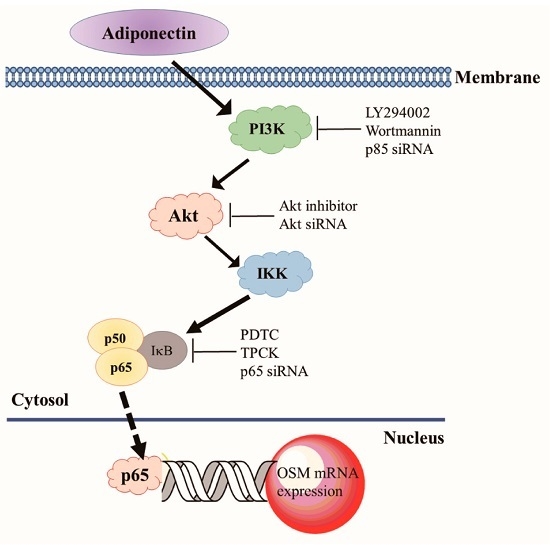
Adiponectin Induces Oncostatin M Expression in Osteoblasts through the PI3K/Akt Signaling Pathway
Abstract
:1. Introduction
2. Results
2.1. Adiponectin Increased OSM Production in Human Osteoblasts
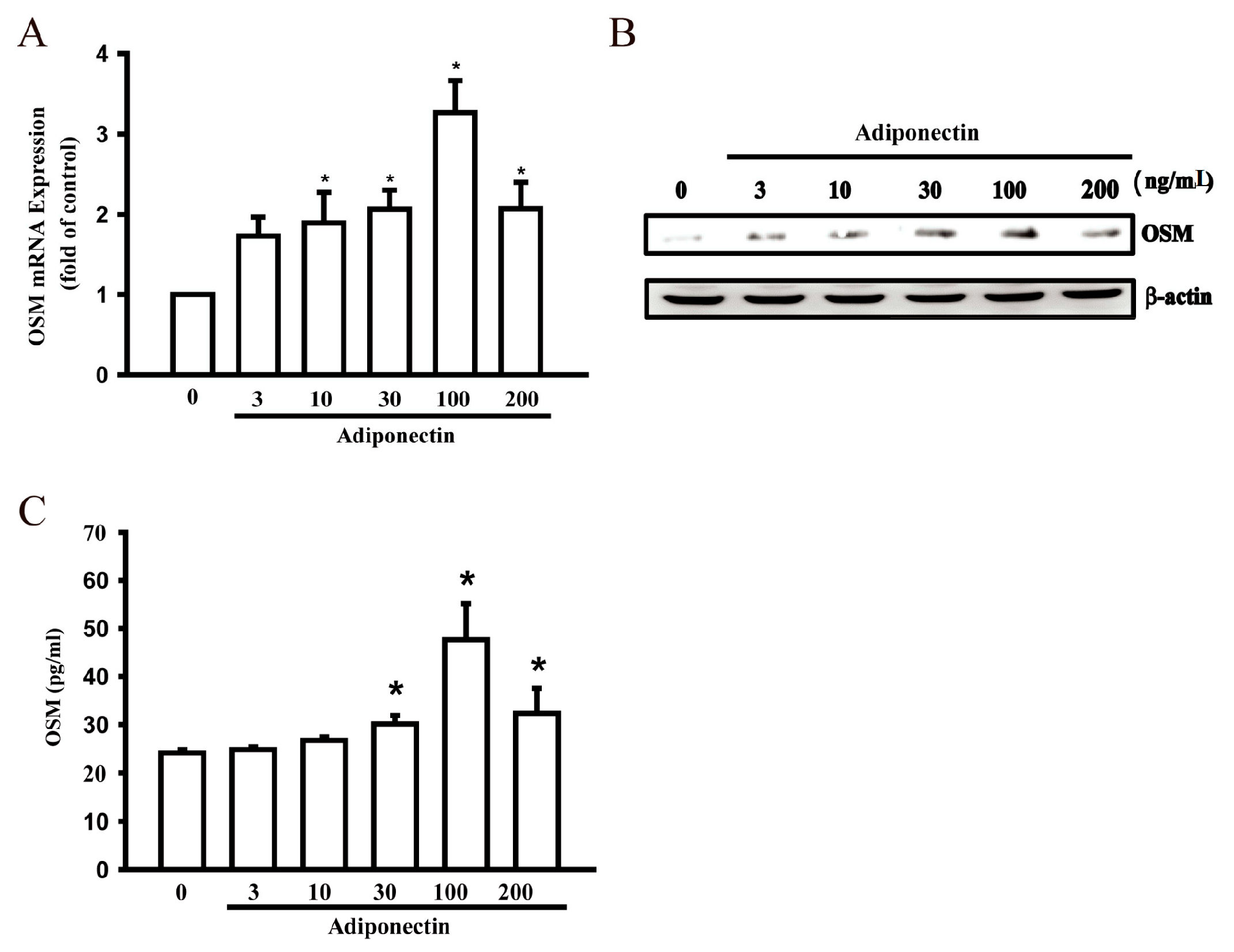
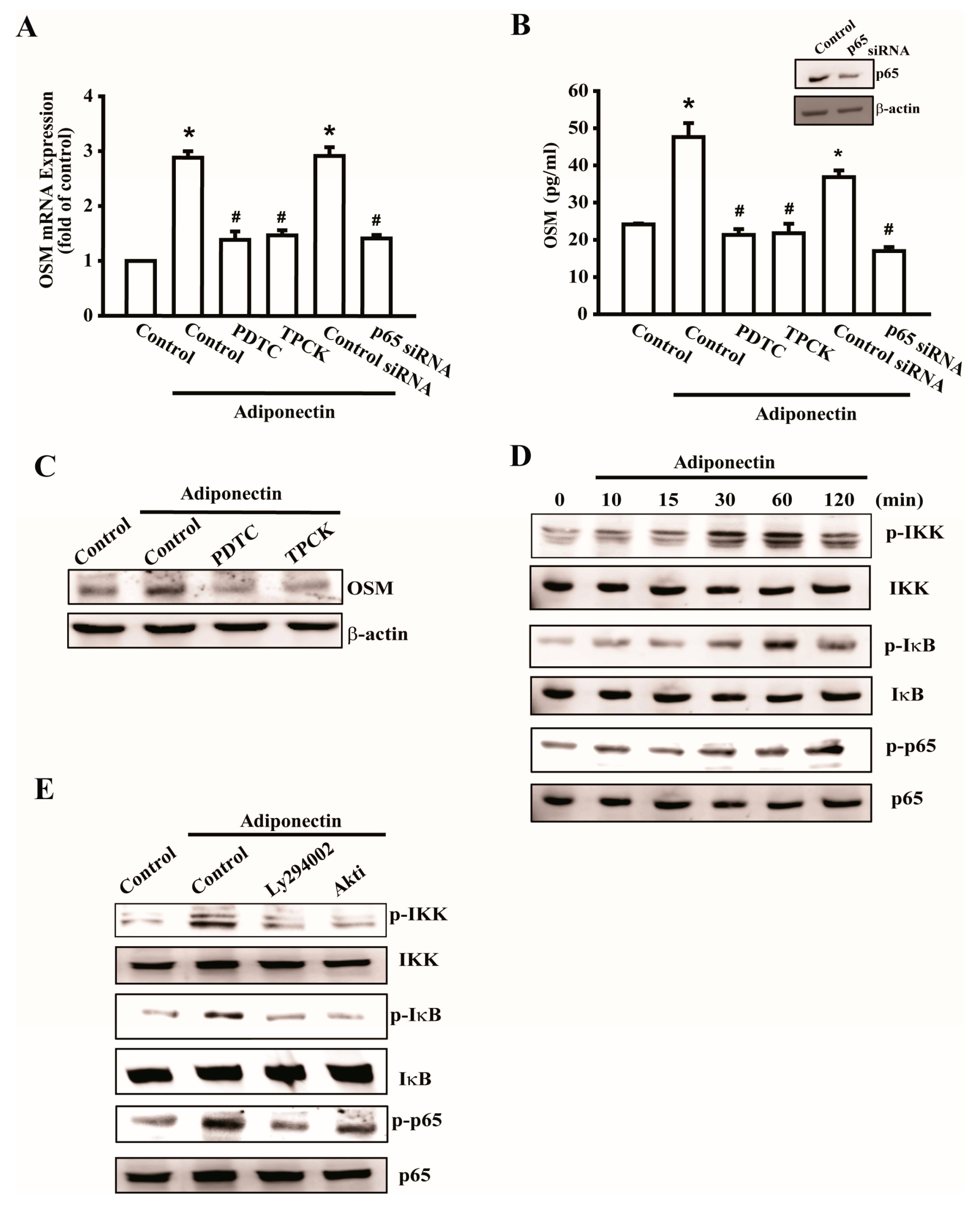
2.2. Signaling Pathways of PI3K Were Involved in Potentiating the Action of Adiponectin

2.3. Involvement of Akt in Adiponectin-Induced OSM Expression in Osteoblasts

2.4. Adiponectin Increased OSM Expression through the NF-κB Pathway

3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Western Blot Analysis
4.4. Quantitative Real-Time Polymerase Chain Reaction
4.5. Enzyme-Linked Immunosorbent Assay
4.6. Plasmid Construction
4.7. Transfection and Reporter Gene Assay
4.8. Chromatin Immunoprecipitation Assay
4.9. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McInnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.C.; Gravallese, E.M. Bone remodeling in rheumatic disease: A question of balance. Immunol. Rev. 2010, 233, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Versini, M.; Jeandel, P.Y.; Rosenthal, E.; Shoenfeld, Y. Obesity in autoimmune diseases: Not a passive bystander. Autoimmun. Rev. 2014, 13, 981–1000. [Google Scholar] [CrossRef] [PubMed]
- Otero, M.; Lago, R.; Gomez, R.; Lago, F.; Dieguez, C.; Gomez-Reino, J.J.; Gualillo, O. Changes in plasma levels of fat-derived hormones adiponectin, leptin, resistin and visfatin in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2006, 65, 1198–1201. [Google Scholar] [CrossRef] [PubMed]
- Kusunoki, N.; Kitahara, K.; Kojima, F.; Tanaka, N.; Kaneko, K.; Endo, H.; Suguro, T.; Kawai, S. Adiponectin stimulates prostaglandin E(2) production in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2010, 62, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.A.; Ji, H.I.; Lee, S.H.; Hong, S.J.; Yang, H.I.; Chul Yoo, M.; Kim, K.S. The role of adiponectin in the production of IL-6, IL-8, VEGF and MMPs in human endothelial cells and osteoblasts: Implications for arthritic joints. Exp. Mol. Med. 2014, 46. [Google Scholar] [CrossRef] [PubMed]
