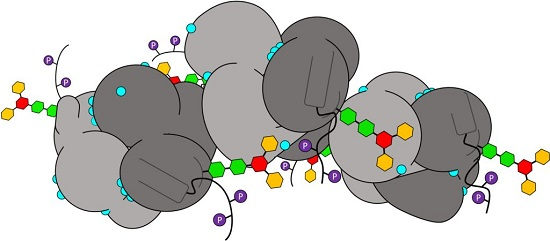
Characterization of Post-Translational Modifications to Calsequestrins of Cardiac and Skeletal Muscle
Abstract
:1. Introduction
2. Results
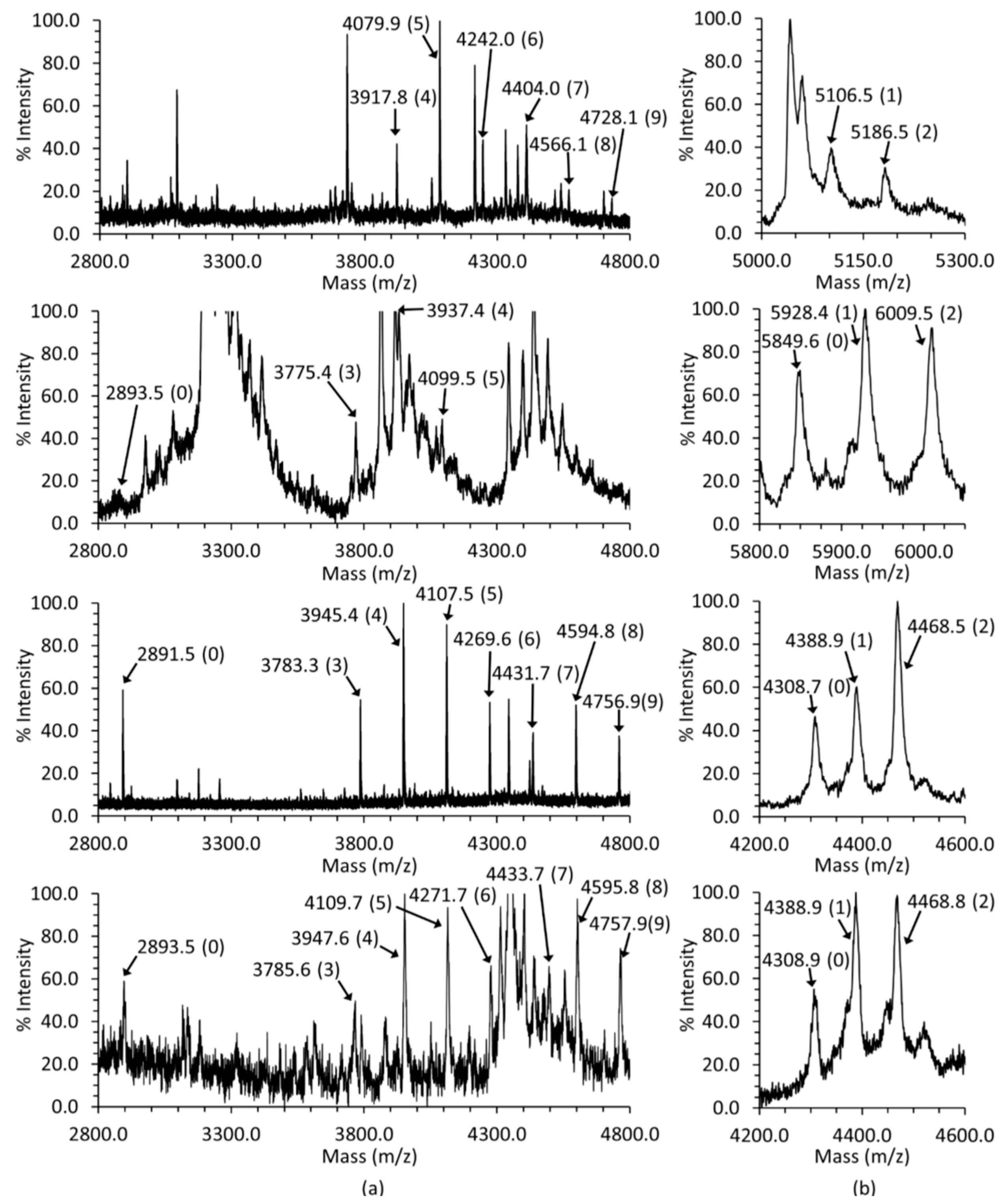
2.1. Glycosylation and Phosphorylation of Casq1
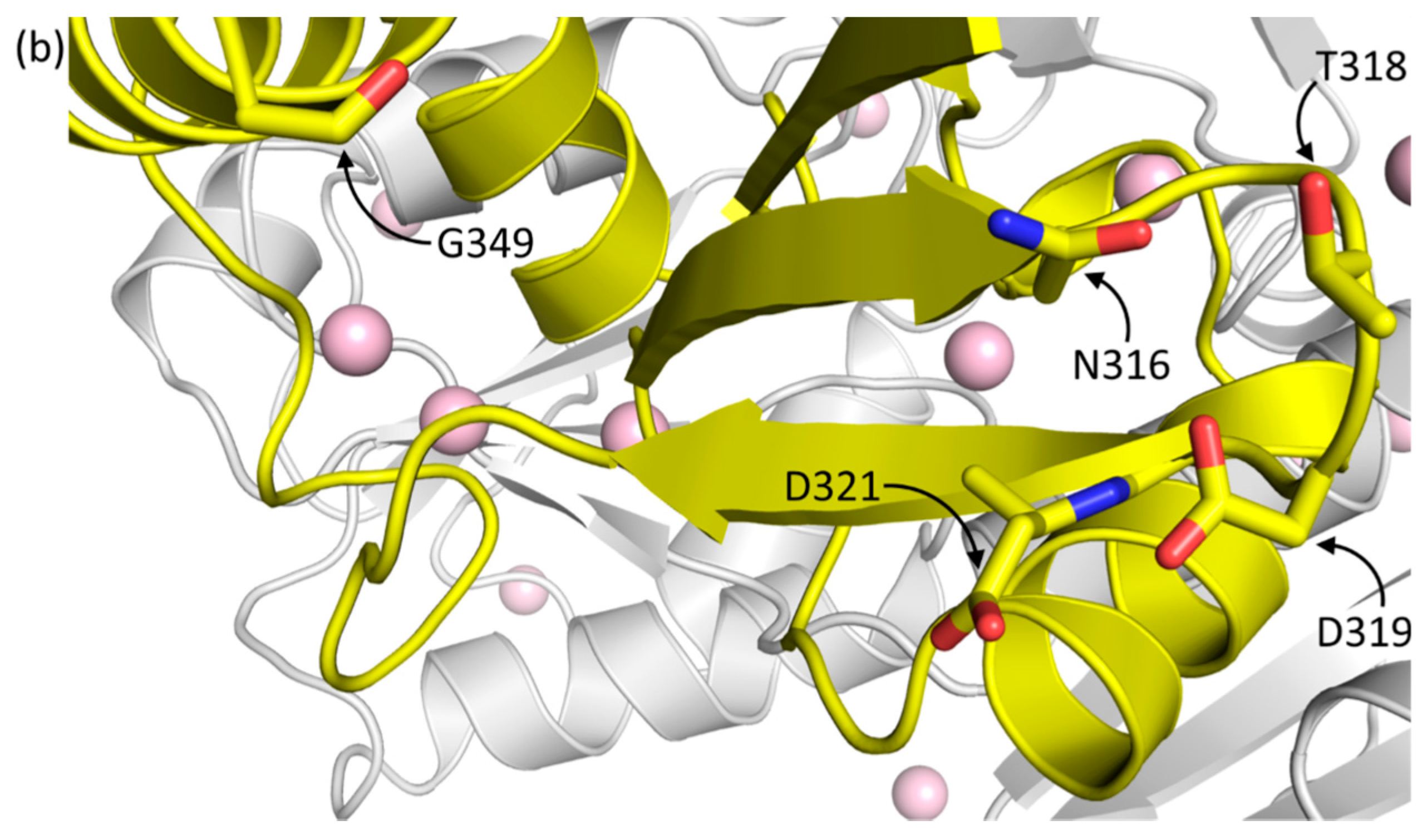
2.1.1. Casq1 Glycosylation
2.1.2. Casq1 Phosphorylation
2.2. Glycosylation and Phosphorylation of Casq2
2.2.1. Casq2 Glycosylation
2.2.2. Casq2 Phosphorylation
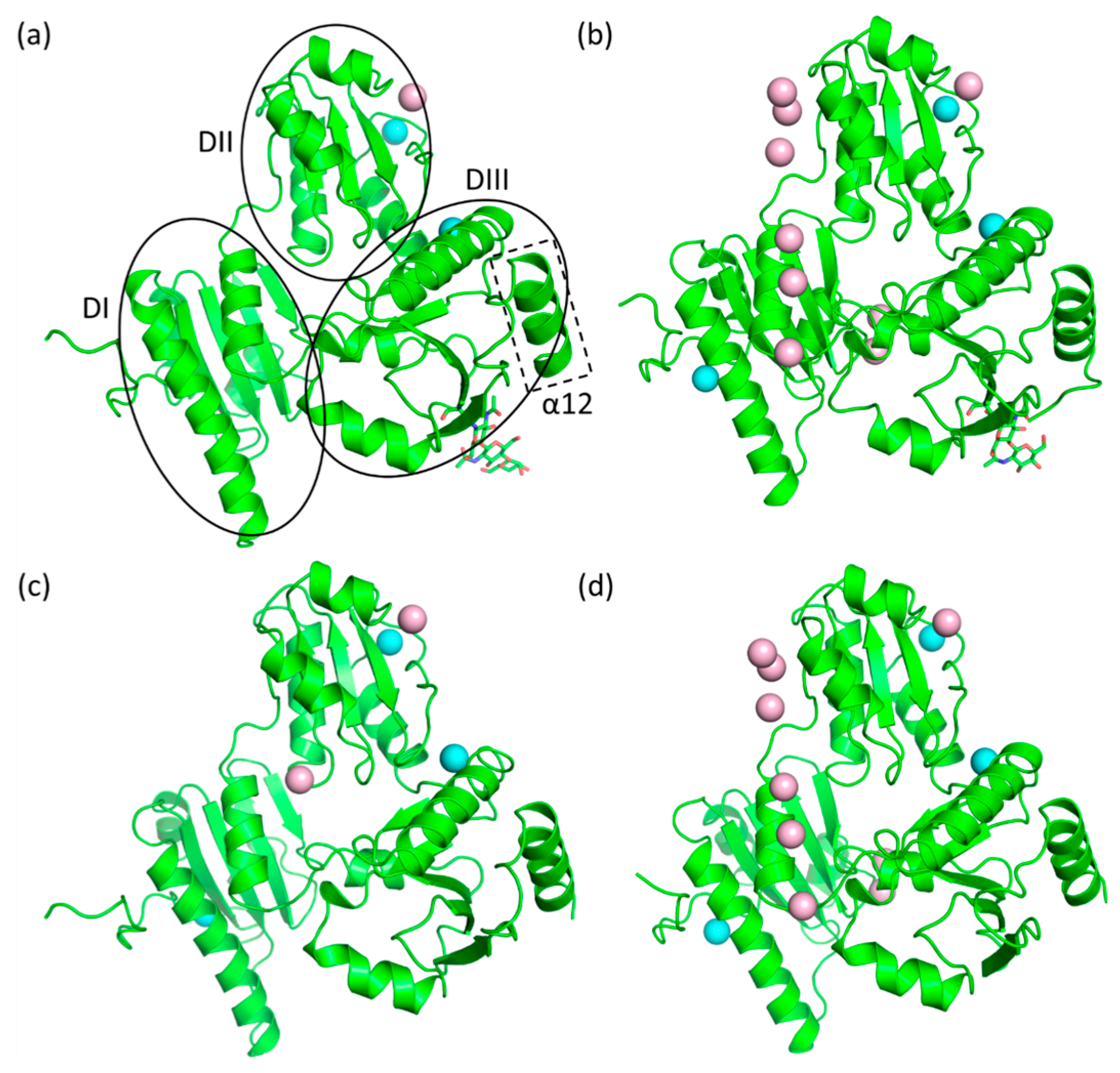
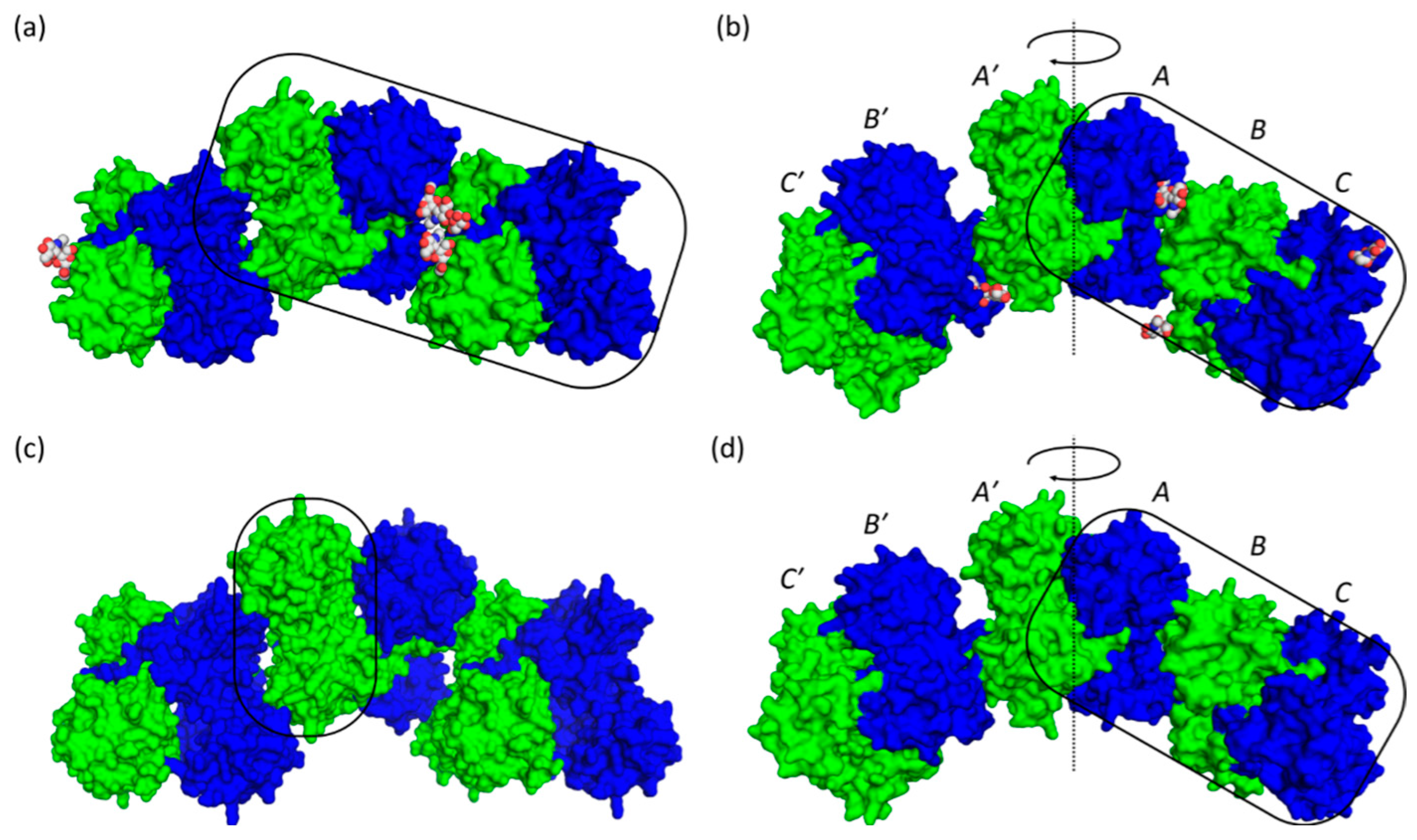
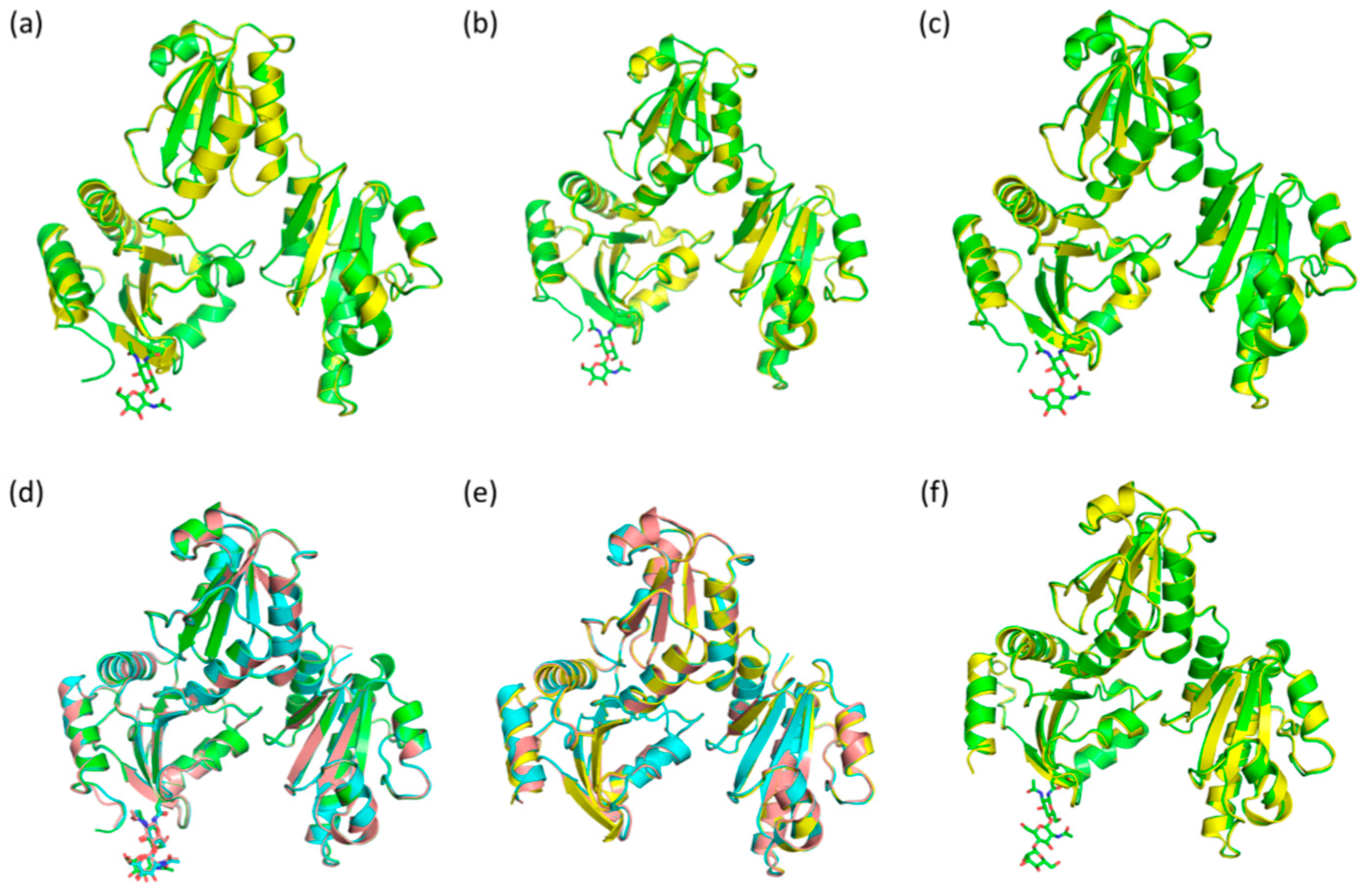
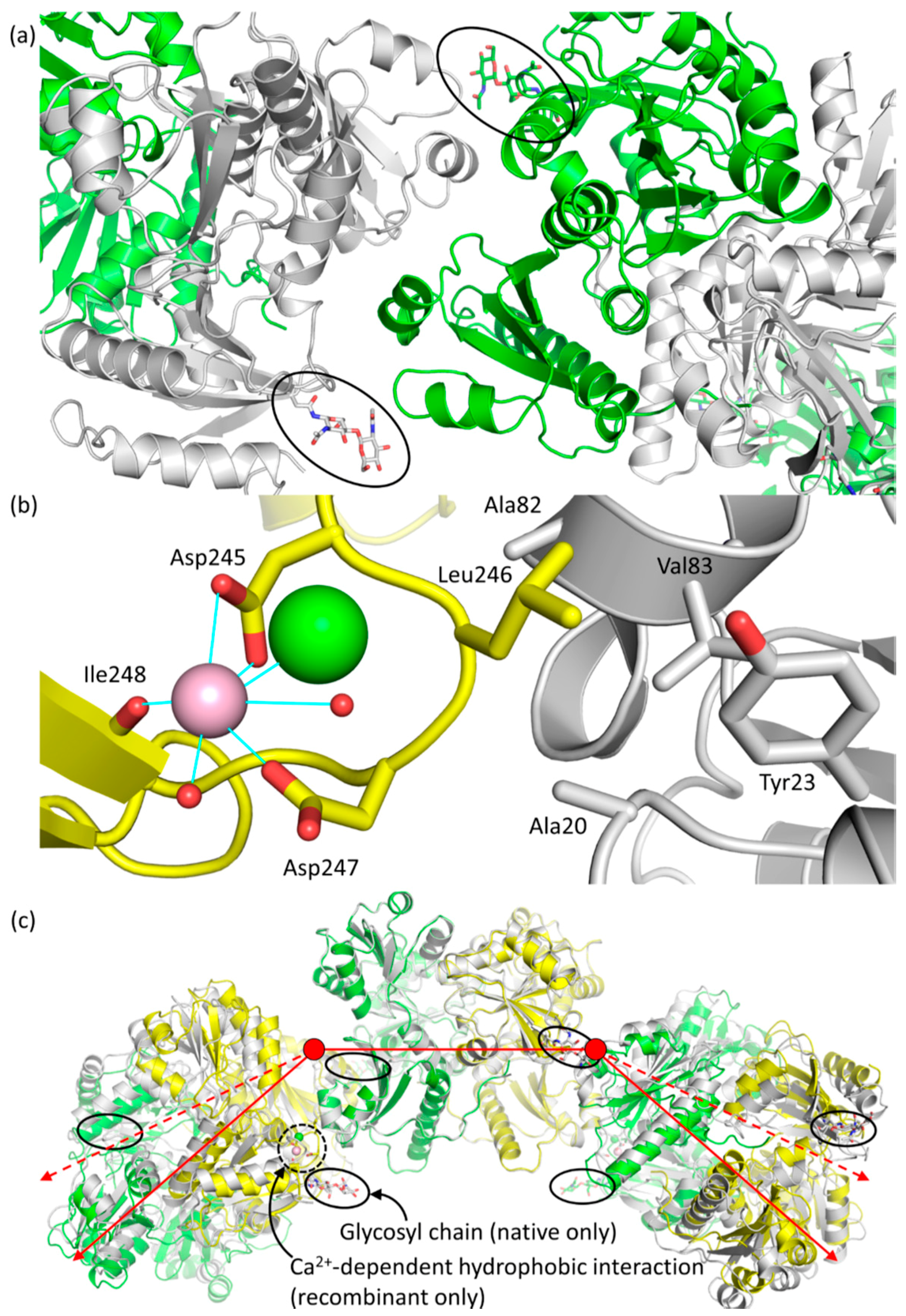
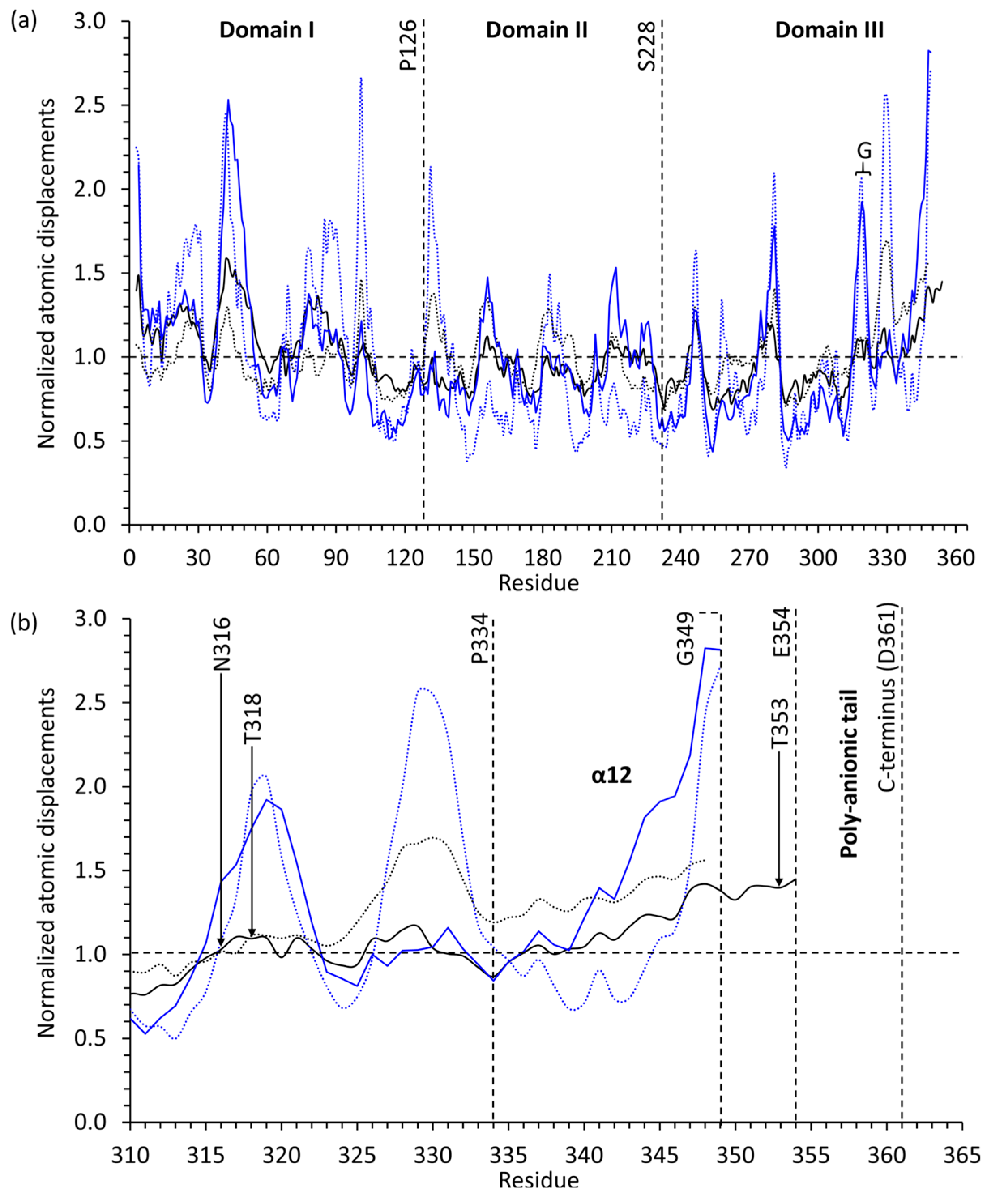
2.3. Casq1 Crystal Structures
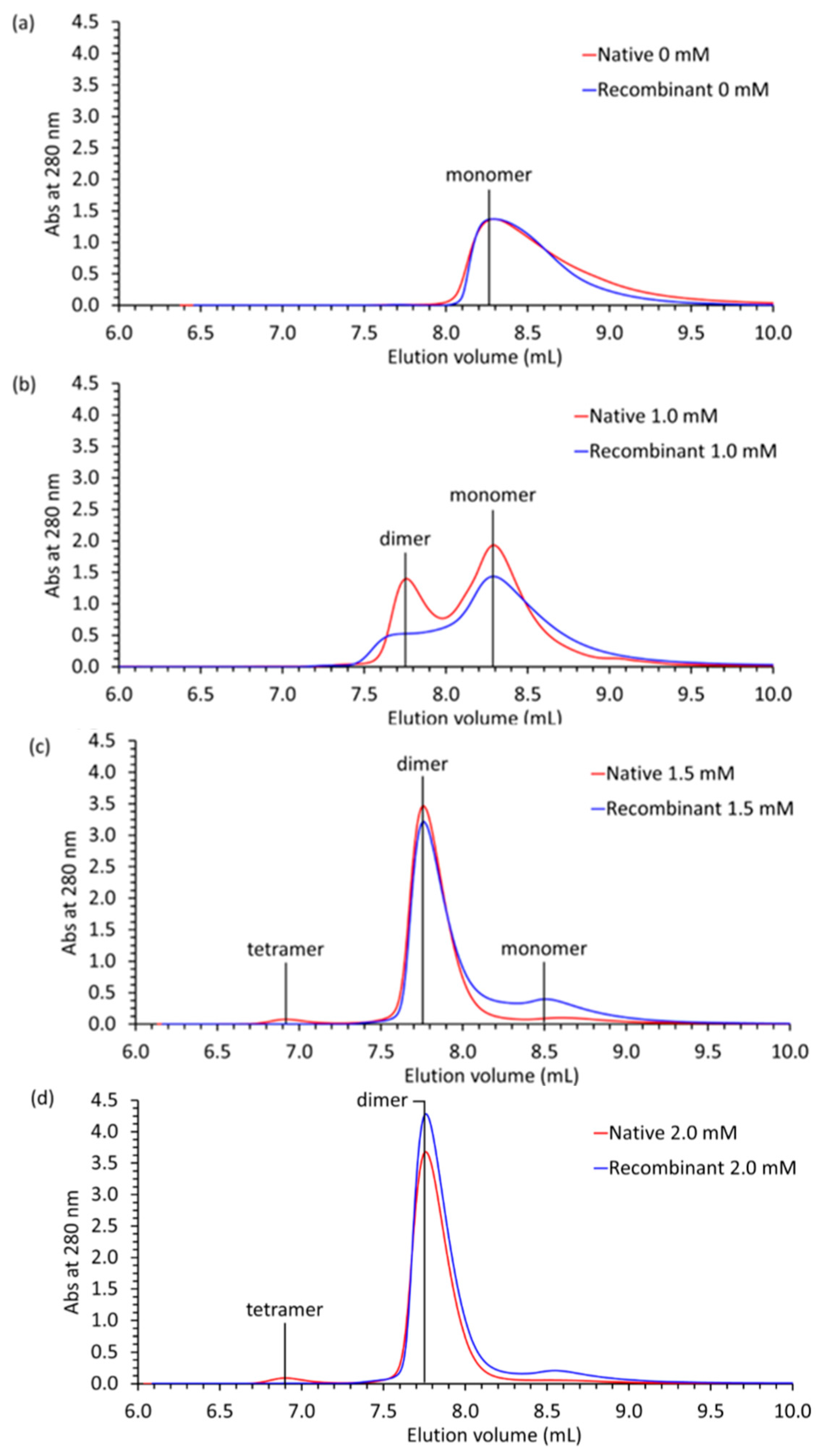
2.4. Multi-Angle Light Scattering
3. Discussion
3.1. Evolution
3.2. Glycosylation
3.3. Phosphorylation
3.4. Clinical Implications
4. Materials and Methods
4.1. Isolation of Cardiac and Skeletal Muscle Tissue
4.2. Recombinant Bovine Casq1 Gene Synthesis
4.3. Purification of Native Casq1 and Casq2
4.4. Purification of Recombinant Bovine Casq1
4.5. Mass Spectrometry
4.6. Multi-Angle Light Scattering
4.7. Crystallization
Acknowledgments
Author Contributions
Conflicts of Interest
References
- MacLennan, D.H.; Wong, P.T. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc. Natl. Acad. Sci. USA 1971, 68, 1231–1235. [Google Scholar] [CrossRef] [PubMed]
- Cozens, B.; Reithmeier, R.A. Size and shape of rabbit skeletal muscle calsequestrin. J. Biol. Chem. 1984, 259, 6248–6252. [Google Scholar] [PubMed]
- Royer, L.; Rios, E. Deconstructing calsequestrin. Complex buffering in the calcium store of skeletal muscle. J. Physiol. 2009, 587, 3101–3111. [Google Scholar] [CrossRef] [PubMed]
