Molecular Mechanisms of Nickel Allergy
Abstract
:1. Introduction
2. Metal Allergy
3. Animal Models and Molecular Mechanism of Metal Allergy
3.1. Keratinocytes and APCs in Ni Allergy Models
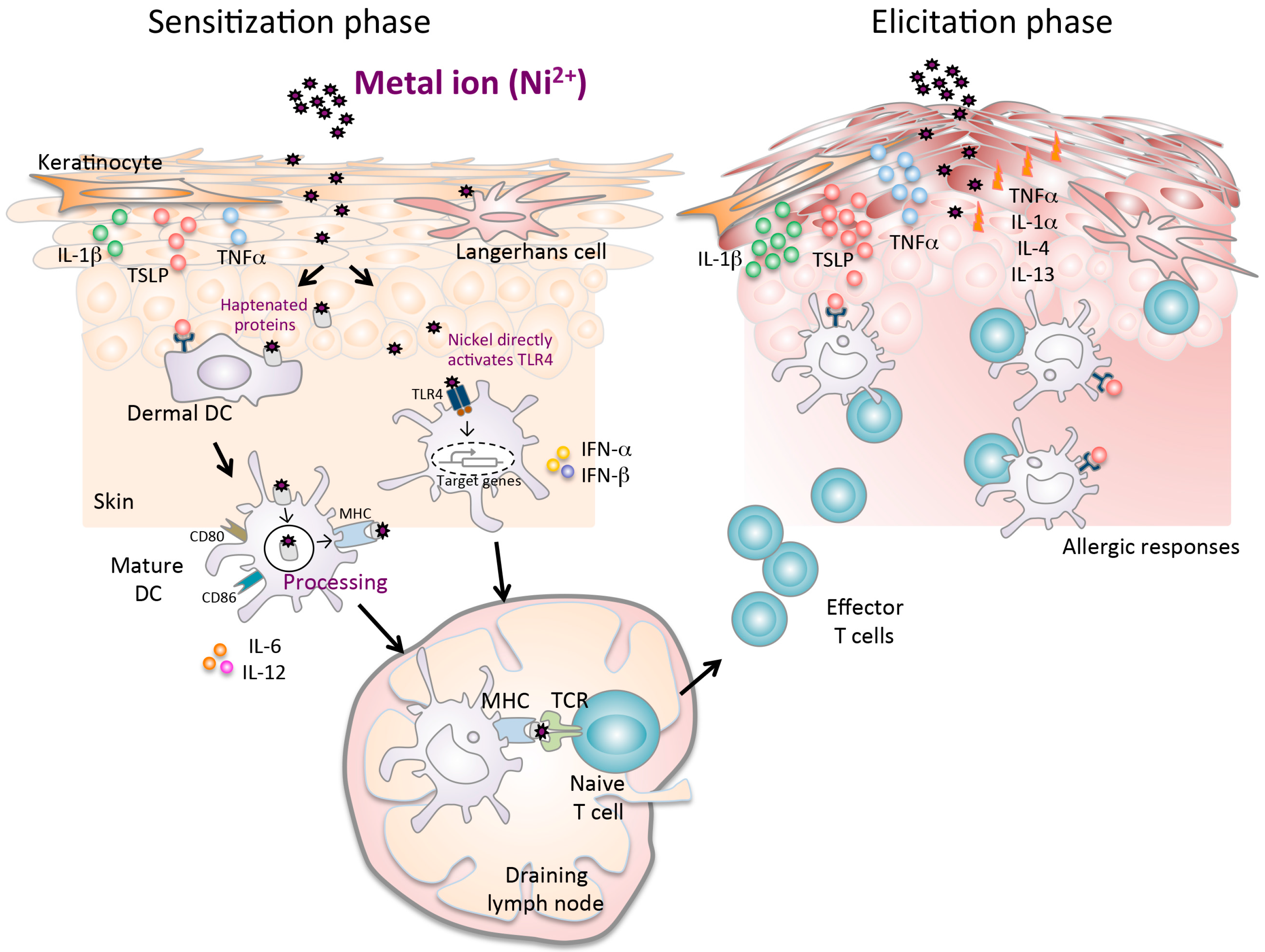
3.2. Critical Role of Toll-Like Receptor 4 in Ni Allergy
3.3. Thymic Stromal Lymphopoietin and Its Receptor in Ni Allergy
4. Adsorption and Excretion of Metals
5. Conclusions and Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Loh, J.; Fraser, J. Metal-derivatized major histocompatibility complex: Zeroing in on contact hypersensitivity. J. Exp. Med. 2003, 197, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Budinger, L.; Hertl, M. Immunologic mechanisms in hypersensitivity reactions to metal ions: An overview. Allergy 2000, 55, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, L. Nickel sensitivity in the general population. Contact Dermat. 1979, 5, 27–32. [Google Scholar] [CrossRef]
- Nielsen, N.H.; Menne, T. Allergic contact sensitization in an unselected Danish population. Acta Derm. Venereol. 1992, 72, 456–460. [Google Scholar] [PubMed]
- Pigatto, P.D.; Guzzi, G. Systemic allergic dermatitis syndrome caused by mercury. Contact Dermat. 2008, 59, 66. [Google Scholar]
- Yoshihisa, Y.; Shimizu, T. Metal allergy and systemic contact dermatitis: An overview. Dermatol. Res. Pract. 2012, 2012, 749561. [Google Scholar] [CrossRef] [PubMed]
- Yokozeki, H.; Katayama, I.; Nishioka, K.; Kinoshita, M.; Nishiyama, S. The role of metal allergy and local hyperhidrosis in the pathogenesis of pompholyx. J. Dermatol. 1992, 19, 964–967. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yin, W.; Ma, Q. Allergic palmoplantar pustulosis caused by cobalt in cast dental crowns: A case report. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, e8–e10. [Google Scholar] [CrossRef] [PubMed]
- Stejskal, V.; Reynolds, T.; Bjorklund, G. Increased frequency of delayed type hypersensitivity to metals in patients with connective tissue disease. J. Trace Elem. Med. Biol. 2015, 31, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Mahler, V.; Geier, J.; Schnuch, A. Current trends in patch testing—new data from the German Contact Dermatitis Research Group (DKG) and the Information Network of Departments of Dermatology (IVDK). JDDG 2014, 12, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Garner, L.A. Contact dermatitis to metals. Dermatol. Ther. 2004, 17, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Peiser, M.; Tralau, T.; Heidler, J.; Api, A.M.; Arts, J.H.; Basketter, D.A.; English, J.; Diepgen, T.L.; Fuhlbrigge, R.C.; Gaspari, A.A.; et al. Allergic contact dermatitis: Epidemiology, molecular mechanisms, in vitro methods and regulatory aspects. Current knowledge assembled at an international workshop at BfR, Germany. Cell. Mol. Life Sci. 2012, 69, 763–781. [Google Scholar] [CrossRef] [PubMed]