- Baum, R.; Gravallese, E.M. Impact of inflammation on the osteoblast in rheumatic diseases. Curr. Osteop. Rep. 2014, 12, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Bellido, T.; Stahl, N.; Farruggella, T.J.; Borba, V.; Yancopoulos, G.D.; Manolagas, S.C. Detection of receptors for interleukin-6, interleukin-11, leukemia inhibitory factor, oncostatin M, and ciliary neurotrophic factor in bone marrow stromal/osteoblastic cells. J. Clin. Investig. 1996, 97, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Cross, A.; Edwards, S.W.; Bucknall, R.C.; Moots, R.J. Secretion of oncostatin M by neutrophils in rheumatoid arthritis. Arthritis Rheum. 2004, 50, 1430–1436. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, H.; Yamamura, M.; Morita, Y.; Harada, S.; Makino, H.; Ota, Z. The synovial expression and serum levels of interleukin-6, interleukin-11, leukemia inhibitory factor, and oncostatin M in rheumatoid arthritis. Arthritis Rheum. 1997, 40, 1096–1105. [Google Scholar] [CrossRef] [PubMed]
- Su, C.M.; Chiang, Y.C.; Huang, C.Y.; Hsu, C.J.; Fong, Y.C.; Tang, C.H. Osteopontin promotes oncostatin m production in human osteoblasts: Implication of rheumatoid arthritis therapy. J. Immunol. 2015, 195, 3355–3364. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.H.; Tsai, C.H.; Fong, Y.C.; Huang, Y.L.; Wang, S.J.; Chang, Y.S.; Tang, C.H. Leptin induces oncostatin M production in osteoblasts by downregulating miR-93 through the Akt signaling pathway. Int. J. Mol. Sci. 2014, 15, 15778–15790. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Folco, E.J.; Shimizu, K.; Libby, P. Adiponectin induces pro-inflammatory programs in human macrophages and CD4+ T cells. J. Biol. Chem. 2012, 287, 36896–36904. [Google Scholar] [CrossRef] [PubMed]
