Patterns of Apoptosis and Proliferation throughout the Biennial Reproductive Cycle of Viviparous Female Typhlonectes compressicauda (Amphibia, Gymnophiona)
Abstract
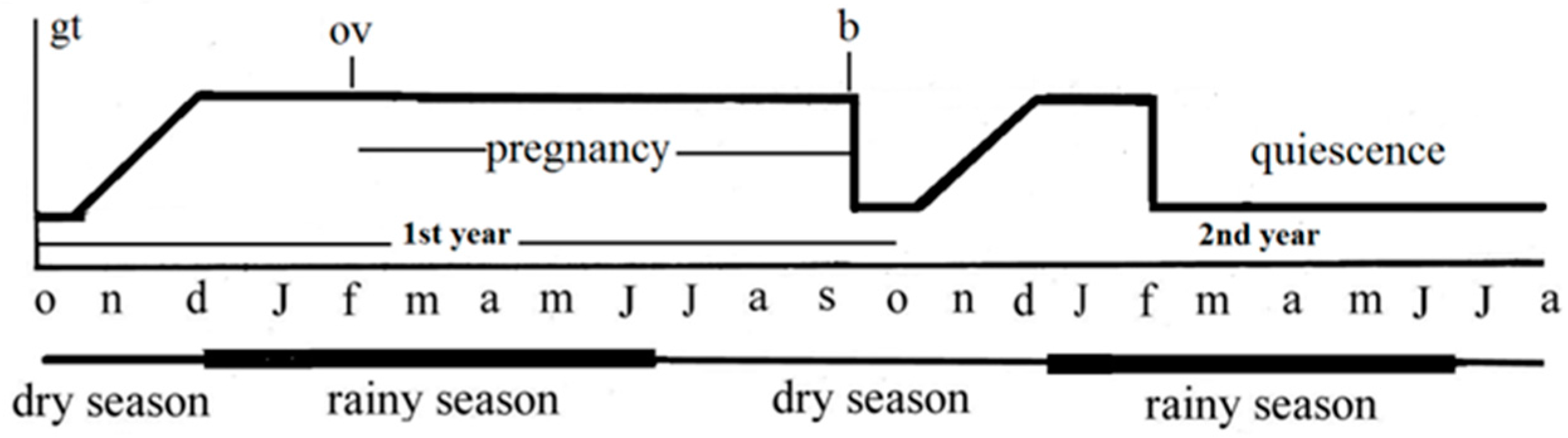
:1. Introduction
1.1. Cell Proliferation and Apoptosis
1.2. The Species Typhlonectes comzpressicauda
1.2.1. Ovaries
1.2.2. Funnels and Oviducts
2. Results
2.1. Proliferation and Apoptosis in the Ovaries
2.1.1. Germinal Nests
2.1.2. Growing Follicles
2.1.3. Vitellogenic Follicles
2.1.4. Atretic Follicles
2.1.5. Corpora Lutea
2.2. Proliferation and Apoptosis in the Different Parts of Oviducts
2.2.1. Ostium (Funnel)
2.2.2. Oviduct
Anterior Part (Tubal Part)
Posterior Part (Uterus)
3. Discussion
3.1. Ovaries
3.2. Funnels and Oviducts
4. Materials and Methods
4.1. Animals
4.2. Histology and Histochemistry
4.3. Detection of Ki67 by Immunohistochemistry
4.4. Visualization of Apoptosis
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Estabel, J.; König, N.; Shiokawa, K.; Exbrayat, J.-M. Apoptosis in Xenopus genus. In Apoptosis; Scovassi, I., Ed.; Research Signpost: Kerala, India, 2005; pp. 147–156. [Google Scholar]
- Exbrayat, J.-M.; Moudilou, E.N.; Abrouk, L.; Brun, C. Apoptosis in amphibian development. Adv. Biosci. Biotechnol. 2012, 3, 669–678. [Google Scholar] [CrossRef]
- Loeb, L.; Fleisher, M.S. The growth of tissues in the test tube under experimentally varied conditions with special reference to mitotic cell proliferation. J. Med. Res. 1919, 40, 509–550. [Google Scholar] [PubMed]
- Hammett, F.S. The natural chemical equilibrium regulative of growth by increase in cell number. Protoplasma 1930, 11, 382–411. [Google Scholar] [CrossRef]
- Barratt, J.W. On mitosis in proliferating epithelium. Proc. R. Soc. Lond. B 1907, 72, 372–377. [Google Scholar] [CrossRef]
- Pardee, A.B. G1 events and regulation of cell proliferation. Science 1989, 246, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Graña, X.; Reddy, E.P. Cell cycle control in mammalian cells: Role of cyclins, cyclin dependent kinases (CDKs), growth suppressor genes and cyclin-dependent kinase inhibitors (CKIs). Oncogene 1995, 11, 211–219. [Google Scholar] [PubMed]
- Van Den Heuvel, S.; Harlow, E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science 1993, 262, 2050–2054. [Google Scholar] [CrossRef] [PubMed]
- Karp, G. Biologie Cellulaire et Moléculaire; De Boeck Université: Paris, France, 2010. [Google Scholar]
- Cosetta Bertoli, J.; Skotheim, M.; de Bruin, R.A. Control of cell cycle transcription during G1 and S phases. Nat. Rev. Mol. Cell Biol. 2013, 14, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Janz, D.M.; van der Kraak, G. Suppression of apoptosis by gonadotropin, 17β-estradiol, and epidermal growth factor in rainbow trout preovulatory ovarian follicles. Gen. Comp. Endocrinol. 1997, 105, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Clarke, C.L.; Sutherland, R.L. 1990 Progestin regulation of cellular proliferation. Endocr. Rev. 1990, 11, 266–301. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Golstein, P. Morts cellulaires et système immunitaire. Med. Sci. 1989, 5, 546–553. [Google Scholar] [CrossRef]