- Schram, S.E.; Warshaw, E.M.; Laumann, A. Nickel hypersensitivity: A clinical review and call to action. Int. J. Dermatol. 2010, 49, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Wataha, J.C.; Drury, J.L.; Chung, W.O. Nickel alloys in the oral environment. Expert Rev. Med. Devices 2013, 10, 519–539. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaka, K.; Mabuchi, R.; Nagasaka, H.; Yoshinari, M.; Inoue, T. Improvement of eczematous symptoms after removal of amalgam-like metal in alveolar bone. Bull. Tokyo Dent. Coll. 2006, 47, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Laeijendecker, R.; Dekker, S.K.; Burger, P.M.; Mulder, P.G.; Van Joost, T.; Neumann, M.H. Oral lichen planus and allergy to dental amalgam restorations. Arch. Dermatol. 2004, 140, 1434–1438. [Google Scholar] [CrossRef] [PubMed]
- Yaqob, A.; Danersund, A.; Stejskal, V.D.; Lindvall, A.; Hudecek, R.; Lindh, U. Metal-specific lymphocyte reactivity is downregulated after dental metal replacement. Neuro Endocrinol. Lett. 2006, 27, 189–197. [Google Scholar] [PubMed]
- Kapsenberg, M.L.; Wierenga, E.A.; Stiekema, F.E.; Tiggelman, A.M.; Bos, J.D. Th1 lymphokine production profiles of nickel-specific CD4+T-lymphocyte clones from nickel contact allergic and non-allergic individuals. J. Investig. Dermatol. 1992, 98, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Mortz, C.G.; Lauritsen, J.M.; Bindslev-Jensen, C.; Andersen, K.E. Prevalence of atopic dermatitis, asthma, allergic rhinitis, and hand and contact dermatitis in adolescents. The Odense Adolescence Cohort Study on Atopic Diseases and Dermatitis. Br. J. Dermatol. 2001, 144, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Thierse, H.J.; Gamerdinger, K.; Junkes, C.; Guerreiro, N.; Weltzien, H.U. T cell receptor (TCR) interaction with haptens: Metal ions as non-classical haptens. Toxicology 2005, 209, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Curtis, A.; Morton, J.; Balafa, C.; MacNeil, S.; Gawkrodger, D.J.; Warren, N.D.; Evans, G.S. The effects of nickel and chromium on human keratinocytes: Differences in viability, cell associated metal and IL-1alpha release. Toxicol. In Vitro 2007, 21, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Larsen, J.M.; Bonefeld, C.M.; Poulsen, S.S.; Geisler, C.; Skov, L. IL-23 and T(H)17-mediated inflammation in human allergic contact dermatitis. J. Allergy Clin. Immunol. 2009, 123, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, S.; Albanesi, C.; Nasorri, F.; Girolomoni, G.; Cavani, A. Nickel-specific CD4(+) and CD8(+) T cells display distinct migratory responses to chemokines produced during allergic contact dermatitis. J. Investig. Dermatol. 2002, 118, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Steinman, R.M.; Pack, M.; Inaba, K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol. Rev. 1997, 156, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.; Ishimaru, N.; Ashrin, M.N.; Arakaki, R.; Yamada, A.; Ichikawa, T.; Hayashi, Y. A novel DC therapy with manipulation of MKK6 gene on nickel allergy in mice. PLoS ONE 2011, 6, e19017. [Google Scholar] [CrossRef] [PubMed]