- Perni, S.; Close, M.; Franzini-Armstrong, C. Novel details of calsequestrin gel conformation in situ. J. Biol. Chem. 2013, 288, 31358–31362. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.J.; Lewis, K.M.; Danna, B.R.; Kang, C. High-capacity Ca2+ binding of human skeletal calsequestrin. J. Biol. Chem. 2012, 287, 11592–11601. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Park, I.Y.; Kim, E.; Youn, B.; Fields, K.; Dunker, A.K.; Kang, C. Comparing skeletal and cardiac calsequestrin structures and their calcium binding: A proposed mechanism for coupled calcium binding and protein polymerization. J. Biol. Chem. 2004, 279, 18026–18033. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Wu, S.; Dunker, A.K.; Kang, C. Polymerization of calsequestrin. Implications for Ca2+ regulation. J. Biol. Chem. 2003, 278, 16176–16182. [Google Scholar] [CrossRef] [PubMed]
- Tijskens, P.; Jones, L.R.; Franzini-Armstrong, C. Junctin and calsequestrin overexpression in cardiac muscle: The role of junctin and the synthetic and delivery pathways for the two proteins. J. Mol. Cell Cardiol. 2003, 35, 961–974. [Google Scholar] [CrossRef]
- Franzini-Armstrong, C.; Protasi, F.; Tijskens, P. The assembly of calcium release units in cardiac muscle. Ann. N. Y. Acad. Sci. 2005, 1047, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Manno, C.; Figueroa, L.; Gillespie, D.; Rios, E. Calsequestrin depolymerizes when Ca2+ concentration decays in the sarcoplasmic reticulum of skeletal muscle. Biophys. J. 2016. [Google Scholar] [CrossRef]
- Adam, G.; Delbrück, M. Reduction of dimensionality in biological diffusion processes. In Structural Chemistry and Molecular Biology; Rich, A., Davidson, N.R., Eds.; W.H. Freeman and Company: San Francisco, CA, USA, 1968; pp. 198–215. [Google Scholar]
- Wang, S.; Trumble, W.R.; Liao, H.; Wesson, C.R.; Dunker, A.K.; Kang, C.H. Crystal structure of calsequestrin from rabbit skeletal muscle sarcoplasmic reticulum. Nat. Struct. Biol. 1998, 5, 476–483. [Google Scholar] [CrossRef] [PubMed]
