Antithymocyte Globulin Induces a Tolerogenic Phenotype in Human Dendritic Cells
Abstract
:1. Introduction
2. Results
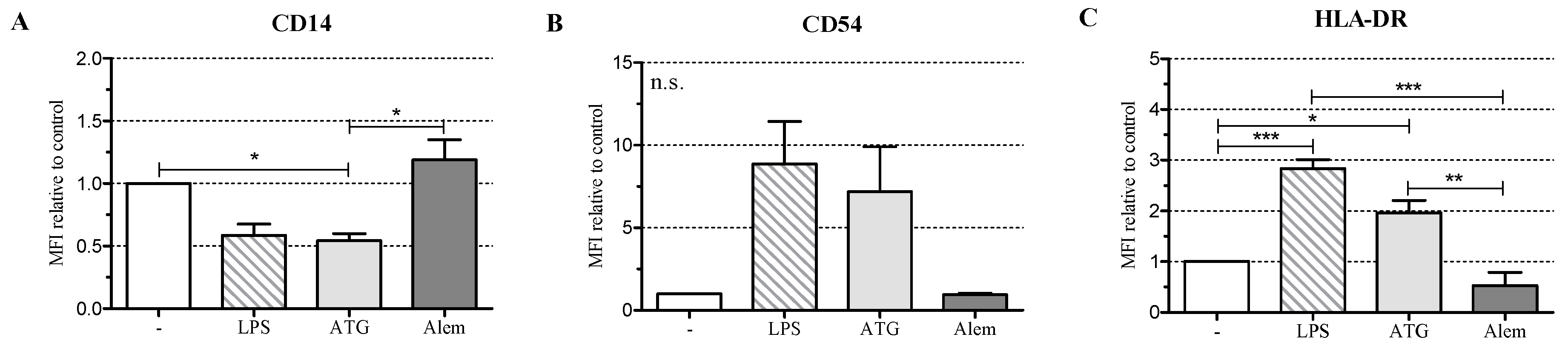
2.1. ATG Induces a Semi-Mature Phenotype in DC
2.2. ATG Induces IL-10 Secretion in DC
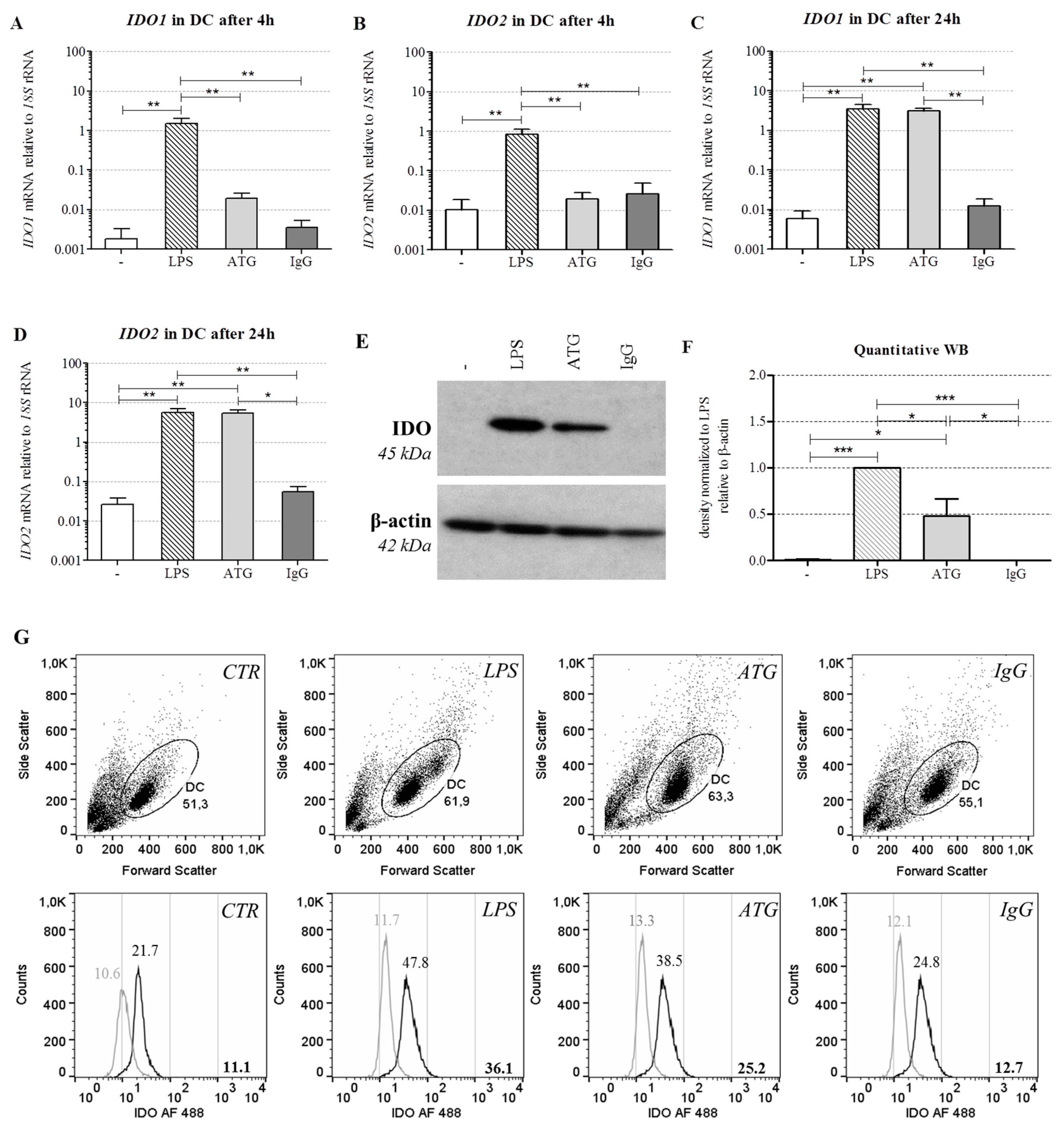
2.3. ATG Induces mRNA Expression of IDO in DC
2.4. ATG Induces IDO at the Protein Level
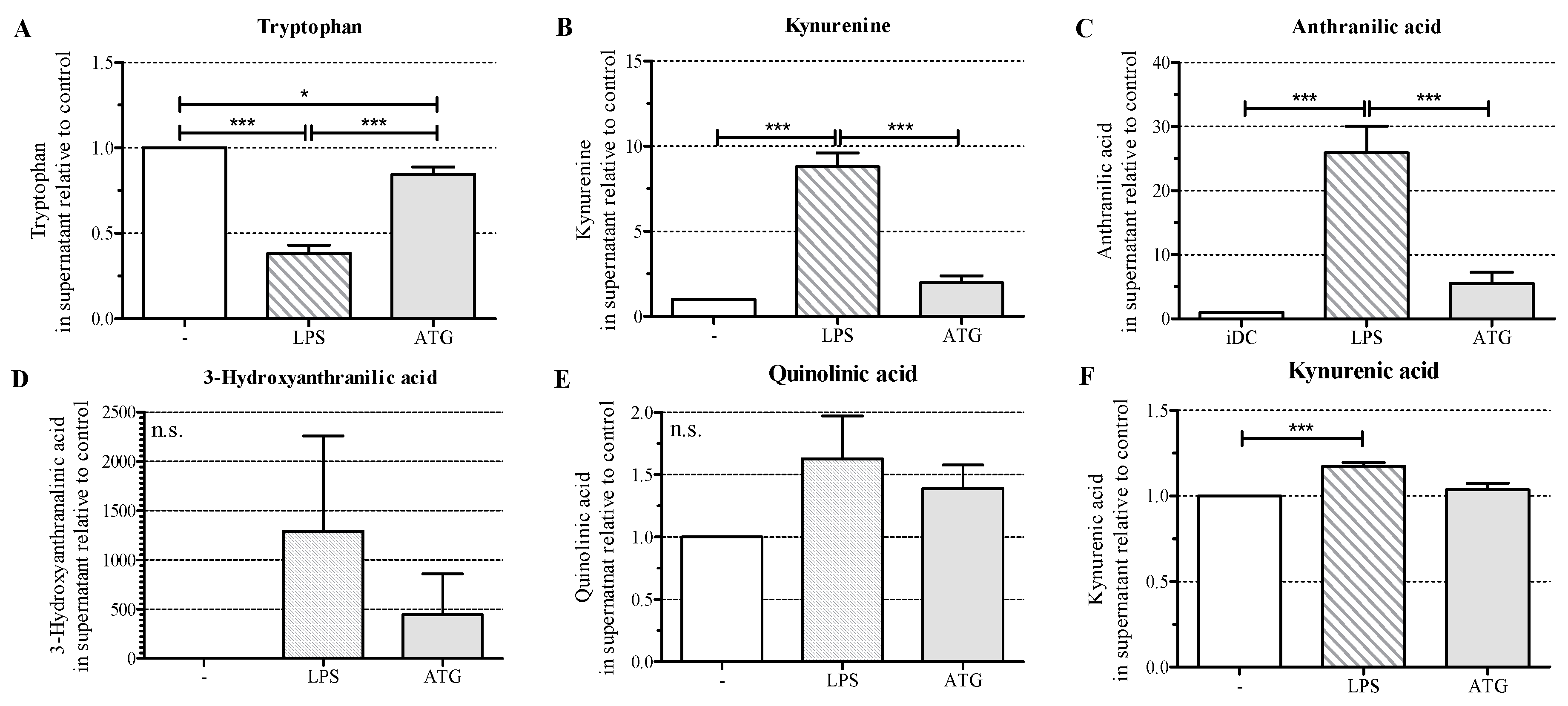
2.5. ATG Stimulates Tryptophan Metabolism
2.6. ATG-Stimulated DC Suppress the Proliferation of Allogeneic T Cells
3. Discussion
4. Material and Methods
4.1. Chemicals
4.2. Isolation of Monocytes and T Lymphocytes
4.3. Culture of Monocytes and Monocyte-Derived DC
4.4. Determination of Cytokines
4.5. Preparation of RNA, Reverse Transcription, and Quantitative Real-Time PCR
4.6. Preparation of Whole Cell Lysates and Western Blotting
4.7. Analysis of Tryptophan Metabolites
4.8. Flow Cytometry Analysis
4.9. Mixed Lymphocyte Reaction (MLR) and 3H-Thymidine Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stenger, E.O.; Turnquist, H.R.; Mapara, M.Y.; Thomson, A.W. Dendritic cells and regulation of graft-versus-host disease and graft-versus-leukemia activity. Blood 2012, 119, 5088–5103. [Google Scholar] [CrossRef] [PubMed]
- Svajger, U.; Rozman, P. Tolerogenic dendritic cells: Molecular and cellular mechanisms in transplantation. J. Leukoc. Biol. 2014, 95, 53–69. [Google Scholar] [CrossRef] [PubMed]
- Prescribing Information ATG-Fresenius S 20 mg/mL, 2011. Available online: http://www.pharmaline.co.il/images/newsletterregistration/neopharm/2012/11052012/atgdr.pdf (accessed on 17 August 2016).
- Mohty, M.; Gaugler, B. Mechanisms of action of antithymocyte globulin: Old dogs with new tricks! Leuk. Lymphoma 2009, 49, 1664–1667. [Google Scholar] [CrossRef] [PubMed]
