Endogenous Sulfur Dioxide Inhibits Vascular Calcification in Association with the TGF-β/Smad Signaling Pathway
Abstract
:1. Introduction
2. Results
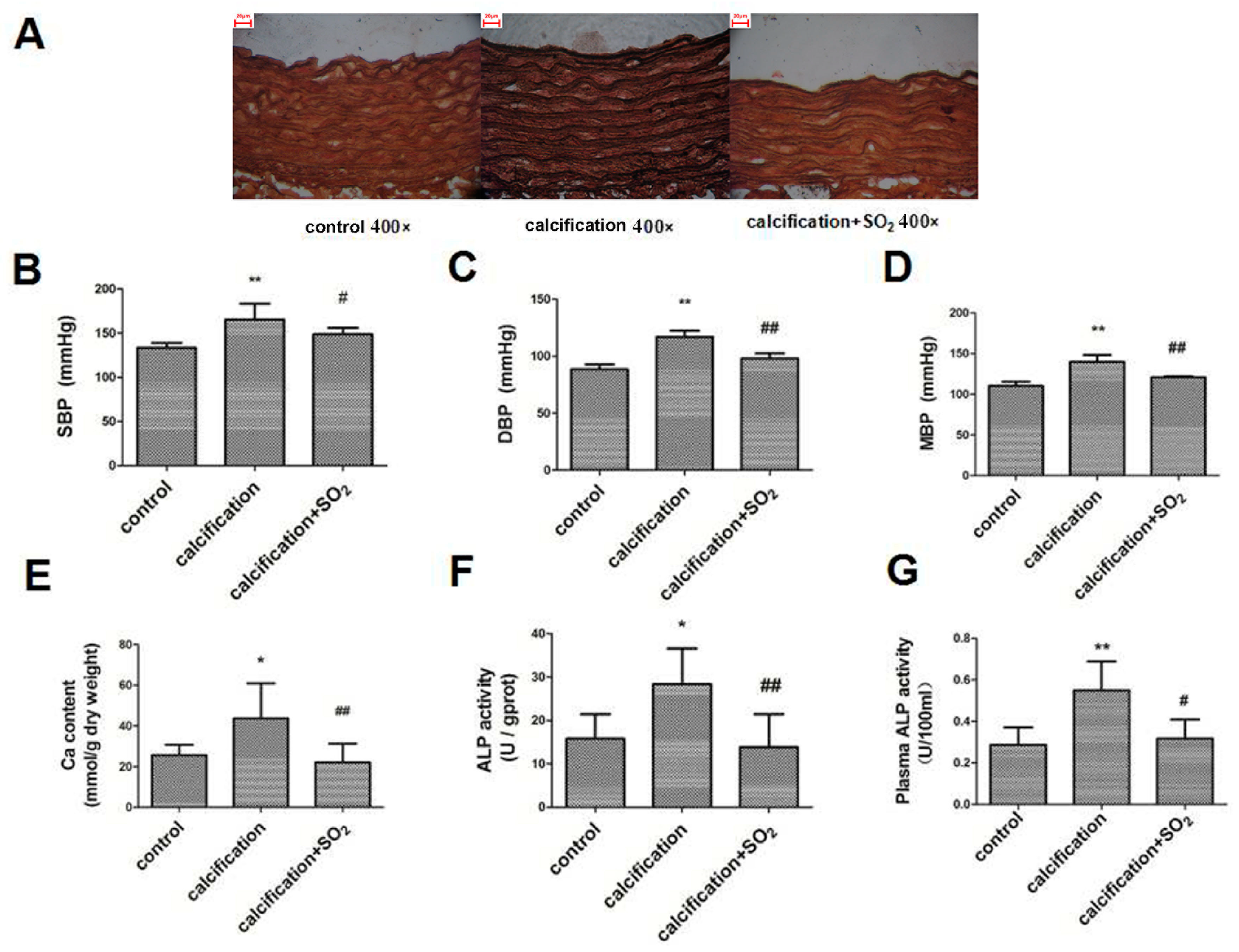
2.1. Establishment of the Rat Vascular Calcification Rat Model
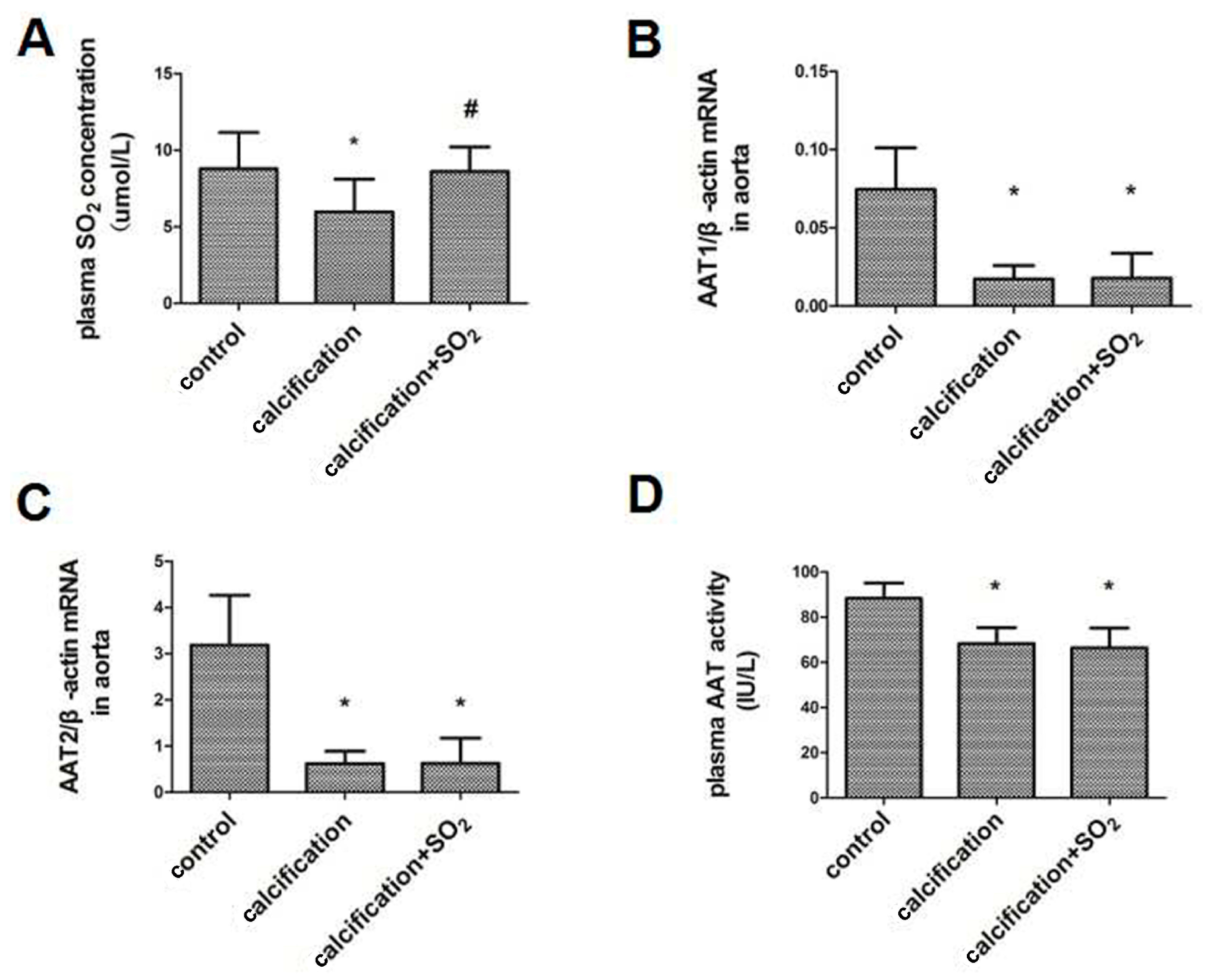
2.2. The SO2/AAT Pathway Is Downregulated in Calcified Arteries
2.3. SO2 Treatment Attenuated Vascular Calcification in Vivo
2.4. SO2 Attenuates Osteoblastic Differentiation of Vascular Smooth Muscle Cells in Vivo
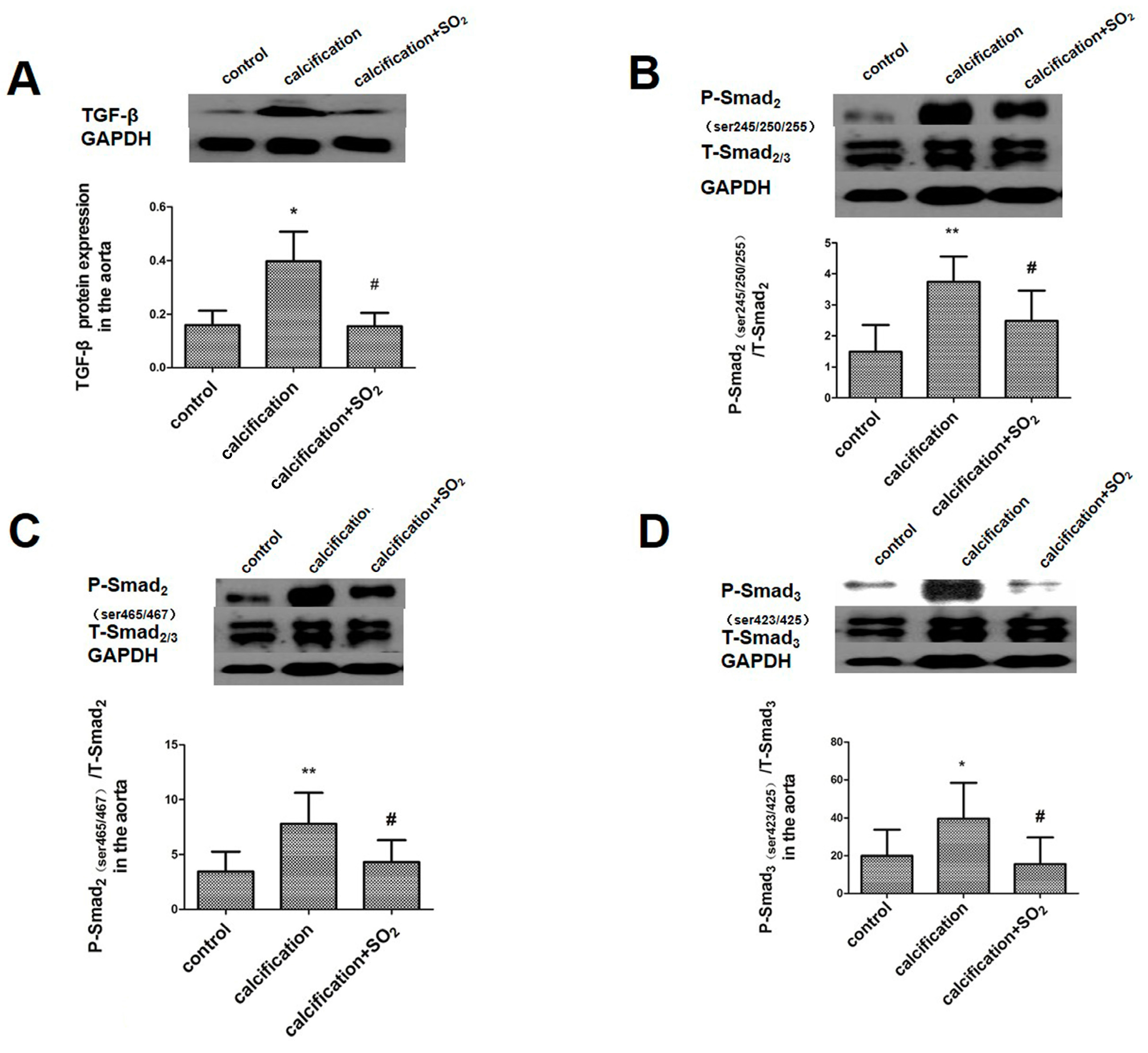
2.5. SO2 Inhibits Activation of the TGF-β Signaling Pathway during the Vascular Calcification Process
2.6. Establishment of the VSMC Calcification Model in Vitro
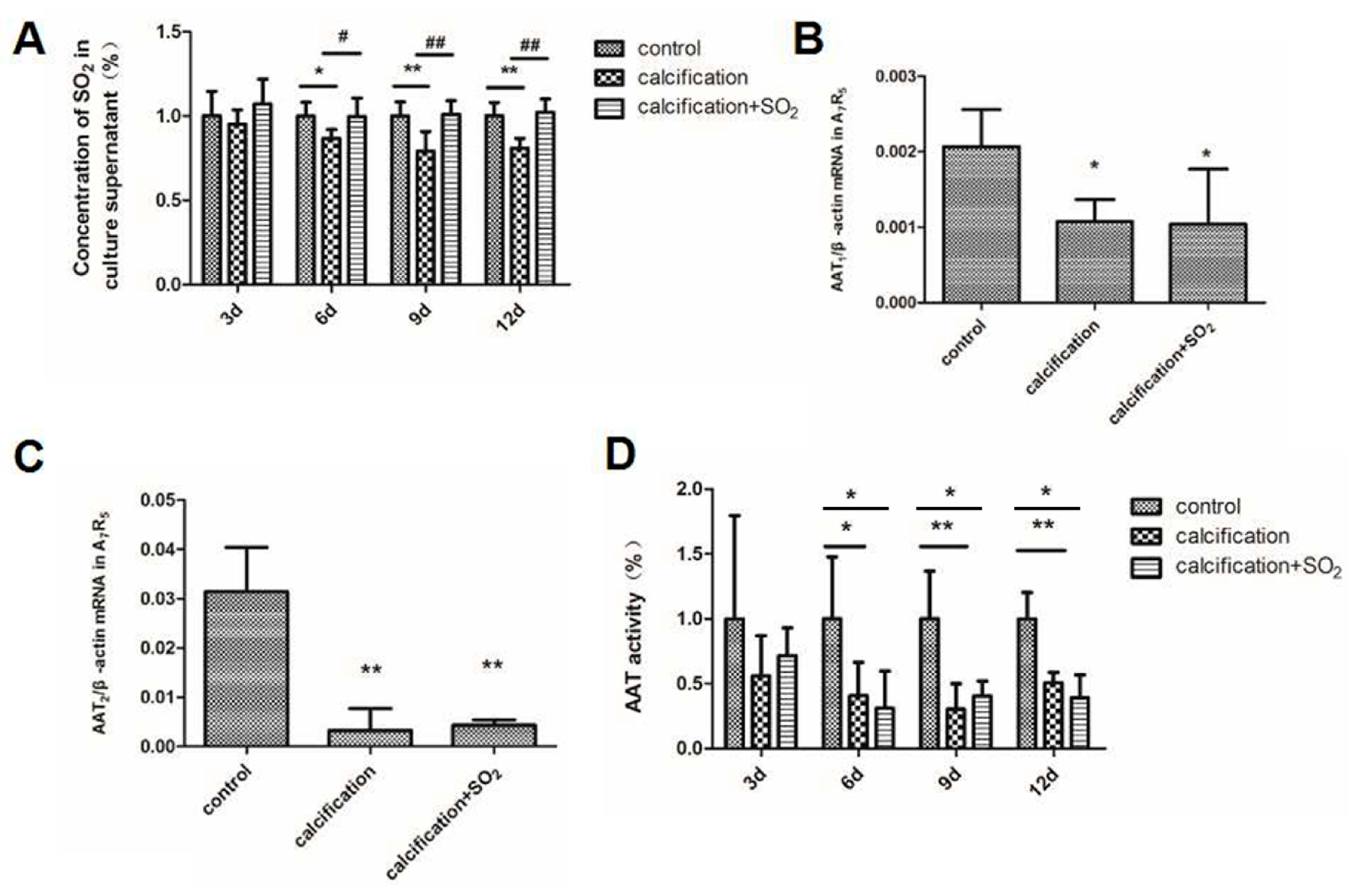
2.7. The Expression of the SO2/AAT Pathway in Calcified VSMCs
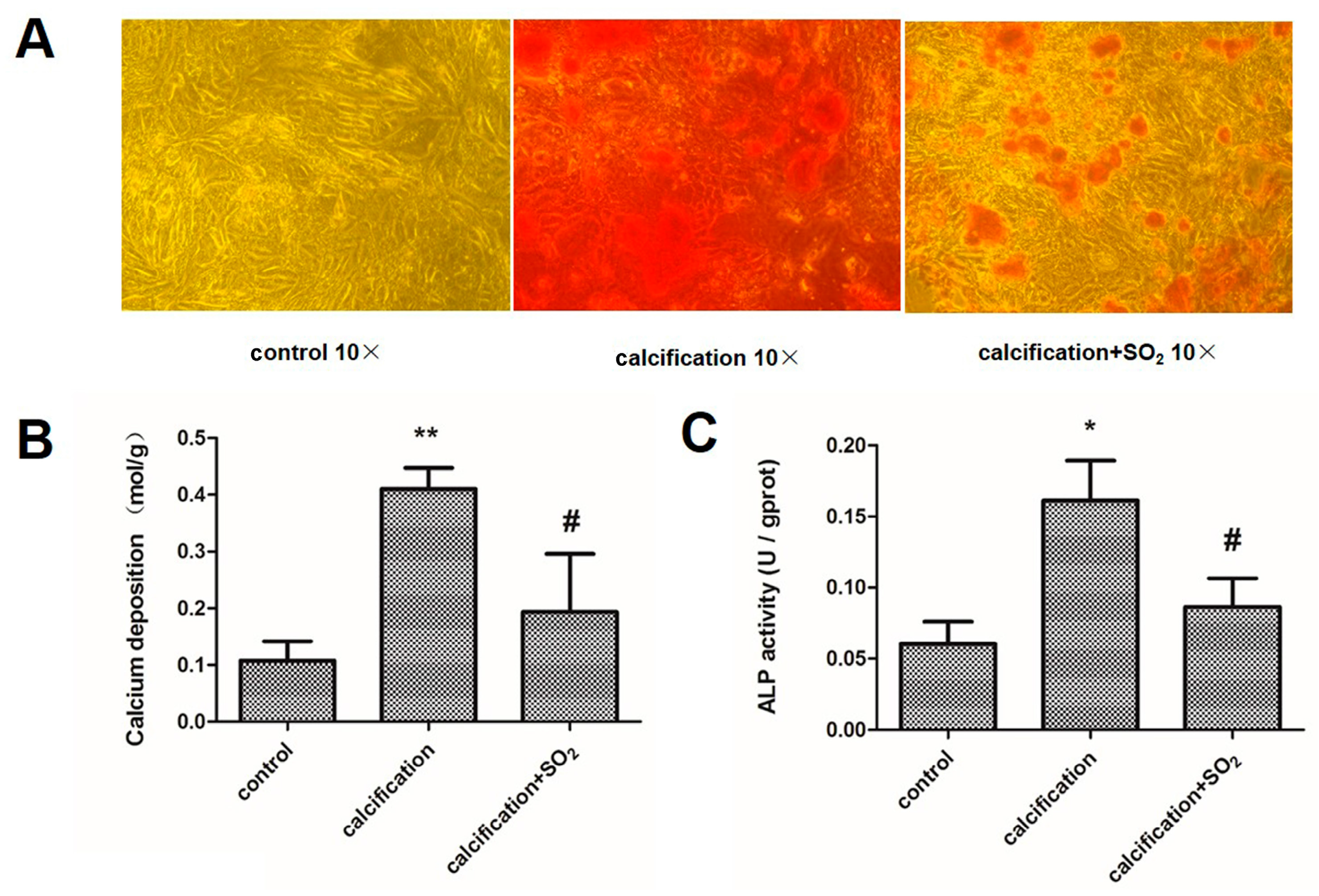
2.8. SO2 Treatment Attenuates Cell Calcification in Vitro
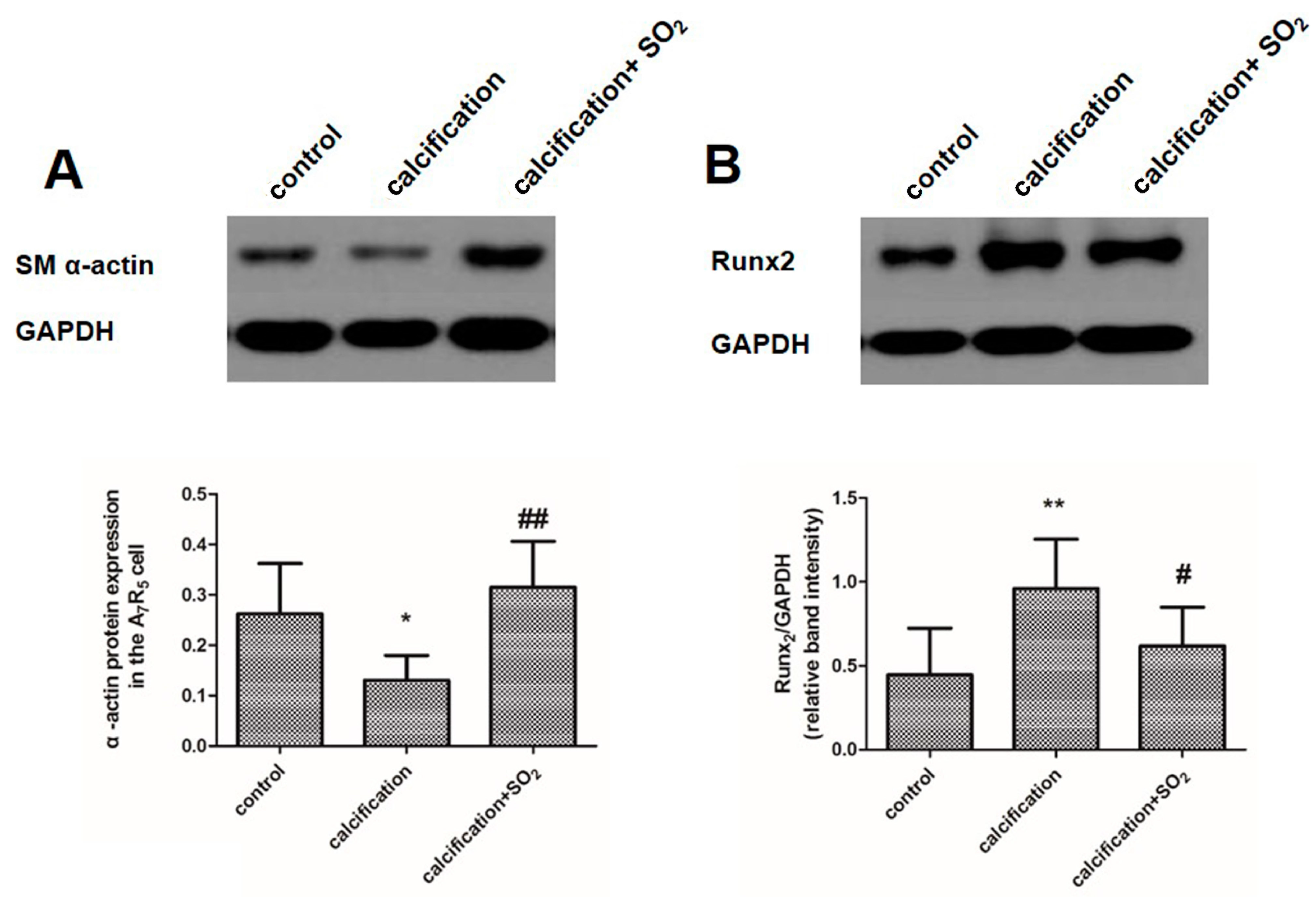
2.9. SO2 Inhibits Osteoblastic Differentiation of VSMCs in Vitro
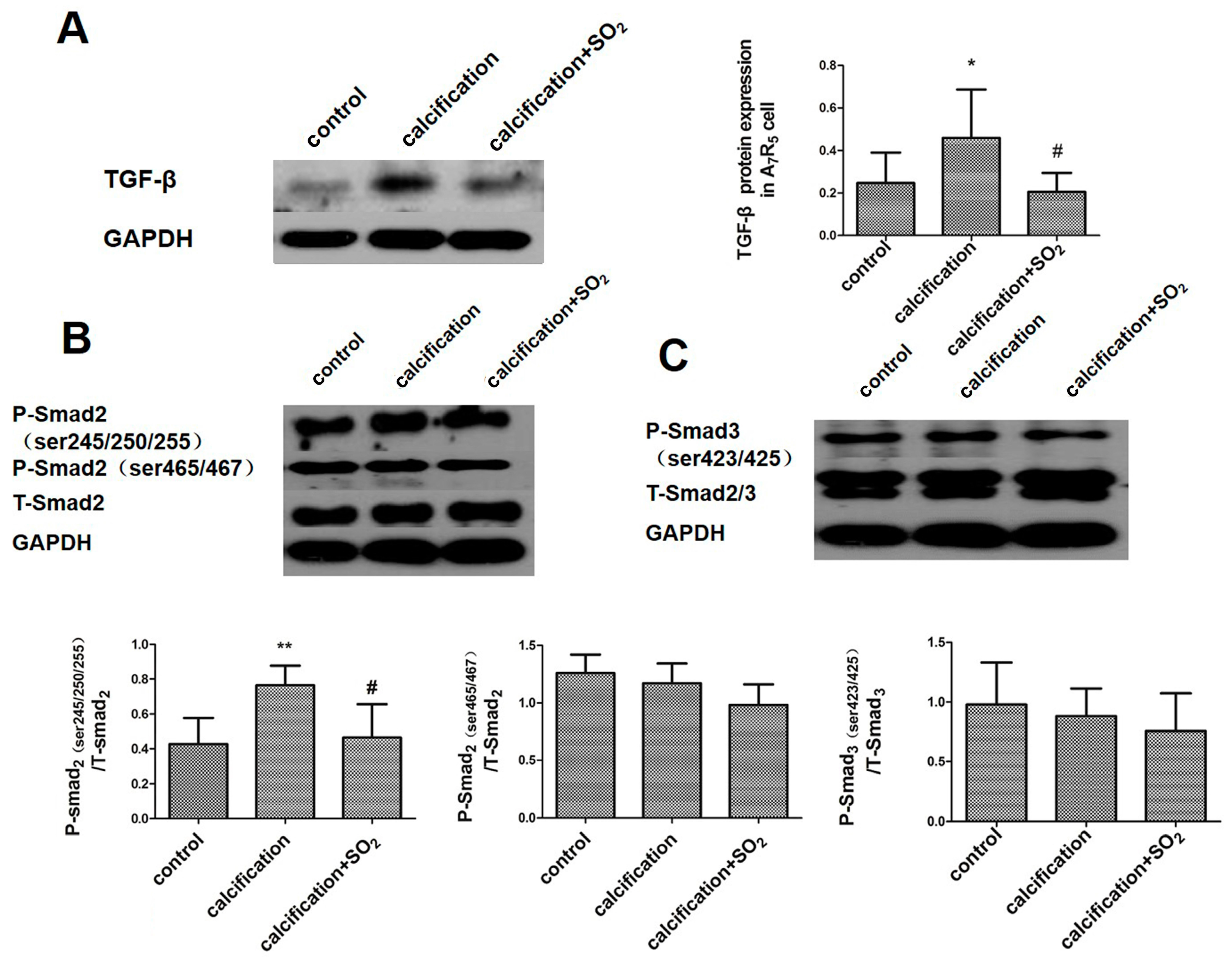
2.10. SO2 Inhibits Activation of the TGF-β Signaling Pathway during the Process of VSMCs Calcification
3. Discussion
4. Materials and Methods
4.1. Preparation of the Animal Model
4.2. A7r5 VSMC Culture and Calcification Inducement
4.3. Measurement of Hemodynamic Features in Vivo