- White, B.C.; Sullivan, J.M. Apoptosis. Acad. Emerg. Med. 1998, 5, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- Edinger, A.L.; Thompson, C.B. Death by design: Apoptosis, necrosis and autophagy. Curr. Opin. Cell Biol. 2004, 16, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, L.; Shi, Z.; Zhao, S.; Wang, F.T.; Zhou, T.T.; Liu, B.; Bao, J.K. Programmed cell death pathways in cancer: A review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 2012, 45, 487–498. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Meier, P.; Finch, A.; Evan, G. Apoptosis in development. Nature 2000, 12, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Brill, A.; Torchinsky, A.; Carp, H.; Toder, V. The role of apoptosis in normal and abnormal embryonic development. J. Assist. Reprod. Genet. 1999, 16, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Hay, B.A. Cell proliferation and apoptosis. Curr. Opin. Cell Biol. 1999, 11, 745–752. [Google Scholar] [CrossRef]
- Mattern, J.; Volm, M. Imbalance of cell proliferation and apoptosis during progression of lung carcinomas. Anticancer Res. 2004, 24, 4243–4246. [Google Scholar] [PubMed]
- Kroemer, G. Early work on the role of mitochondria in apoptosis, an interview with Guido Kroemer. Cell Death Differ. 2004, 11, S33–S36. [Google Scholar] [CrossRef] [PubMed]
- Wood, A.W.; van der Kraak, G.J. Apoptosis and ovarian function: Novel perspectives from the teleosts. Biol. Reprod. 2001, 64, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Santos, H.B.; Rizzo, E.R.; Bazzoli, N.B.; Sato, Y.; Moro, L. Ovarian regression and apoptosis in the South American teleost Leporinus taeniatus Lutken (Characiformes, Anostomidae) from the Sao Francisco Basin. J. Fish Biol. 2005, 67, 1446–1459. [Google Scholar] [CrossRef]
- Drummond, C.D.; Bazzoli, N.; Rizzo, E.; Sato, Y. Postovulatory follicle: A model for experimental studies of programmed cell death or apoptosis in teleosts. J. Exp. Zool. 2000, 287, 176–182. [Google Scholar] [CrossRef]
- Maillet, G.; Feral, C.; Benhaim, A. Apoptosis of the follicular cells: Its implication in ovarian induction protocols. Gynecol. Obstet. Fertil. 2005, 33, 653–658. [Google Scholar] [CrossRef] [PubMed]
- Terranova, T.; Taylor, C. Apoptosis (cell death). In Encyclopedia of Reproduction; Knobil, E., Neill, J.D., Eds.; Academic Press: New York, NY, USA, 1998; pp. 261–273. [Google Scholar]
- Abrahamsohn, P.A.; Zorn, T.M.T. Implantation and decidualization in rodents. J. Exp. Zool. 1993, 266, 603–628. [Google Scholar] [CrossRef] [PubMed]