- MacLennan, D.H.; Reithmeier, R.A. Ion tamers. Nat. Struct. Biol. 1998, 5, 409–411. [Google Scholar] [CrossRef] [PubMed]
- McFarland, T.P.; Milstein, M.L.; Cala, S.E. Rough endoplasmic reticulum to junctional sarcoplasmic reticulum trafficking of calsequestrin in adult cardiomyocytes. J. Mol. Cell. Cardiol. 2010, 49, 556–564. [Google Scholar] [CrossRef] [PubMed]
- O'Brian, J.J.; Ram, M.L.; Kiarash, A.; Cala, S.E. Mass spectrometry of cardiac calsequestrin characterizes microheterogeneity unique to heart and indicative of complex intracellular transit. J. Biol. Chem. 2002, 277, 37154–37160. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.J.; Lewis, K.M.; Munske, G.R.; Nissen, M.S.; Kang, C. Glycosylation of skeletal calsequestrin: Implications for its function. J. Biol. Chem. 2012, 287, 3042–3050. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.J.; Munske, G.R.; Criswell, A.; Milting, H.; Dunker, A.K.; Kang, C. Phosphorylation of human calsequestrin: Implications for calcium regulation. Mol. Cell. Biochem. 2011, 353, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Faggioni, M.; Kryshtal, D.O.; Knollmann, B.C. Calsequestrin mutations and catecholaminergic polymorphic ventricular tachycardia. Pediatr. Cardiol. 2012, 33, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Faggioni, M.; Knollmann, B.C. Calsequestrin 2 and arrhythmias. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, 1250–1260. [Google Scholar] [CrossRef] [PubMed]
- Kirchhefer, U.; Wehrmeister, D.; Postma, A.V.; Pohlentz, G.; Mormann, M.; Kucerova, D.; Muller, F.U.; Schmitz, W.; Schulze-Bahr, E.; Wilde, A.A.; et al. The human Casq2 mutation K206N is associated with hyperglycosylation and altered cellular calcium handling. J. Mol. Cell. Cardiol. 2010, 49, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K.M.; Ronish, L.A.; Rios, E.; Kang, C. Characterization of two human skeletal calsequestrin mutants implicated in malignant hyperthermia and vacuolar aggregate myopathy. J. Biol. Chem. 2015, 290, 28665–28674. [Google Scholar] [CrossRef] [PubMed]
