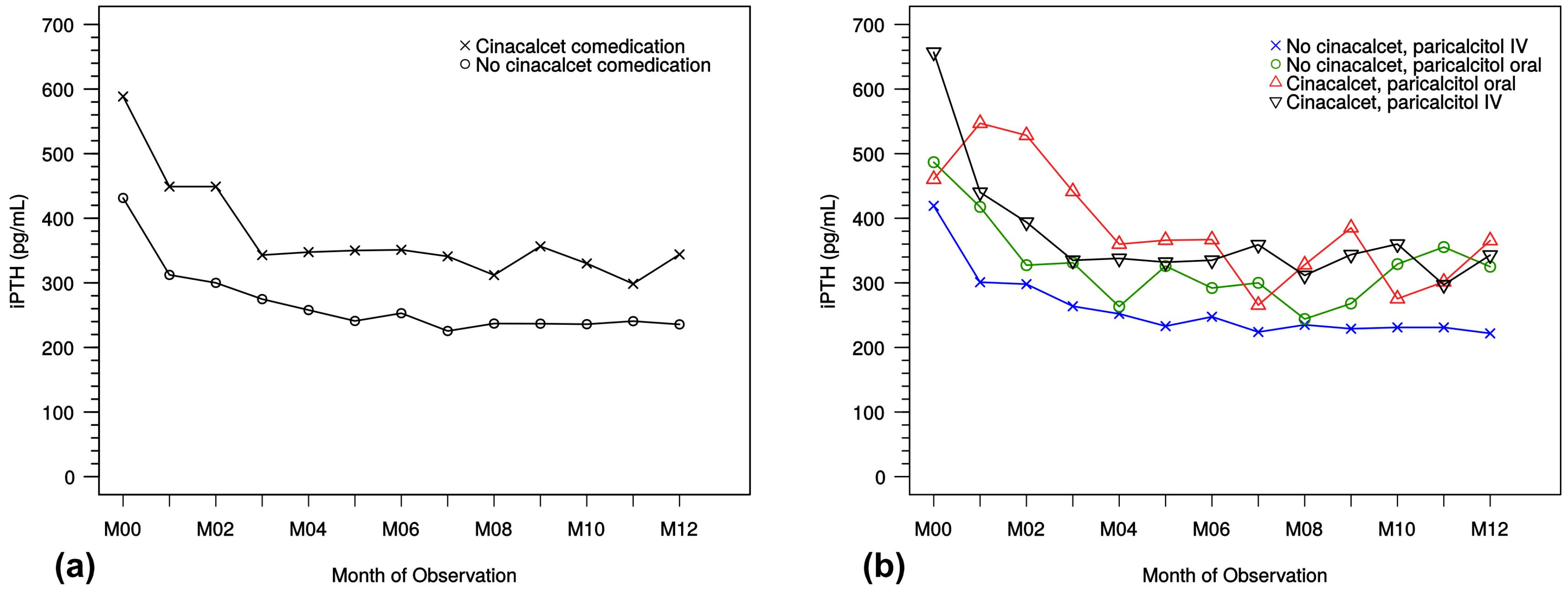
Calcimimetics, like cinacalcet, are a class of drugs that suppress PTH secretion by increasing the sensitivity of the parathyroid gland calcium-sensing receptors without increasing plasma calcium levels. The impact of cinacalcet on different parameters has been investigated as well. Although the influence of calcimimetic use in parallel to paricalcitol treatment can only be described with caution, in this study, there were no significant changes in comparison with patients receiving paricalcitol as monotherapy. iPTH, serum calcium, and phosphate levesl and the decrease in PTH levels was similar between the combination therapy and paricalcitol monotherapy groups. This supports the use of cinacalcet in daily routine. The observation that initial median iPTH levels were higher in the group taking cinacalcet (see
Table 5 and
Table 6) may indicate that it is common practice to prescribe cinacalcet in patients with strongly elevated iPTH levels and possibly refractory disease. Although the initial slope in iPTH decrease was comparable in both groups, the absolute median iPTH values remained on a higher level in patients with cinacalcet. Nevertheless, paricalcitol proved to be effective also in these patients.