4.4. Sample Preparation
4.5. Quantification of Calcium Content
4.6. ALP Activity Assay
4.7. Von Kossa Staining and Alizarin Red S Staining
4.8. Real-Time PCR Analysis
4.9. Western Blot Analysis
4.10. Determination of SO2 Content in Plasma and Cell Supernatant
4.11. AAT Activity Assay
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shanahan, C.M.; Crouthamel, M.H.; Kapustin, A.; Giachelli, C.M. Arterial calcification in chronic kidney disease: Key roles for calcium and phosphate. Circ. Res. 2011, 109, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, Y.; Wang, L.; Liu, B.; Tian, Q.Y.; Liu, C.J.; Zhang, T.; Xu, Q.B.; Zhu, Y.; Ake, O.; et al. Cartilage oligomeric matrix protein inhibits vascular smooth muscle calcification by interacting with bone morphogenetic protein-2. Circ. Res. 2011, 108, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Towler, D.A.; Demer, L.L. Thematic series on the pathobiology of vascular calcification: An introduction. Circ. Res. 2011, 108, 1378–1380. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A. MicroRNAs regulate vascular medial calcification. Cells 2014, 3, 963–980. [Google Scholar] [CrossRef] [PubMed]
- Qiao, W.; Chen, L.; Zhang, M. MicroRNA-205 regulates the calcification and osteoblastic differentiation of vascular smooth muscle cells. Cell. Physiol. Biochem. 2014, 33, 1945–1953. [Google Scholar] [CrossRef] [PubMed]