- Kraeva, N.; Zvaritch, E.; Frodis, W.; Sizova, O.; Kraev, A.; MacLennan, D.H.; Riazi, S. Casq1 gene is an unlikely candidate for malignant hyperthermia susceptibility in the north american population. Anesthesiology 2013, 118, 344–349. [Google Scholar] [PubMed]
- Maclennan, D.H.; Zvaritch, E. Mechanistic models for muscle diseases and disorders originating in the sarcoplasmic reticulum. Biochim. Biophys. Acta 2011, 1813, 948–964. [Google Scholar] [CrossRef] [PubMed]
- Benton, M.J.; Donoghue, P.C. Paleontological evidence to date the tree of life. Mol. Biol. Evol. 2007, 24, 26–53. [Google Scholar] [CrossRef] [PubMed]
- Churakov, G.; Sadasivuni, M.K.; Rosenbloom, K.R.; Huchon, D.; Brosius, J.; Schmitz, J. Rodent evolution: Back to the root. Mol. Biol. Evol. 2010, 27, 1315–1326. [Google Scholar] [CrossRef] [PubMed]
- Kiarash, A.; Kelly, C.E.; Phinney, B.S.; Valdivia, H.H.; Abrams, J.; Cala, S.E. Defective glycosylation of calsequestrin in heart failure. Cardiovasc. Res. 2004, 63, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Beard, N.A.; Wei, L.; Cheung, S.N.; Kimura, T.; Varsanyi, M.; Dulhunty, A.F. Phosphorylation of skeletal muscle calsequestrin enhances its Ca2+ binding capacity and promotes its association with junctin. Cell Calcium 2008, 44, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Cala, S.E.; Miles, K. Phosphorylation of the cardiac isoform of calsequestrin in cultured rat myotubes and rat skeletal muscle. Biochim. Biophys. Acta 1992, 1118, 277–287. [Google Scholar] [CrossRef]
- Rodriguez, M.M.; Chen, C.H.; Smith, B.L.; Mochly-Rosen, D. Characterization of the binding and phosphorylation of cardiac calsequestrin by epsilon protein kinase C. FEBS Lett. 1999, 454, 240–246. [Google Scholar] [PubMed]
