SOX2 Promotes the Epithelial to Mesenchymal Transition of Esophageal Squamous Cells by Modulating Slug Expression through the Activation of STAT3/HIF-α Signaling
Abstract
:1. Introduction
2. Results
2.1. SOX2 over Expression Promotes the Migration and Invasion of Eca-109 Cells
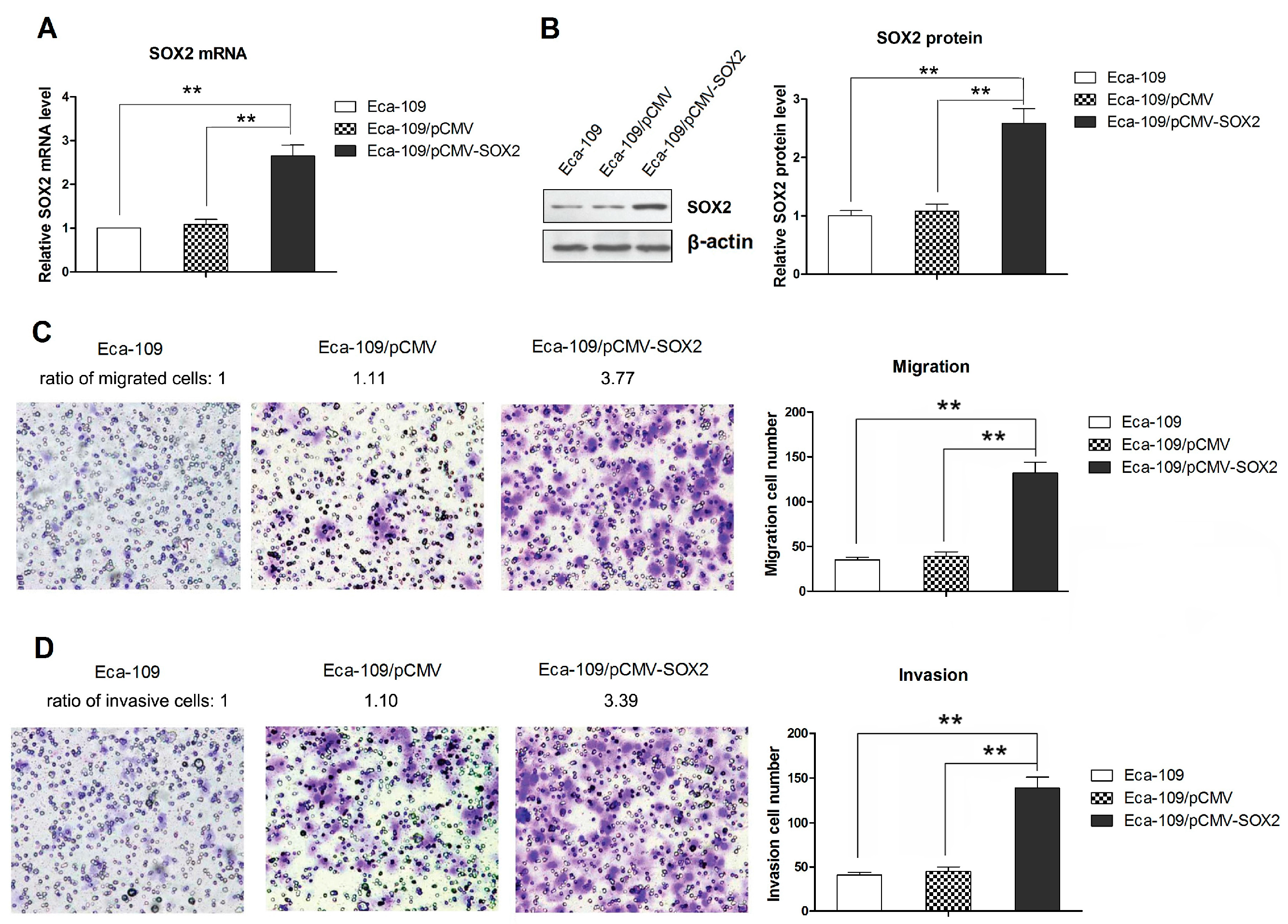