- Roake, J.A.; Rao, A.S.; Morris, P.J.; Larsen, C.P.; Hankins, D.F.; Austyn, J.M. Dendritic cell loss from nonlymphoid tissues after systemic administration of lipopolysaccharide, tumor necrosis factor, and interleukin 1. J. Exp. Med. 1995, 181, 2237–2247. [Google Scholar] [CrossRef] [PubMed]
- Lore, K.; Sonnerborg, A.; Spetz, A.L.; Andersson, U.; Andersson, J. Immunocytochemical detection of cytokines and chemokines in Langerhans cells and in vitro derived dendritic cells. J. Immunol. Methods 1998, 214, 97–111. [Google Scholar] [CrossRef]
- Riedl, E.; Stockl, J.; Majdic, O.; Scheinecker, C.; Rappersberger, K.; Knapp, W.; Strobl, H. Functional involvement of E-cadherin in TGF-beta 1-induced cell cluster formation of in vitro developing human Langerhans-type dendritic cells. J. Immunol. 2000, 165, 1381–1386. [Google Scholar] [CrossRef] [PubMed]
- Geissmann, F.; Dieu-Nosjean, M.C.; Dezutter, C.; Valladeau, J.; Kayal, S.; Leborgne, M.; Brousse, N.; Saeland, S.; Davoust, J. Accumulation of immature Langerhans cells in human lymph nodes draining chronically inflamed skin. J. Exp. Med. 2002, 196, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Villadangos, J.A.; Cardoso, M.; Steptoe, R.J.; van Berkel, D.; Pooley, J.; Carbone, F.R.; Shortman, K. MHC class II expression is regulated in dendritic cells independently of invariant chain degradation. Immunity 2001, 14, 739–749. [Google Scholar] [CrossRef]
- Verhasselt, V.; Buelens, C.; Willems, F.; De Groote, D.; Haeffner-Cavaillon, N.; Goldman, M. Bacterial lipopolysaccharide stimulates the production of cytokines and the expression of costimulatory molecules by human peripheral blood dendritic cells: Evidence for a soluble CD14-dependent pathway. J. Immunol. 1997, 158, 2919–2925. [Google Scholar] [PubMed]
- Kyriakis, J.M. Life-or-death decisions. Nature 2001, 414, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Arrighi, J.F.; Rebsamen, M.; Rousset, F.; Kindler, V.; Hauser, C. A critical role for p38 mitogen-activated protein kinase in the maturation of human blood-derived dendritic cells induced by lipopolysaccharide, TNF-alpha, and contact sensitizers. J. Immunol. 2001, 166, 3837–3845. [Google Scholar] [CrossRef] [PubMed]
- Jorgl, A.; Platzer, B.; Taschner, S.; Heinz, L.X.; Hocher, B.; Reisner, P.M.; Gobel, F.; Strobl, H. Human Langerhans-cell activation triggered in vitro by conditionally expressed MKK6 is counterregulated by the downstream effector RelB. Blood 2007, 109, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Raghavan, B.; Muller, V.; Vogl, T.; Fejer, G.; Tchaptchet, S.; Keck, S.; Kalis, C.; Nielsen, P.J.; Galanos, C.; et al. Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nat. Immunol. 2010, 11, 814–819. [Google Scholar] [CrossRef] [PubMed]