- Shoshan-Barmatz, V.; Orr, I.; Weil, S.; Meyer, H.; Varsanyi, M.; Heilmeyer, L.M.G. The identification of the phosphorylated 150/160-kDa proteins of sarcoplasmic reticulum, their kinase and their association with the ryanodine receptor. Biochim. Biophys. Acta 1996, 1283, 89–100. [Google Scholar] [CrossRef]
- Varsanyi, M.; Heilmeyer, L.M. Autocatalytic phosphorylation of calsequestrin. FEBS Lett. 1980, 122, 227–230. [Google Scholar] [CrossRef]
- Cala, S.E.; Jones, L.R. Phosphorylation of cardiac and skeletal muscle calsequestrin isoforms by casein kinase II. Demonstration of a cluster of unique rapidly phosphorylated sites in cardiac calsequestrin. J. Biol. Chem. 1991, 266, 391–398. [Google Scholar] [PubMed]
- Beard, N.A.; Dulhunty, A.F. C-terminal residues of skeletal muscle calsequestrin are essential for calcium binding and for skeletal ryanodine receptor inhibition. Skelet Muscle 2015, 5, 6–18. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Youn, B.; Kemper, L.; Campbell, C.; Milting, H.; Varsanyi, M.; Kang, C. Characterization of human cardiac calsequestrin and its deleterious mutants. J. Mol. Biol. 2007, 373, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Park, I.Y.; Kim, E.J.; Park, H.; Fields, K.; Dunker, A.K.; Kang, C. Interaction between cardiac calsequestrin and drugs with known cardiotoxicity. Mol. Pharmacol. 2005, 67, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Tam, M.; Siems, W.F.; Kang, C. Effects of drugs with muscle-related side effects and affinity for calsequestrin on the calcium regulatory function of sarcoplasmic reticulum microsomes. Mol. Pharmacol. 2005, 68, 1708–1715. [Google Scholar] [PubMed]