- Sanders, E.J.; Wride, M.A. Programmed cell death in development. Int. Rev. Cytol. 1995, 163, 105–173. [Google Scholar] [PubMed]
- Assisi, L.; Raucci, F.; di Fiore, M.M. Seasonal study of apoptotic markers in lizard oviduct. J. Exp. Zool. A Ecol. Genet. Physiol. 2011, 315, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Bagwill, A.; Sever, D.M.; Elsey, R.M. Seasonal variation of the oviduct of the American alligator, Alligator mississippiensis (Reptilia: Crocodylia). J. Morph. 2009, 270, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Steffl, M.; Schweiger, M.; Amselgruber, W.M. Expression of transforming growth factor-β3 (TGF-β3) in the porcine ovary during the oestrus cycle. Histol. Histopathol. 2008, 23, 665–671. [Google Scholar] [PubMed]
- Ogielska, M.; Rozenblut, B.; Augustyńska, R.; Kotusz, A. Degeneration of germ line cells in amphibian ovary. Acta Zool. 2010, 91, 319–327. [Google Scholar] [CrossRef]
- Scaia, M.F.; Czuchlej, S.C.; Cervino, N.; Ceballos, N.R. Apoptosis, proliferation and presence of estradiol receptors in the testes and Bidder’s organ of the toad Rhinella arenarum (Amphibia, Anura). J. Morph. 2015, 277, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Greven, H. The urodele oviduct and its secretions in and after G. von Wahlert’s doctoral thesis “Eileiter, Laich und Kloake der Salamandriden”. Bonner Zool. Monogr. 2002, 50, 25–61. [Google Scholar]
- Medina, M.F.; Ramos, I.; Crespo, C.A.; González-Calvar, S.; Fernández, S.N. Changes in serum sex steroid levels throughout the reproductive cycle of Bufo arenarum females. Gen. Comp. Endocrinol. 2004, 136, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Rose, C.S. Hormonal control in larval development and evolution—amphibians. In The Origin and Evolution of Larval Forms; Hall, B.K., Wake, M.H., Eds.; Academic Press: San Diego, CA, USA, 1999; pp. 167–216. [Google Scholar]
- Exbrayat, J.-M. Quelques aspects de la biologie de la reproduction chez Typhlonectes compressicaudus (Duméril et Bibron, 1841). Ph.D. Thesis, Paris VI University, Paris, France, 1986. [Google Scholar]
- Exbrayat, J.-M. Le testicule de Typhlonectes compressicaudus. Structure, ultrastructure, croissance et cycle de reproduction. Mém. Soc. Zool. Fr. 1986, 43, 121–132. [Google Scholar]
- Exbrayat, J.-M. Premières observations sur le cycle annuel de l’ovaire de Typhlonectes compressicaudus (Duméril et Bibron, 1841), Batracien Apode vivipare. C R Acad. Sci. 1983, 296, 493–498. [Google Scholar]
- Exbrayat, J.-M. Variations histologiques des voies génitales femelles au cours de la reproduction de Typhlonectes compressicaudus, Amphibien Apode. Bull. Soc. Herpetol. Fr. 1984, 29, 67–68. [Google Scholar]
- Exbrayat, J.-M. Croissance et cycle des voies génitales femelles de Typhlonectes compressicaudus (Duméril et Bibron, 1841), Amphibien Apode vivipare. Amphibia Reptilia 1988, 9, 117–134. [Google Scholar] [CrossRef]