2.2. SOX2 Promotes the Process of EMT in Eca-109 Cells
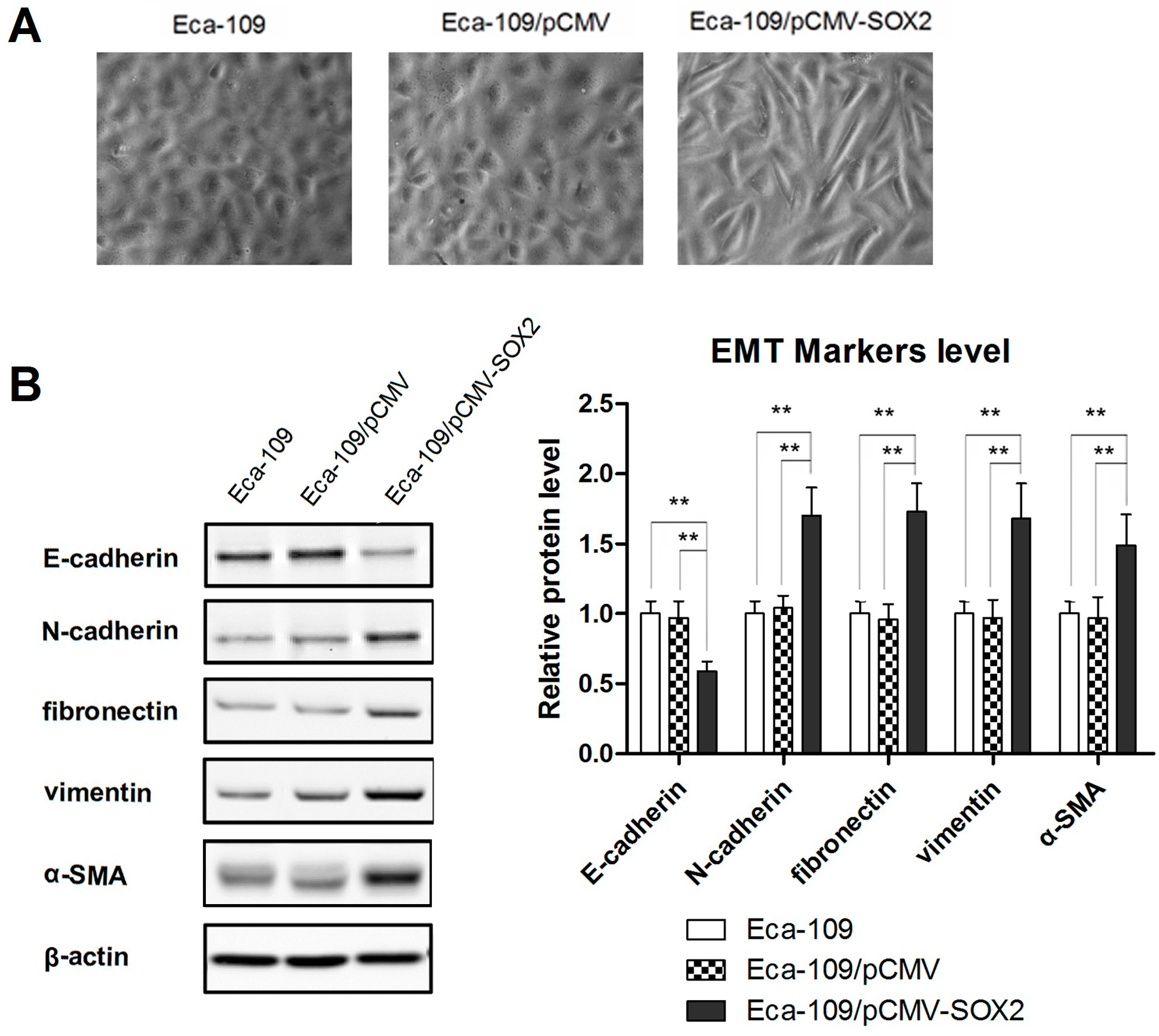
2.3. SOX2-Promoted EMT Is Dependent on the Activity of Slug
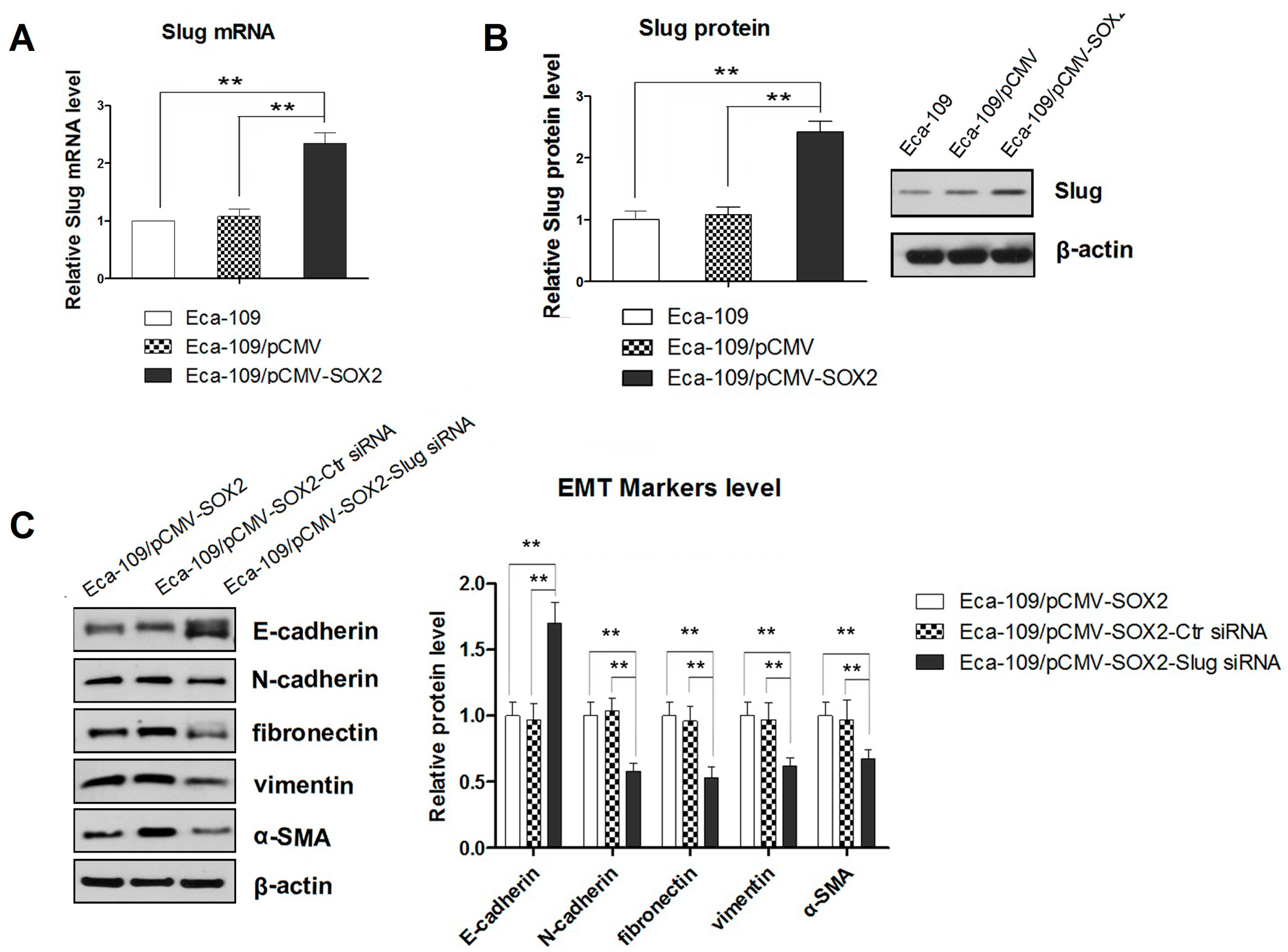