- Kang, C.; Nissen, M.S.; Sanchez, E.J.; Lam, K.S.; Milting, H. Potential adverse interaction of human cardiac calsequestrin. J. Pharmacol. 2010, 646, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.D.; Afonine, P.V.; Bunkoczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. Phenix: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Painter, J.; Merritt, E.A. TLSMD web server for the generation of multi-group TLS models. J. Appl. Crystallogr. 2006, 39, 109–111. [Google Scholar] [CrossRef]




| Protein and Chain Identity | High-Ca2+ Native | High-Ca2+ Recombinant | |||||
|---|---|---|---|---|---|---|---|
| Chain A | Chain B | Chain C | Chain A | Chain B | Chain C | ||
| High-Ca2+ recombinant | Chain C | 0.245 | 0.269 | 0.234 | 0.401 | 0.391 | 0 |
| Chain B | 0.380 | 0.412 | 0.399 | 0.535 | 0 | – | |
| Chain A | 0.438 | 0.464 | 0.435 | 0 | – | – | |
| High-Ca2+ native | Chain C | 0.205 | 0.228 | 0 | – | – | – |
| Chain B | 0.234 | 0 | – | – | – | – | |
| Chain A | 0 | – | – | – | – | – | |
| Protein | btCasq2 | cfCasq2 | hsCasq2 | mmCasq2 | ocCasq2 | rnCasq2 | rnCasq1 | ocCasq1 | mmCasq1 | hsCasq1 | cfCasq1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| btCasq1 | 69.53 | 69.81 | 70.08 | 68.70 | 68.98 | 68.70 | 95.84 | 94.74 | 95.01 | 94.18 | 95.84 |
| (93.35) | (92.80) | (93.35) | (92.80) | (91.97) | (92.24) | (99.45) | (98.89) | (99.45) | (99.17) | (99.17) | |
| cfCasq1 | 69.21 | 70.30 | 69.75 | 68.39 | 68.94 | 68.39 | 96.19 | 96.73 | 95.37 | 96.41 | – |
| (93.73) | (93.19) | (94.01) | (92.64) | (92.64) | (92.64) | (99.73) | (99.18) | (99.73) | (99.45) | – | |
| hsCasq1 | 68.51 | 68.51 | 69.06 | 66.57 | 67.68 | 66.85 | 97.51 | 96.41 | 96.41 | – | – |
| (93.37) | (93.09) | (93.65) | (92.54) | (92.27) | (92.54) | (100) | (99.17) | (99.72) | – | – | |
| mmCasq1 | 69.00 | 68.73 | 68.46 | 67.39 | 68.19 | 66.85 | 99.19 | 95.37 | – | – | – |
| (93.90) | (93.26) | (94.07) | (92.72) | (92.45) | (92.72) | (100) | (99.46) | – | – | – | |
| ocCasq1 | 69.48 | 70.03 | 69.75 | 68.94 | 69.21 | 66.21 | 96.19 | – | – | – | – |
| (94.01) | (93.46) | (94.28) | (92.92) | (92.92) | (90.19) | (99.46) | – | – | – | – | |