- Exbrayat, J.-M. Reproduction and development of Typhlonectes compressicauda as a contribution to the knowledge of the biology of caecilians, the least known amphibian order. Recent Res. Dev. Life Sci. 2005, 3, 215–239. [Google Scholar]
- Wake, M.H. A brief history of research on Gymnophionan. In Reproductive Biology and Phylogeny of Gymnophiona: Caecilians; Jamieson, B.G.M., Exbrayat, J.-M., Eds.; Science Publishers: Enfield, NH, USA, 2006; pp. 1–37. [Google Scholar]
- Wilkinson, M.; Nussbaum, R.A. Caecilian phylogeny and classification. In Reproductive Biology and Phylogeny of Gymnophiona: Caecilians; Jamieson, B.G.M., Exbrayat, J.-M., Eds.; Science Publishers: Enfield, NH, USA, 2006; pp. 39–78. [Google Scholar]
- Exbrayat, J.-M.; Estabel, J. Anatomy to particular reference to the reproductive system. In Reproductive Biology and Phylogeny of Gymnophiona: Caecilians; Jamieson, B.G.M., Exbrayat, J.-M., Eds.; Science Publishers: Enfield, NH, USA, 2006; pp. 79–155. [Google Scholar]
- Exbrayat, J.-M.; Raquet, M. Vertebrate evolution: The strange case of gymnophionan amphibians. In Evolutionary Biology from Concepts to Application; Pontarotti, P., Ed.; Springer Berlin Heidelberg: Berlin, Germany, 2009; pp. 71–89. [Google Scholar]
- Taylor, E.H. The Caecilians of the World; University Kansas Press: Lawrence, KS, USA, 1968. [Google Scholar]
- San Mauro, D.; Gower, D.J.; Müller, H.S.; Loader, P.; Zardoya, R.A.; Nussbaum, R.; Wilkinson, M. Life-history evolution and mitogenomic phylogeny of caecilian amphibians. Mol. Phylogenet. Evol. 2014, 73, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Kupfer, A.; Maxwell, E.; Reinhard, S.; Kuehnel, S. The evolution of parental investment in caecilian amphibians: A comparative approach. Biol. J. Linn. Soc. 2016, 119, 4–14. [Google Scholar] [CrossRef]
- Exbrayat, J.-M. Endocrinology of reproduction. In Reproductive Biology and Phylogeny of Gymnophiona: Caecilians; Jamieson, B.G.M., Exbrayat, J.-M., Eds.; Science Publishers: Enfield, NH, USA, 2006; pp. 183–229. [Google Scholar]
- Exbrayat, J.-M. Oogenesis and folliculogenesis. In Reproductive Biology and Phylogeny of Gymnophiona: Caecilians; Jamieson, B.G.M., Exbrayat, J.-M., Eds.; Science Publishers: Enfield, NH, USA, 2006; pp. 275–290. [Google Scholar]
- Exbrayat, J.-M. Modes of parity. In Reproductive Biology and Phylogeny of Gymnophiona: Caecilians; Jamieson, B.G.M., Exbrayat, J.-M., Eds.; Science Publishers: Enfield, NH, USA, 2006; pp. 303–323. [Google Scholar]
- Anjubault, E.; Exbrayat, J.-M. Embryonic development of gonads and sexual maturity. In Reproductive Biology and Phylogeny of Gymnophiona: Caecilians; Jamieson, B.G.M., Exbrayat, J.-M., Eds.; Science Publishers: Enfield, NH, USA, 2006; pp. 291–302. [Google Scholar]