- Leitner, J.; Grabmeier-Pfistershammer, K.; Majdic, O.; Zlabinger, G.; Steinberger, P. Interaction of antithymocyte globulins with dendritic cell antigens. Am. J. Transplant. 2011, 11, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Naujokat, C.; Berges, C.; Fuchs, D.; Sadeghi, M.; Opelz, G.; Daniel, V. Antithymocyte globulins suppress dendritic cell function by multiple mechanisms. Transplantation 2007, 83, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Fehse, B.; Engel, M.; Zander, A.; Kröger, N. Antithymocyte globulin induces ex vivo and in vivo depletion of myeloid and plasmacytoid dendritic cells. Transplantation 2005, 79, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, D.; Merad, M. Harnessing dendritic cells to improve allogeneic hematopoietic cell transplantation outcome. Semin. Immunol. 2011, 23, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Monti, P.; Allavena, P.; di Carlo, V.; Piemonti, L. Effects of anti-lymphocytes and anti-thymocytes globulin on human dendritic cells. Int. Immunopharmacol. 2003, 3, 189–196. [Google Scholar] [CrossRef]
- Gillet-Hladky, S.; de Carvalho, C.M.; Bernaud, J.; Bendahou, C.; Bloy, C.; Rigal, D. Rabbit Antithymocyte globulin inhibits monocyte-derived dendritic cells maturation in vitro and polarizes monocyte-derived dendritic cells towards tolerogenic dendritic cells expressing indoleamine 2,3-dioxygenase. Transplantation 2006, 82, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Landfried, K.; Zhu, W.; Waldhier, M.C.; Schulz, U.; Ammer, J.; Holler, B.; Wolff, D.; Edinger, M.; Peter, K.; Kreutz, M.; et al. Tryptophan catabolism is associated with acute GVHD after human allogeneic stem cell transplantation and indicates activation of indoleamine 2,3-dioxygenase. Blood 2011, 118, 6971–6974. [Google Scholar] [CrossRef] [PubMed]
- Mándi, Y.; Vécsei, L. The kynurenine system and immunoregulation. J. Neural Transm. 2012, 119, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Mellor, A.L.; Munn, D.H. IDO expression by dendritic cells: Tolerance and tryptophan catabolism. Nat. Rev. Immunol. 2004, 4, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Fallarino, F.; Grohmann, U.; Vacca, C.; Bianchi, R.; Orabona, C.; Spreca, A.; Fioretti, M.C.; Puccetti, P. T cell apoptosis by tryptophan catabolism. Cell Death Differ. 2002, 9, 1069–1077. [Google Scholar] [CrossRef] [PubMed]