Phosphate levels are considered an independent predictor of survival in patients on dialysis and start increasing as eGFR drops below 30 mL/min/1.73 m2. Among different factors determining phosphate levels (i.e., residual renal function, dietary intake of phosphate, dialysis adequacy, dose of phosphate binders), the use of vitamin D analogues might play a major role in this scenario.
A recent meta-analysis of CKD in patients who were not undergoing treatment with HD also indicates the efficacy of paricalcitol in lowering PTH levels, but with reference to a careful use of vitamin D analogues because of hypercalcemia and calcification risks [
26]. However, in patients with early CKD, including subjects with mild sHPT, those treated even with high doses of native (inactive) cholecalciferol reported no hypercalcemic events [
27], indicative of the tolerance of native vitamin D treatment in this group. This is also important for combinations with active vitamin D compounds. In addition, Coyne et al. reported a low incidence of hypercalcemia in patients with stages 3 and 4 CKD treated with paricalcitol while achieving sustained PTH suppression [
24].
Although KDIGO guideline recommendations have liberalized maximum permissible values for PTH in sHPT, data from ARO [
28] and FARO [
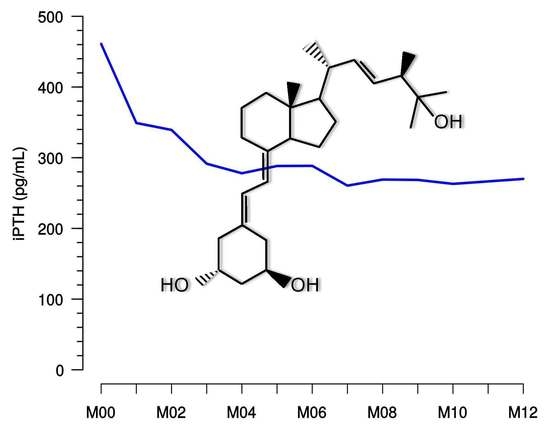
29] studies in dialysis patients argue for the achievement of only modestly elevated PTH levels to attain favorable outcomes. This target range is corresponding to the monitoring plan of our observational study according to KDOQI and was reached with paricalcitol in many dialysis patients, but treating physicians may have pursued individual target goals in their patients. Given the sustained reduction as shown by median iPTH levels after 4 or 5 months and in the sequential follow-up (see
Figure 4), it does not appear that a marked dose reduction or even cessation of treatment has taken place. Moreover, the data show that in the hands of the treating physicians, paricalcitol dose corrections were not necessary in the majority of patients after several months, indicating an overall safe and sustained treatment effect.
The inability to suppress severe sHPT with the use of traditional VDRA agents can lead to the need for parathyroidectomy, with the patient still at risk for the development of recurrent hyperparathyroidism or aplastic bone disease. In this regard, potent pharmacologic treatment is mandatory, with paricalcitol offering an effective treatment option in the setting of daily clinical practice.
Rapid and significant reduction of iPTH in patients with stage 5 CKD undergoing HD/PD was observed under investigational study conditions [
15] within a few weeks. Our observational data support that under real-world conditions, stable results can be obtained within two to three months of treatment and can be maintained for 12 months.
The present observational data add valuable information to phase III clinical trial data investigating the use of paricalcitol in appropriate patients [
14,
16,
17,
30] and demonstrated a safety profile that did not reveal any new safety concerns during its use in daily routine practice.
Moreover, compared to historical comparison groups, there was no incidence of increased specific AEs [
31]. Regarding the occurrence of deaths, our data do not differ from reported numbers generated from the US DOPPS (Dialysis Outcomes Practice Patterns Study) analysis arm [
32], from DOPPS data analysis worldwide [
33], or from European DOPPS cohorts [
34] or those in Germany [
35].
The present observational data regarding PTH suppression are highlighted by the concomitant amelioration of symptoms such as itching, bone pain, and fatigue, which was documented during 12 months of treatment.
Although compliance is generally not a challenge with IV administration, there is a risk of nonadherence to treatment with oral paricalcitol. In 19 patients (7.6%) receiving oral treatment, a total of 33 cases of noncompliance were reported by physicians.