- Exbrayat, J.-M.; Hraoui-Bloquet, S. Viviparity in Typhlonectes compressicauda. In Reproductive Biology and Phylogeny of Gymnophiona: Caecilians; Jamieson, B.G.M., Exbrayat, J.-M., Eds.; Science Publishers: Enfield, NH, USA, 2006; pp. 325–357. [Google Scholar]
- Hraoui-Bloquet, S. Nutrition embryonnaire et relations materno-fœtales chez Typhlonectes compressicauda (Duméril et Bibron, 1841), Amphibien Gymnophione vivipare. Ph.D. Thesis, Ecole Pratique des Hautes Etudes, Paris, France, 1995. [Google Scholar]
- Key, G.; Kubbutat, M.H.; Gerdes, J. Assessment of cell proliferation by means of an enzyme-linked immunosorbent assay based on the detection of the Ki-67 protein. J. Immunol. Methods 1994, 177, 113–117. [Google Scholar] [CrossRef]
- Brun, C.; Moudilou, E. Visualization of apoptosis. In Histohemical and Cytochemical Methods of Visualization; Exbrayat, J.-M., Ed.; CRC Press: Boca Raton, FL, USA, 2013; pp. 165–174. [Google Scholar]
- Kanamadi, R.D.; Saidapur, S.K. Pattern of ovarian activity in the Indian toad Bufo melanostictus (Schn). Proc. Natl. Sci. Acad. India B 1982, 48, 307–316. [Google Scholar]
- Tilly, J.L.; Kowalski, K.I.; Johnson, A.L.; Hsueh, A.J. Involvement of apoptosis in ovarian follicular atresia and postovulatory regression. Endocrinology 1991, 129, 2799–2801. [Google Scholar] [CrossRef] [PubMed]
- Kaipia, A.; Hsueh, A.J. Regulation of ovarian follicle atresia. Annu. Rev. Physiol. 1997, 59, 349–363. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, F.; Inoue, N.; Manabe, N.; Ohkura, S. Follicular growth and atresia in mammalian ovaries: Regulation by survival and death of granulosa cells. J. Reprod. Dev. 2012, 58, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, M.; Prasad, S.; Tripathi, A.; Pandey, A.N.; Ali, I.; Singh, A.K.; Chaube, S.K. Apoptosis in mammalian oocytes: A review. Apoptosis 2015, 20, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Havelka, P.; Marciniaková, A.; Lichnovsky, V. The role of programmed cell death and the genes involved in regulation of programmed cell death in differentiation of human embryo myocard. Biomed. Pap. Palacky Univ. Olomouc 2001, 145, 106. [Google Scholar]
- Kubínyiová, M.; Pospísilová, E. Role of apoptosis and proliferation in differentiation of human retina, regio olfactoria and regio respiratoria. Biomed. Pap. Palacky Univ. Olomouc 2001, 145, 65–68. [Google Scholar] [CrossRef]
- Prochazkova, J.; Kylarova, D.; Vranka, P.; Lichnovsky, V. Comparative study of apoptosis-detecting techniques: TUNEL, apostain, and lamin B. Biotechniques 2003, 35, 528–533. [Google Scholar] [PubMed]
- Teshima, T.H.N.; Wells, K.L.; Lourenço, S.V.; Tucker, A.S. Apoptosis in early salivary gland duct morphogenesis and lumen formation. J. Dent. Res. 2015. Available online: http://78.39.227.9/handle/Hannan/72388 (accessed on 16 December 2016). [Google Scholar]