- Rachmawati, D.; Bontkes, H.J.; Verstege, M.I.; Muris, J.; von Blomberg, B.M.; Scheper, R.J.; van Hoogstraten, I.M. Transition metal sensing by Toll-like receptor-4: Next to nickel, cobalt and palladium are potent human dendritic cell stimulators. Contact Dermat. 2013, 68, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Sato, N.; Kinbara, M.; Kuroishi, T.; Kimura, K.; Iwakura, Y.; Ohtsu, H.; Sugawara, S.; Endo, Y. Lipopolysaccharide promotes and augments metal allergies in mice, dependent on innate immunity and histidine decarboxylase. Clin. Exp. Allergy 2007, 37, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Suga, H.; Sugaya, M.; Fujita, H.; Asano, Y.; Tada, Y.; Kadono, T.; Sato, S. TLR4, rather than TLR2, regulates wound healing through TGF-beta and CCL5 expression. J. Dermatol. Sci. 2014, 73, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Reche, P.A.; Soumelis, V.; Gorman, D.M.; Clifford, T.; Liu, M.; Travis, M.; Zurawski, S.M.; Johnston, J.; Liu, Y.J.; Spits, H.; et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J. Immunol. 2001, 167, 336–343. [Google Scholar] [CrossRef] [PubMed]
- Rachmawati, D.; Buskermolen, J.K.; Scheper, R.J.; Gibbs, S.; von Blomberg, B.M.; van Hoogstraten, I.M. Dental metal-induced innate reactivity in keratinocytes. Toxicol. In Vitro 2015, 30, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Ashrin, M.N.; Arakaki, R.; Yamada, A.; Kondo, T.; Kurosawa, M.; Kudo, Y.; Watanabe, M.; Ichikawa, T.; Hayashi, Y.; Ishimaru, N. A critical role for thymic stromal lymphopoietin in nickel-induced allergy in mice. J. Immunol. 2014, 192, 4025–4031. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; O'Connor, B.; Ratoff, J.; Meng, Q.; Mallett, K.; Cousins, D.; Robinson, D.; Zhang, G.; Zhao, J.; Lee, T.H.; et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J. Immunol. 2005, 174, 8183–8190. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Omori, M.; Gyarmati, D.; Zhou, B.; Aye, T.; Brewer, A.; Comeau, M.R.; Campbell, D.J.; Ziegler, S.F. Spontaneous atopic dermatitis in mice expressing an inducible thymic stromal lymphopoietin transgene specifically in the skin. J. Exp. Med. 2005, 202, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Cavani, A. Breaking tolerance to nickel. Toxicology 2005, 209, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Cavani, A.; Nasorri, F.; Ottaviani, C.; Sebastiani, S.; De Pita, O.; Girolomoni, G. Human CD25+ regulatory T cells maintain immune tolerance to nickel in healthy, nonallergic individuals. J. Immunol. 2003, 171, 5760–5768. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.M. Human exposure to toxic metals: Factors influencing interpretation of biomonitoring results. Sci. Total Environ. 1995, 166, 89–135. [Google Scholar] [CrossRef]