- Frumento, G.; Rotondo, R.; Tonetti, M.; Damonte, G.; Benatti, U.; Ferrara, G.B. Tryptophan-derived catabolites are responsible for inhibition of T and natural killer cell proliferation induced by indoleamine 2,3-dioxygenase. J. Exp. Med. 2002, 196, 459–468. [Google Scholar] [CrossRef] [PubMed]
- Cook, C.H.; Bickerstaff, A.A.; Wang, J.J.; Nadasdy, T.; Della Pelle, P.; Colvin, R.B.; Orosz, C.G. Spontaneous renal allograft acceptance associated with “regulatory” dendritic cells and IDO. J. Immunol. 2008, 180, 3103–3112. [Google Scholar] [CrossRef] [PubMed]
- Keating, M.J.; O’Brien, S.; Ferrajoli, A. Alemtuzumab: A novel monoclonal antibody. Expert Opin. Biol. Ther. 2005, 1, 1059–1065. [Google Scholar] [CrossRef] [PubMed]
- Mezrich, J.D.; Fechner, J.H.; Zhang, X.; Johnson, B.P.; Burlingham, W.J.; Bradfield, C.A. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J. Immunol. 2010, 185, 3190–3198. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.; Clarkson, M.R.; Albin, M.; Sayegh, M.H.; Najafian, N. A novel mechanism of action for anti-thymocyte globulin: Induction of CD4+CD25+Foxp3+ regulatory T cells. J. Am. Soc. Nephrol. 2006, 17, 2844–2853. [Google Scholar] [CrossRef] [PubMed]
- Michallet, M.-C.; Preville, X.; Flacher, M.; Fournel, S.; Genestier, L.; Revillard, J.-P. Functional antibodies to leukocyte adhesion molecules in antithymocyte globulins1. Transplantation 2003, 75, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Okubo, Y.; Mera, T.; Wang, L.; Faustman, D.L. Homogeneous expansion of human T-regulatory cells via tumor necrosis factor receptor 2. Sci. Rep. 2013, 3, 3153. [Google Scholar] [CrossRef] [PubMed]