- Greven, H. Oviduct and egg-jelly. In Reproductive Biology and Phylogeny of Urodela; Jamieson, B.G.M., Sever, D.M., Eds.; CRC Press: Boca Raton, FL, USA, 2003; pp. 151–181. [Google Scholar]
- Thompson, E.B. Apoptosis and steroid hormones. Mol. Endocrinol. 1994, 8, 665–673. [Google Scholar] [PubMed]
- Ji, Z.-S.; Kubokawa, K.; Abé, S.-I. Promotion of differentiation of newt primary spermatocytes into spermatids by mammalian FSH via Sertoli cells. J. Exp. Zool. 1995, 272, 374–383. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, R.; Iela, L.; di Meglio, M.; di Fiore, M.M.; D’Aniello, B.; Pinelli, C.; Fiorentino, M. Hormonal regulation of reproductive cycles in amphibians. In Amphibian Zoology; Heatwole, H., Ed.; Surrey Betty and Sons: Chipping Norton, Australia, 2005; Volume 6, pp. 2045–2177. [Google Scholar]
- Shao, R.; Weijdegard, B.; Ljungström, K.; Friberg, A.; Zhu, C.; Wang, X.; Zhu, Y.; Fernandez-Rodriguez, J.; Egecioglu, E.; Rung, E.; et al. Nuclear progesterone receptor A and B isoforms in mouse Fallopian tube and uterus: Implications for expression, regulation, and cellular function. Am. J. Physiol. Endocr. Metab. 2006, 291, E59–E72. [Google Scholar] [CrossRef] [PubMed]
- Mintz, P.J.; Habib, N.A.; Jones, L.J.; Giamas, G.; Lewis, J.S.; Bowen, R.L.; Coombes, R.C.; Stebbing, J. The phosphorylated membrane estrogen receptor and cytoplasmic signaling an apoptosis protein human breast cancer. Cancer 2008, 113, 1489–1495. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, S.; Ohara, N.; Xu, Q.; Chen, W.; Wang, J.; Nakabayashi, K.; Sasaki, H.; Morikawa, A.; Maruo, T. Cell-type specific actions of progesterone receptor modulators in the regulationof uterine Leiomyoma growth. Semin. Reprod. Med. 2010, 57, 88–98. [Google Scholar]
- Wilkanowska, A.; Mazurowski, A.; Mroczkowski, S.; Kokoszyński, D. Prolactin (PRL) and prolactin receptor (PRLR) genes and their role in poultry production traits. Folia Biol. 2014, 62, 1–8. [Google Scholar] [CrossRef]
- Ghosh, J.; Dhara, S.K.; Malik, P.K.; Bhatta, R.; Takahashi, J.; Kohn, R.A.; Prasad, C.S. Climate change: Effects on animal reproduction. In Livestock Production and Climate Change; CABI: Wallingford, UK, 2015; p. 183. [Google Scholar]
- Exbrayat, J.-M.; Lescure, J. Quelques aperçus sur l’écologie et l’éthologie des Gymnophiones. Bull. Soc. Herpetol. Fr. 1995, 69, 1–18. [Google Scholar]
- Exbrayat, J.-M. Variations pondérales des organes de réserve (corps adipeux et foie) chez Typhlonectes compressicaudus, Amphien Apode vivipare, au cours des alternances saisonnières et des cycles de reproduction. Ann. Sci. Nat. Zool. Biol. Anim. 1988, 9, 45–53. [Google Scholar]

| Season | N | Tissue | Proliferation | Apoptosis |
|---|---|---|---|---|
| Reproduction (pregnant females) | 2 | Connective tissue | 15.8 ± 1.2 | 14.3 ± 3.3 |
| Oogonia | 43.3 ± 9.4 | 8.3 ± 2.3 | ||
| Sexual rest (quiescent females) | 2 | Connective tissue | 0 | 10.7 ± 2.5 (A) |