- Cui, R.R.; Li, S.J.; Liu, L.J.; Yi, L.; Liang, Q.H.; Zhu, X.; Liu, G.Y.; Liu, Y.; Wu, S.S.; Liao, X.B.; et al. MicroRNA-204 regulates vascular smooth muscle cell calcification in vitro and in vivo. Cardiovasc. Res. 2012, 96, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.B.; Zhang, Z.Y.; Yuan, K.; Liu, Y.; Feng, X.; Cui, R.R.; Hu, Y.R.; Yuan, Z.S.; Gu, L.; Li, S.J.; et al. MiR-133a modulates osteogenic differentiation of vascular smooth muscle cells. Endocrinology 2013, 154, 3344–3352. [Google Scholar] [CrossRef] [PubMed]
- Du, S.X.; Jin, H.F.; Bu, D.F.; Zhao, X.; Geng, B.; Tang, C.S.; Du, J.B. Endogenously generated sulfur dioxide and its vasorelaxant effect in rats. Acta Pharmacol. Sin. 2008, 29, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Tang, C.S.; Jin, H.F.; Du, J.B. Sulfur dioxide acts as a novel endogenous gaseous signaling molecule in the cardiovascular system. Chin. Med. J. (Engl.) 2011, 124, 1901–1905. [Google Scholar] [PubMed]
- Wang, X.B.; Jin, H.F.; Tang, C.S.; Du, J.B. The biological effect of endogenous sulfur dioxide in the cardiovascular system. Eur. J. Pharmacol. 