- Pierini, A.; Strober, W.; Moffett, C.; Baker, J.; Nishikii, H.; Alvarez, M.; Pan, Y.; Schneidawind, D.; Meyer, E.; Negrin, R.S. TNF-α priming enhances CD4+FoxP3+ regulatory T-cell suppressive function in murine GVHD prevention and treatment. Blood 2016, 128, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.S.; Alegre, E.; LeMaoult, J.; Carosella, E.; Gonzalez, A. Regulatory role of tryptophan degradation pathway in HLA-G expression by human monocyte-derived dendritic cells. Mol. Immunol. 2006, 43, 2151–2160. [Google Scholar] [CrossRef] [PubMed]
- Shimony, O.; Nagler, A.; Gellman, Y.N.; Refaeli, E.; Rosenblum, N.; Eshkar-Sebban, L.; Yerushalmi, R.; Shimoni, A.; Lytton, S.D.; Stanevsky, A.; et al. Anti-T lymphocyte globulin (ATG) induces generation of regulatory T cells, at least part of them express activated CD44. J. Clin. Immunol. 2012, 32, 173–188. [Google Scholar] [CrossRef] [PubMed]
- Vogel, C.F.A.; Goth, S.R.; Dong, B.; Pessah, I.N.; Matsumura, F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem. Biophys. Res. Commun. 2008, 375, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Chen, G.; Shen, B.; Li, Y. Tim-3: An activation marker and activation limiter of innate immune cells. Front. Immunol. 2013, 4, 449. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Bai, X.; Cao, Y.; Wu, J.; Huang, M.; Tang, D.; Tao, S.; Zhu, T.; Liu, Y.; Yang, Y.; et al. Lymphoma endothelium preferentially expresses Tim-3 and facilitates the progression of lymphoma by mediating immune evasion. J. Exp. Med. 2010, 207, 505–520. [Google Scholar] [CrossRef] [PubMed]
- Andreesen, R.; Scheibenbogen, C.; Brugger, W.; Krause, S.; Meerpohl, H.G.; Leser, H.G.; Engler, H.; Lohr, G.W. Adoptive transfer of tumor cytotoxic macrophages generated in vitro from circulating blood monocytes: A new approach to cancer immunotherapy. Cancer Res. 1990, 50, 7450–7456. [Google Scholar] [PubMed]