| Oogonia | 14.6 ± 2.9 | 0 | ||
| After parturition | 2 | Connective tissue | 13.5 ± 2.1 | 73.1 ± 9.7 |
| Season | N | Tissue | Proliferation | Apoptosis |
|---|---|---|---|---|
| Reproduction (pregnant females) | 2 | Theca | 11.2 ± 1.8 | 14.5 ± 4.9 |
| Granulosa | 28.2 ± 4.6 | 11.3 ± 5.2 | ||
| Sexual rest (quiescent females) | 2 | Theca | 2.5 ± 3.5 | 32.5 ± 3.5 |
| Granulosa | 22.5 ± 3.5 | 26.2 ± 5.3 | ||
| After parturition or at the end of the second year | 2 | Theca | 24.0 ± 1.4 | 13.5 ± 2.1 |
| Granulosa | 29.2 ± 2.0 | 23.3 ± 4.7 | ||
| Preparation for reproduction | 2/0 | Theca | 27.5 ± 3.5 | |
| Granulosa | 18.3 ± 2.3 |
| Season | N | Tissue | Young Follicles | Mature Follicles | ||
|---|---|---|---|---|---|---|
| Proliferation | Apoptosis | Proliferation | Apoptosis | |||
| Reproduction (pregnant females) | 2 | Theca | 13.3 ± 4.7 | 10.2 ± 3.2 | 14.7 ± 3.9 | 13.3 ± 4.7 |
| Granulosa | 36.5 ± 4.7 | 39.1 ± 8.3 | 41.2 ± 1.8 | 43.3 ± 4.7 | ||
| Sexual rest (quiescent females) | 2 | Theca | 0 | 25.5 ± 2.5 25 ± 1.4 (A) | 0 | 16.5 ± 2.1 17.5 ± 3.5 (A) |
| Granulosa | 17.5 ± 3.5 | 23 ± 4.2 21 ± 2.8 (A) | 34.5 ± 2.1 | 16.5 ± 2 | ||
| After parturition or at the end of the second year | 2/0 | Theca | 15.8 ± 1.2 | 3.2 ± 2.4 | ||
| Granulosa | 48.5 ± 4.9 | 21.5 ± 4.9 | ||||
| Preparation for reproduction | 2 | Theca | 14.9 ± 2.5 | 14.2 ± 5 | ||
| Granulosa | 55 ± 7.1 | 33.3 ± 4.7 | ||||
| Season | N | Tissue | Proliferation | Apoptosis |
|---|---|---|---|---|
| Reproduction (pregnant females) | 2 | Peripheral cells | 0 | 49.8 ± 5.3 |
| Invasive cells | 77.5 ± 3.5 | 0 | ||
| Sexual rest (quiescent females) | 2 | Peripheral cells | 0 | 36.5 ± 4.9 (A) |
| Invasive cells | 61.0 ± 5.6 | 15.2 ± 4.6 (A) | ||
| After parturition or at the end of the second year | 2 | Peripheral cells | 0 | 8.7 ± 1.8 |
| Invasive cells | 25.8 ± 1.2 | 29.1 ± 5.9 | ||
| Preparation for reproduction | 2 | Peripheral cells | 27.5 ± 3.5 | 31.3 ± 5.2 |
| Invasive cells | 31.2 ± 2.3 | 40.8 ± 5.9 |
| N | Cell Type | Proliferation | Apoptosis |
|---|---|---|---|
| 2 | Peripheral cells | 14.7 ± 0.3 | 18.5 ± 2.31 |
| Internal cells | 35.8 ± 1.2 | 20.8 ± 5.9 |
| Season | N | Tissue | Proliferation | Apoptosis |
|---|---|---|---|---|
| Reproduction (pregnant females) | 2 | Connective cells | 0 | 18.3 ± 2.3 |
| Ciliated cells | 31.6 ± 2.3 | 27.6 ± 2.3 | ||
| Sexual rest (quiescent females) | 2 | Connective cells | 7.2 ± 3.2 | 22.5 ± 3.2 18.4 ± 2.2 (A) |
| Ciliated cells | 36.8 ± 4.5 | 18.3 ± 2.3 | ||
| After parturition or at the end of the second year | 2 | Connective cells | 18.2 ± 1.9 | 38.5 ± 4.9 |
| Ciliated cells | 37.5 ± 3.5 | 51.7 ± 2.5 | ||
| Preparation for reproduction | 2 | Connective cells | 38.3 ± 2.3 | |
| Ciliated cells | 43.5 ± 2.1 | 75.0 ± 7.1 (A) |
| Season | N | Tissue | Proliferation | Apoptosis |
|---|---|---|---|---|