- Van Stijn, C.M.; Kim, J.; Lusis, A.J.; Barish, G.D.; Tangirala, R.K. Macrophage polarization phenotype regulates adiponectin receptor expression and adiponectin anti-inflammatory response. FASEB J. 2015, 29, 636–649. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.; Kiba, A.; Zong, Y.; Witte, O.N. Interleukin-6 and oncostatin-M synergize with the PI3K/AKT pathway to promote aggressive prostate malignancy in mouse and human tissues. Mol. Cancer Res. 2013, 11, 1159–1165. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cohen, J.I. The role of PI3K/Akt in human herpesvirus infection: From the bench to the bedside. Virology 2015, 479, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Karsenty, G. Transcription factors in bone: Developmental and pathological aspects. Trends Mol. Med. 2002, 8, 340–345. [Google Scholar] [CrossRef]
- Persson, E.; Voznesensky, O.S.; Huang, Y.F.; Lerner, U.H. Increased expression of interleukin-6 by vasoactive intestinal peptide is associated with regulation of CREB, AP-1 and C/EBP, but not NF-κB, in mouse calvarial osteoblasts. Bone 2005, 37, 513–529. [Google Scholar] [CrossRef] [PubMed]
- Bugatti, S.; Manzo, A.; Caporali, R.; Montecucco, C. Inflammatory lesions in the bone marrow of rheumatoid arthritis patients: A morphological perspective. Arthritis Res. Ther. 2012, 14. [Google Scholar] [CrossRef] [PubMed]
- Haavardsholm, E.A.; Boyesen, P.; Ostergaard, M.; Schildvold, A.; Kvien, T.K. Magnetic resonance imaging findings in 84 patients with early rheumatoid arthritis: Bone marrow oedema predicts erosive progression. Ann. Rheum. Dis. 2008, 67, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Amarasekara, D.S.; Yu, J.; Rho, J. Bone loss triggered by the cytokine network in inflammatory autoimmune diseases. J. Immunol. Res. 2015, 2015, 832127. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Y.; Chen, C.Y.; Chuang, T.Y.; Lin, Y.; Liu, H.Y.; Mersmann, H.J.; Wu, S.C.; Ding, S.T. Adiponectin receptor 1 regulates bone formation and osteoblast differentiation by GSK-3β/β-catenin signaling in mice. Bone 2014, 64, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.T.; Tsou, H.K.; Chen, J.C.; Shih, J.M.; Chen, Y.J.; Tang, C.H. Adiponectin enhances intercellular adhesion molecule-1 expression and promotes monocyte adhesion in human synovial fibroblasts. PLoS ONE 2014, 9, e92741. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.C.; Shieh, D.C.; Tong, K.M.; Chen, C.P.; Huang, K.C.; Chen, P.C.; Fong, Y.C.; Hsu, H.C.; Tang, C.H. Involvement of AdipoR receptor in adiponectin-induced motility and α2β1 integrin upregulation in human chondrosarcoma cells. Carcinogenesis 2009, 30, 1651–1659. [Google Scholar] [CrossRef] [PubMed]
- Gatselis, N.K.; Ntaios, G.; Makaritsis, K.; Dalekos, G.N. Adiponectin: A key playmaker adipocytokine in non-alcoholic fatty liver disease. Clin. Exp. Med. 2014, 14, 121–131. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Lu, J.; Bao, J.; Guo, J.; Shi, J.; Wang, Y. Adiponectin: A biomarker for rheumatoid arthritis? Cytokine Growth Factor Rev. 2013, 24, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Senolt, L.; Pavelka, K.; Housa, D.; Haluzik, M. Increased adiponectin is negatively linked to the local inflammatory process in patients with rheumatoid arthritis. Cytokine 2006, 35, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Jay, P.R.; Centrella, M.; Lorenzo, J.; Bruce, A.G.; Horowitz, M.C. Oncostatin-M: A new bone active cytokine that activates osteoblasts and inhibits bone resorption. Endocrinology 1996, 137, 1151–1158. [Google Scholar] [PubMed]
- Ciou, S.Y.; Hsu, C.C.; Kuo, Y.H.; Chao, C.Y. Effect of wild bitter gourd treatment on inflammatory responses in BALB/c mice with sepsis. Biomedicine 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Sims, N.A.; Quinn, J.M. Osteoimmunology: Oncostatin M as a pleiotropic regulator of bone formation and resorption in health and disease. BoneKEy Rep. 2014, 3, 527. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, L.C.; Chen, H.; Lu, X.; Nanes, M.S. Chronic low dose tumor necrosis factor-α (TNF) suppresses early bone accrual in young mice by inhibiting osteoblasts without affecting osteoclasts. Bone 2013, 56, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.Y.; Su, C.M.; Huang, Y.L.; Tsai, C.H.; Fuh, L.J.; Tang, C.H. CCN1 induces oncostatin M production in osteoblasts via integrin-dependent signal pathways. PLoS ONE 2014, 9, e106632. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, T.; Kadowaki, T. Adiponectin receptor as a key player in healthy longevity and obesity-related diseases. Cell Metab. 2013, 17, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.P.; Lin, C.Y.; Shih, J.S.; Fong, Y.C.; Wang, S.W.; Li, T.M.; Tang, C.H. Adiponectin promotes VEGF-A-dependent angiogenesis in human chondrosarcoma through PI3K, Akt, mTOR, and HIF-α pathway. Oncotarget 2015, 6, 36746–36761. [Google Scholar] [PubMed]
- Wang, W.; Abbruzzese, J.L.; Evans, D.B.; Larry, L.; Cleary, K.R.; Chiao, P.J. The nuclear factor-κB RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin. Cancer Res. 1999, 5, 119–127. [Google Scholar] [PubMed]
- Su, C.M.; Hsu, C.J.; Tsai, C.H.; Huang, C.Y.; Wang, S.W.; Tang, C.H. Resistin promotes angiogenesis in endothelial progenitor cells through inhibition of MicroRNA206: Potential implications for rheumatoid arthritis. Stem Cells 2015, 33, 2243–2255. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, C.-M.; Lee, W.-L.; Hsu, C.-J.; Lu, T.-T.; Wang, L.-H.; Xu, G.-H.; Tang, C.-H. Adiponectin Induces Oncostatin M Expression in Osteoblasts through the PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2016, 17, 29. https://doi.org/10.3390/ijms17010029
Su C-M, Lee W-L, Hsu C-J, Lu T-T, Wang L-H, Xu G-H, Tang C-H. Adiponectin Induces Oncostatin M Expression in Osteoblasts through the PI3K/Akt Signaling Pathway. International Journal of Molecular Sciences. 2016; 17(1):29. https://doi.org/10.3390/ijms17010029
Chicago/Turabian StyleSu, Chen-Ming, Wei-Lin Lee, Chin-Jung Hsu, Ting-Ting Lu, Li-Hong Wang, Guo-Hong Xu, and Chih-Hsin Tang. 2016. "Adiponectin Induces Oncostatin M Expression in Osteoblasts through the PI3K/Akt Signaling Pathway" International Journal of Molecular Sciences 17, no. 1: 29. https://doi.org/10.3390/ijms17010029