2011, 670, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.Y.; Du, J.B.; Sun, Y.; Chen, S.; Tsai, H.J.; Yuan, L.; Li, L.; Tang, C.S.; Jin, H.F. Sulfur dioxide derivatives depress l-type calcium channel in rat cardiomyocytes. Clin. Exp. Pharmacol. Physiol. 2011, 38, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Meng, Z. The vasodilator mechanism of sulfur dioxide on isolated aortic rings of rats: Involvement of the K+ and Ca2+ channels. Eur. J. Pharmacol. 2009, 602, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Tian, Y.; Prabha, M.; Liu, D.; Chen, S.; Zhang, R.; Liu, X.; Tang, C.; Tang, X.; Jin, H.; et al. Effects of sulfur dioxide on hypoxic pulmonary vascular structural remodeling. Lab. Investig. 2010, 90, 68–82. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.F.; Du, S.X.; Zhao, X.; Wei, H.L.; Wang, Y.F.; Liang, Y.F.; Tang, C.S.; Du, J.B. Effects of endogenous sulfur dioxide on monocrotaline-induced pulmonary hypertension in rats. Acta Pharmacol. Sin. 2008, 29, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, Y.; Bu, D.; Liu, A.D.; Holmberg, L.; Jia, Y.; Tang, C.; Du, J.; Jin, H. Sulfur dioxide inhibits vascular smooth muscle cell proliferation via suppressing the Erk/MAP kinase pathway mediated by cAMP/PKA signaling. Cell Death Dis. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Tang, C.; Jin, H.; Du, J. Regulatory effects of sulfur dioxide on the development of atherosclerotic lesions and vascular hydrogen sulfide in atherosclerotic rats. Atherosclerosis 2011, 215, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Liu, D.; Ochs, T.; Tang, C.; Chen, S.; Zhang, S.; Geng, B.; Jin, H.; Du, J. Endogenous sulfur dioxide protects against isoproterenol-induced myocardial injury and increases myocardial antioxidant capacity in rats. Lab. Investig. 2011, 91, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Du, J.; Liang, Y.; Ochs, T.; Liu, D.; Zhu, L.; Tang, X.; Tang, C.; Jin, H. Sulfur dioxide inhibits excessively activated endoplasmic reticulum stress in rats with myocardial injury. Heart Vessels. 2012, 27, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Huang, X.M.; Ochs, T.; Li, X.Y.; Jin, H.F.; Tang, C.S.; Du, J.B. Effect of sulfur dioxide preconditioning on rat myocardial ischemia/reperfusion injury by inducing endoplasmic reticulum stress. Basic Res. Cardiol. 2011, 106, 865–878. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Du, J.; Jin, H.; Li, W.; Liang, Y.; Geng, B.; Li, S.; Zhang, C.; Tang, C. Endogenous sulfur dioxide aggravates myocardial injury in isolated rat heart with ischemia and reperfusion. Transplantation 2009, 87, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Jian, B.; Narula, N.; Li, Q.Y.; Mohler, E.R., III; Levy, R.J. Progression