| rnCasq1 | 69.35 | 69.35 | 69.09 | 68.01 | 68.82 | 67.47 | – | – | – | – | – |
| (93.82) | (93.28) | (94.09) | (92.74) | (97.24) | (92.74) | – | – | – | – | – | |
| rnCasq2 | 90.41 | 91.30 | 91.58 | 97.21 | 92.05 | – | – | – | – | – | – |
| (98.96) | (97.70) | (98.42) | (99.49) | (97.69) | – | – | – | – | – | – | |
| ocCasq2 | 94.04 | 93.85 | 93.95 | 91.28 | – | – | – | – | – | – | – |
| (98.45) | (97.95) | (98.16) | (97.69) | – | – | – | – | – | – | – | |
| mmCasq2 | 89.12 | 90.28 | 90.53 | – | – | – | – | – | – | – | – |
| (98.70) | (97.70) | (98.42) | – | – | – | – | – | – | – | – | |
| hsCasq2 | 95.26 | 93.68 | – | – | – | – | – | – | – | – | – |
| (99.47) | (98.42) | – | – | – | – | – | – | – | – | – | |
| cfCasq2 | 95.60 | – | – | – | – | – | – | – | – | – | – |
| (98.96) | – | – | – | – | – | – | – | – | – | – |
| Protein | Low-Ca2+ Native Bovine Casq1 | High-Ca2+ Native Bovine Casq1 | Low-Ca2+ Recombinant Bovine Casq1 | High-Ca2+ Recombinant Bovine Casq1 |
|---|---|---|---|---|
| PDB ID | 5KN0 | 5KN2 | 5KN3 | 5KN1 |
| Data collection | – | – | – | – |
| Space group | P1 | C2221 | C2221 | C2221 |
| Cell dimensions | – | – | – | – |
| a, b, c (Å) | 60.342, 92.994, 101.849 | 130.363, 169.194, 155.477 | 59.393, 146.06, 110.34 | 135.669, 165.604, 156.626 |
| α, β, γ (°) | 71.122, 84.574, 73.485 | 90.0, 90.0, 90.0 | 90.0, 90.0, 90.0 | 90.0, 90.0, 90.0 |
| Resolution (Å) | 49.66–2.73 (2.83–2.73) | 42.95–2.60 (2.70–2.60) | 49.20–1.85 (1.92–1.85) | 43.63–2.14 (2.21–2.14) |
| Rmerge | 0.0318 (0.3289) | 0.0633 (1.416) | 0.0698 (0.8201) | 0.0922 (0.8878) |
| <I>/<σI> | 14.84 (2.55) | 13.25 (1.34) | 13.40 (2.72) | 27.16 (5.0) |
| Completeness (%) | 0.98 (0.94) | 0.99 (0.97) | 0.100 (0.97) | 0.99 (0.94) |
| Multiplicity | 2.0 (1.9) | 7.3 (7.4) | 7.1 (6.6) | 7.1 (6.8) |
| Refinement | – | – | – | – |