- Dietl, K.; Renner, K.; Dettmer, K.; Timischl, B.; Eberhart, K.; Dorn, C.; Hellerbrand, C.; Kastenberger, M.; Kunz-Schughart, L.A.; Oefner, P.J.; et al. Lactic acid and acidification inhibit TNF secretion and glycolysis of human monocytes. J. Immunol. 2010, 184, 1200–1209. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Stevens, A.P.; Dettmer, K.; Gottfried, E.; Hoves, S.; Kreutz, M.; Holler, E.; Canelas, A.B.; Kema, I.; Oefner, P.J. Quantitative profiling of tryptophan metabolites in serum, urine, and cell culture supernatants by liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2011, 401, 3249–3261. [Google Scholar] [CrossRef] [PubMed]
- Gregori, S.; Passerini, L.; Roncarolo, M.G. Clinical outlook for type-1 and FOXP3+ T regulatory cell-based therapy. Front. Immunol. 2015, 6, 593. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roider, T.; Katzfuß, M.; Matos, C.; Singer, K.; Renner, K.; Oefner, P.J.; Dettmer-Wilde, K.; Herr, W.; Holler, E.; Kreutz, M.; et al. Antithymocyte Globulin Induces a Tolerogenic Phenotype in Human Dendritic Cells. Int. J. Mol. Sci. 2016, 17, 2081. https://doi.org/10.3390/ijms17122081
Roider T, Katzfuß M, Matos C, Singer K, Renner K, Oefner PJ, Dettmer-Wilde K, Herr W, Holler E, Kreutz M, et al. Antithymocyte Globulin Induces a Tolerogenic Phenotype in Human Dendritic Cells. International Journal of Molecular Sciences. 2016; 17(12):2081. https://doi.org/10.3390/ijms17122081
Chicago/Turabian StyleRoider, Tobias, Michael Katzfuß, Carina Matos, Katrin Singer, Kathrin Renner, Peter J. Oefner, Katja Dettmer-Wilde, Wolfgang Herr, Ernst Holler, Marina Kreutz, and et al. 2016. "Antithymocyte Globulin Induces a Tolerogenic Phenotype in Human Dendritic Cells" International Journal of Molecular Sciences 17, no. 12: 2081. https://doi.org/10.3390/ijms17122081
APA StyleRoider, T., Katzfuß, M., Matos, C., Singer, K., Renner, K., Oefner, P. J., Dettmer-Wilde, K., Herr, W., Holler, E., Kreutz, M., & Peter, K. (2016). Antithymocyte Globulin Induces a Tolerogenic Phenotype in Human Dendritic Cells. International Journal of Molecular Sciences, 17(12), 2081. https://doi.org/10.3390/ijms17122081