of aortic valve stenosis: TGF-β1 is present in calcified aortic valve cusps and promotes aortic valve interstitial cell calcification via apoptosis. Ann. Thorac. Surg. 2003, 75, 457–465. [Google Scholar] [CrossRef]
- Kanno, Y.; Into, T.; Lowenstein, C.J.; Matsushita, K. Nitric oxide regulates vascular calcification by interfering with TGF-signalling. Cardiovasc. Res. 2008, 77, 221–230. [Google Scholar] [CrossRef] [PubMed]
- Watson, K.E.; Boström, K.; Ravindranath, R.; Lam, T.; Norton, B.; Demer, L.L. TGF-β1 and 25-hydroxycholesterol stimulate osteoblast-like vascular cells to calcify. J. Clin. Investig. 1994, 93, 2106–2113. [Google Scholar] [CrossRef] [PubMed]
- Grainger, D.J.; Metcalfe, J.C.; Grace, A.A.; Mosedale, D.E. Transforming growth factor-β dynamically regulates vascular smooth muscle differentiation in vivo. J. Cell Sci. 1998, 111, 2977–2988. [Google Scholar] [PubMed]
- Jeon, H.S.; Jen, J. TGF-β signaling and the role of inhibitory Smads in non-small cell lung cancer. J. Thorac. Oncol. 2010, 5, 417–419. [Google Scholar] [CrossRef] [PubMed]
- Speer, M.Y.; Li, X.; Hiremath, P.G.; Giachelli, C.M. Runx2/Cbfa1, but not loss of myocardin, is required for smooth muscle cell lineage reprogramming toward osteochondrogenesis. J. Cell. Biochem. 2010, 110, 935–947. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Kim, H.J.; Li, Q.L.; Chi, X.Z.; Ueta, C.; Komori, T.; Wozney, J.M.; Kim, E.G.; Choi, J.Y.; Ryoo, H.M.; et al. Runx2 is a common target of transforming growth factor β1 and bone morphogenetic protein 2, and cooperation between Runx2 and Smad5 induces osteoblast-specific gene expression in the pluripotent mesenchymal precursor cell line C2C12. Mol. Cell. Biol. 2000, 20, 8783–8792. [Google Scholar] [PubMed]
- Scheiber, D.; Veulemans, V.; Horn, P.; Chatrou, M.L.; Potthoff, S.A.; Kelm, M.; Schurgers, L.J.; Westenfeld, R. High-dose menaquinone-7 supplementation reduces cardiovascular calcification in a murine model of extraosseous calcification. Nutrients 2015, 7, 6991–7011. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhou, Y.B.; Zhang, G.G.; Cai, Y.; Duan, X.H.; Teng, X.; Song, J.Q.; Shi, Y.; Tang, C.S.; Yin, X.H.; et al. Cortistatin attenuates vascular calcification in rats. Regul. Pept. 2010, 159, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, P.; Robert, A.; Capdeville-Atkinson, C.; Atkinson, J.; Lartaud-Idjouadiene, I. Age-related arterial calcification in rats. Life Sci. 2000, 66, 2371–2381. [Google Scholar] [CrossRef]