| Reproduction (pregnant females) | 2 | Connective cells | 36.7 ± 4.7 | 30.0 ± 4.7 38.3 ± 2.3 (A) |
| Secretory cells | 21.9 ± 1.6 | 20.8 ± 5.9 30.6 ± 3.7 (A) | ||
| Ciliated cells | 34.3 ± 8.1 | 20.5 ± 0.7 30.0 ± 4.7 (A) | ||
| Sexual rest (quiescent females) | 2 | Connective cells | 3.6 ± 2.0 | 13.7 ± 1.8 |
| Secretory cells | 16.8 ± 4.5 | 6.7 ± 2.5 | ||
| Ciliated cells | 0 | 5.7 ± 1.0 | ||
| After parturition or at the end of the second year | 2 | Connective cells | 37.5 ± 3.5 | 53.3 ± 4.7 |
| Secretory cells | 14.5 ± 4.3 | 22.5 ± 3.5 | ||
| Ciliated cells | 20.8 ± 5.9 | 42.5 ± 3.5 | ||
| Preparation for reproduction | 2 | Connective cells | 40.3 ± 6.7 | 21.7 ± 4.6 20.8 ± 1.2 (A) |
| Secretory cells | 24.6 ± 2.9 | 29.1 ± 5.9 30.6 ± 3.7 (A) | ||
| Ciliated cells | 66.0 ± 5.7 | 27.5 ± 3.5 25.8 ± 1.2 (A) |
| Season | N | Tissue | Ki67 | TUNEL |
|---|---|---|---|---|
| Reproduction (pregnant females) | 2 | Connective cells | 27.5 ± 3.5 | 36.6 ± 4.7 |
| Secretory cells | 20.4 ± 0.6 | 10.7 ± 1.1 | ||
| Ciliated cells | 35.5 ± 3.5 | 28.3 ± 2.3 | ||
| Sexual rest (quiescent females) | 2 | Connective cells | 16.5 ± 2.1 | 14.3 ± 3.3 |
| Secretory cells | 33.2 ± 2.5 | 18.4 ± 2.7 | ||
| Ciliated cells | 0 | 34.1 ± 1.2 | ||
| After parturition or at the end of the second year | 2 | Connective cells | 58.3 ± 2.6 | 43.7 ± 1.7 41.6 ± 2.3 (A) |
| Secretory cells | 19.6 ± 4.1 | 24.3 ± 3.3 35.0 ± 7.1 (A) | ||
| Ciliated cells | 10.7 ± 1.1 | 33.3 ± 4.7 | ||
| Preparation for reproduction | 2 | Connective cells | 12.5 ± 3.5 | |
| Secretory cells | 36.6 ± 4.7 | |||
| Ciliated cells | 36.8 ± 4.5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raquet, M.; Brun, C.; Exbrayat, J.-M. Patterns of Apoptosis and Proliferation throughout the Biennial Reproductive Cycle of Viviparous Female Typhlonectes compressicauda (Amphibia, Gymnophiona). Int. J. Mol. Sci. 2017, 18, 16. https://doi.org/10.3390/ijms18010016
Raquet M, Brun C, Exbrayat J-M. Patterns of Apoptosis and Proliferation throughout the Biennial Reproductive Cycle of Viviparous Female Typhlonectes compressicauda (Amphibia, Gymnophiona). International Journal of Molecular Sciences. 2017; 18(1):16. https://doi.org/10.3390/ijms18010016
Chicago/Turabian StyleRaquet, Michel, Claire Brun, and Jean-Marie Exbrayat. 2017. "Patterns of Apoptosis and Proliferation throughout the Biennial Reproductive Cycle of Viviparous Female Typhlonectes compressicauda (Amphibia, Gymnophiona)" International Journal of Molecular Sciences 18, no. 1: 16. https://doi.org/10.3390/ijms18010016
APA StyleRaquet, M., Brun, C., & Exbrayat, J.-M. (2017). Patterns of Apoptosis and Proliferation throughout the Biennial Reproductive Cycle of Viviparous Female Typhlonectes compressicauda (Amphibia, Gymnophiona). International Journal of Molecular Sciences, 18(1), 16. https://doi.org/10.3390/ijms18010016