| Resolution | 49.7–2.73 (2.83–2.73) | 42.95–2.60 (2.70–2.60) | 49.20–1.85 (1.92–1.85) | 43.63–2.14 (2.21–2.14) |
| Unique reflections | 52,550 (5026) | 52,807 (5112) | 41,309 (3984) | 96,658 (9078) |
| Rwork/Rfree | 0.1970/0.241 (0.3474/0.3834) | 0.2056/0.2411 (0.3302/0.3536) | 0.1833/0.2106 (0.2936/0.3201) | 0.1836/0.2011 (0.2429/0.2594) |
| Number of atoms | – | – | – | – |
| Macromolecules | 11,172 | 8456 | 2827 | 8466 |
| Ion | 19 | 39 | 5 | 40 |
| Ligand | 175 | 84 | 32 | 33 |
| Water molecules | 33 | 5 | 336 | 461 |
| R.m.s deviations | – | – | – | – |
| Bond lengths (Å) | 0.005 | 0.005 | 0.003 | 0.005 |
| Bond angles (°) | 0.71 | 0.84 | 0.57 | 0.69 |
| Ramachandrans | – | – | – | – |
| Favored (%) | 97.6 | 98.0 | 98.6 | 98.0 |
| Outliers (%) | 0 | 0 | 0 | 0 |
| Clashscore | 1.59 | 1.03 | 2.70 | 1.82 |
| B-factors | – | – | – | – |
| Protein | 76.71 | 108.25 | 43.83 | 59.42 |
| Ligand/Ion | 100.15 | 139.00 | 69.90 | 74.69 |
| Water | 59.89 | 67.90 | 44.92 | 51.10 |
| TLS groups | 27 | 13 | 20 | 60 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lewis, K.M.; Munske, G.R.; Byrd, S.S.; Kang, J.; Cho, H.-J.; Ríos, E.; Kang, C. Characterization of Post-Translational Modifications to Calsequestrins of Cardiac and Skeletal Muscle. Int. J. Mol. Sci. 2016, 17, 1539. https://doi.org/10.3390/ijms17091539
Lewis KM, Munske GR, Byrd SS, Kang J, Cho H-J, Ríos E, Kang C. Characterization of Post-Translational Modifications to Calsequestrins of Cardiac and Skeletal Muscle. International Journal of Molecular Sciences. 2016; 17(9):1539. https://doi.org/10.3390/ijms17091539
Chicago/Turabian StyleLewis, Kevin M., Gerhard R. Munske, Samuel S. Byrd, Jeehoon Kang, Hyun-Jai Cho, Eduardo Ríos, and ChulHee Kang. 2016. "Characterization of Post-Translational Modifications to Calsequestrins of Cardiac and Skeletal Muscle" International Journal of Molecular Sciences 17, no. 9: 1539. https://doi.org/10.3390/ijms17091539