- Cai, Y.; Xu, M.J.; Teng, X.; Zhou, Y.B.; Chen, L.; Zhu, Y.; Wang, X.; Tang, C.S.; Qi, Y.F. Intermedin inhibits vascular calcification by increasing the level of matrix γ-carboxyglutamic acid protein. Cardiovasc. Res. 2010, 85, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Ameer, O.Z.; Salman, I.M.; Avolio, A.P.; Phillips, J.K.; Butlin, M. Opposing changes in thoracic and abdominal aortic biomechanical properties in rodent models of vascular calcification and hypertension. Am. J. Physiol.-Heart Circ. Physiol. 2014, 307, H143–H151. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Z.; Huang, Y.; Du, J.; Liu, A.D.; Tang, C.; Qi, Y.; Jin, H. Endogenous Sulfur Dioxide Inhibits Vascular Calcification in Association with the TGF-β/Smad Signaling Pathway. Int. J. Mol. Sci. 2016, 17, 266. https://doi.org/10.3390/ijms17030266
Li Z, Huang Y, Du J, Liu AD, Tang C, Qi Y, Jin H. Endogenous Sulfur Dioxide Inhibits Vascular Calcification in Association with the TGF-β/Smad Signaling Pathway. International Journal of Molecular Sciences. 2016; 17(3):266. https://doi.org/10.3390/ijms17030266
Chicago/Turabian StyleLi, Zhenzhen, Yaqian Huang, Junbao Du, Angie Dong Liu, Chaoshu Tang, Yongfen Qi, and Hongfang Jin. 2016. "Endogenous Sulfur Dioxide Inhibits Vascular Calcification in Association with the TGF-β/Smad Signaling Pathway" International Journal of Molecular Sciences 17, no. 3: 266. https://doi.org/10.3390/ijms17030266