- Tossavainen, A.; Nurminen, M.; Mutanen, P.; Tola, S. Application of mathematical modelling for assessing the biological half-times of chromium and nickel in field studies. Br. J. Ind. Med. 1980, 37, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Albohn, H. Comparative epicutaneous tests with contact allergens and croton oil in 5 different body rions. Z. Haut Gechlechtskr. 1966, 40, 118–124. (In Gemany) [Google Scholar]
- Fullerton, A.; Menne, T.; Hoelgaard, A. Patch testing with nickel chloride in a hydrogel. Contact Dermat. 1989, 20, 17–20. [Google Scholar] [CrossRef]
- Fullerton, A.; Andersen, J.R.; Hoelgaard, A.; Menne, T. Permeation of nickel salts through human skin in vitro. Contact Dermat. 1986, 15, 173–177. [Google Scholar] [CrossRef]
- Sunderman, F.W., Jr.; Hopfer, S.M.; Sweeney, K.R.; Marcus, A.H.; Most, B.M.; Creason, J. Nickel absorption and kinetics in human volunteers. Proc. Soc. Exp. Biol. Med. 1989, 191, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Leggett, R.W. The biokinetics of inorganic cobalt in the human body. Sci. Total Environ. 2008, 389, 259–269. [Google Scholar] [CrossRef] [PubMed]
- Lauwerys, R.; Lison, D. Health risks associated with cobalt exposure—An overview. Sci. Total Environ. 1994, 150, 1–6. [Google Scholar] [CrossRef]
- Mosconi, G.; Bacis, M.; Vitali, M.T.; Leghissa, P.; Sabbioni, E. Cobalt excretion in urine: Results of a study on workers producing diamond grinding tools and on a control group. Sci. Total Environ. 1994, 150, 133–139. [Google Scholar] [CrossRef]
- Simonsen, L.O.; Harbak, H.; Bennekou, P. Cobalt metabolism and toxicology—A brief update. Sci. Total Environ. 2012, 432, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.; Lind, P.M.; Lindgren, C.; Ingelsson, E.; Mahajan, A.; Morris, A.; Lind, L. Genome-wide association study of toxic metals and trace elements reveals novel associations. Hum. Mol. Genet. 2015, 24, 4739–4745. [Google Scholar] [CrossRef] [PubMed]
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saito, M.; Arakaki, R.; Yamada, A.; Tsunematsu, T.; Kudo, Y.; Ishimaru, N. Molecular Mechanisms of Nickel Allergy. Int. J. Mol. Sci. 2016, 17, 202. https://doi.org/10.3390/ijms17020202
Saito M, Arakaki R, Yamada A, Tsunematsu T, Kudo Y, Ishimaru N. Molecular Mechanisms of Nickel Allergy. International Journal of Molecular Sciences. 2016; 17(2):202. https://doi.org/10.3390/ijms17020202
Chicago/Turabian StyleSaito, Masako, Rieko Arakaki, Akiko Yamada, Takaaki Tsunematsu, Yasusei Kudo, and Naozumi Ishimaru. 2016. "Molecular Mechanisms of Nickel Allergy" International Journal of Molecular Sciences 17, no. 2: 202. https://doi.org/10.3390/ijms17020202