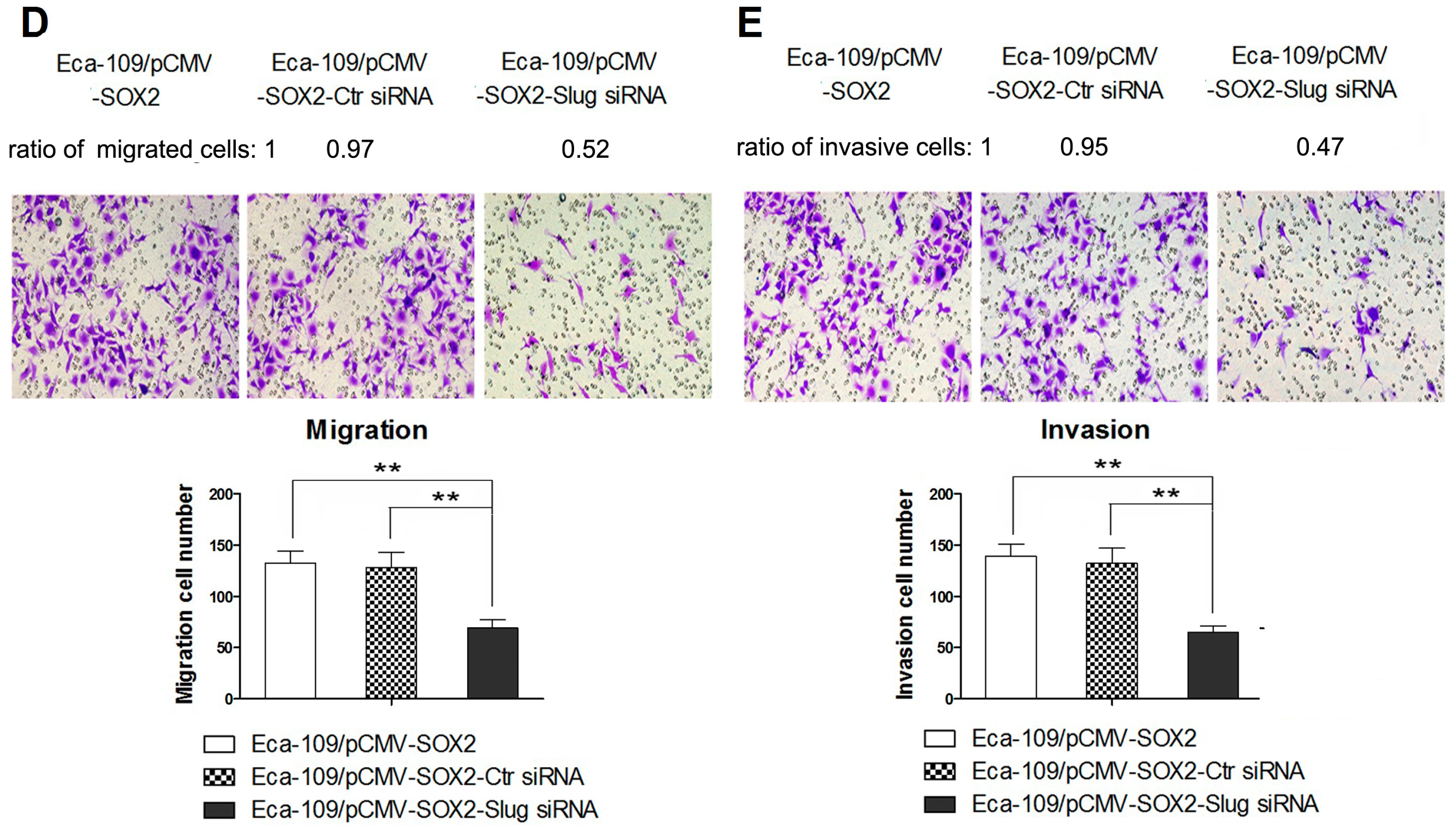
2.4. STAT3/HIF-1α Signaling Is Involved in SOX2-Induced Up-Regulation of Slug Expression in Eca109 Cells
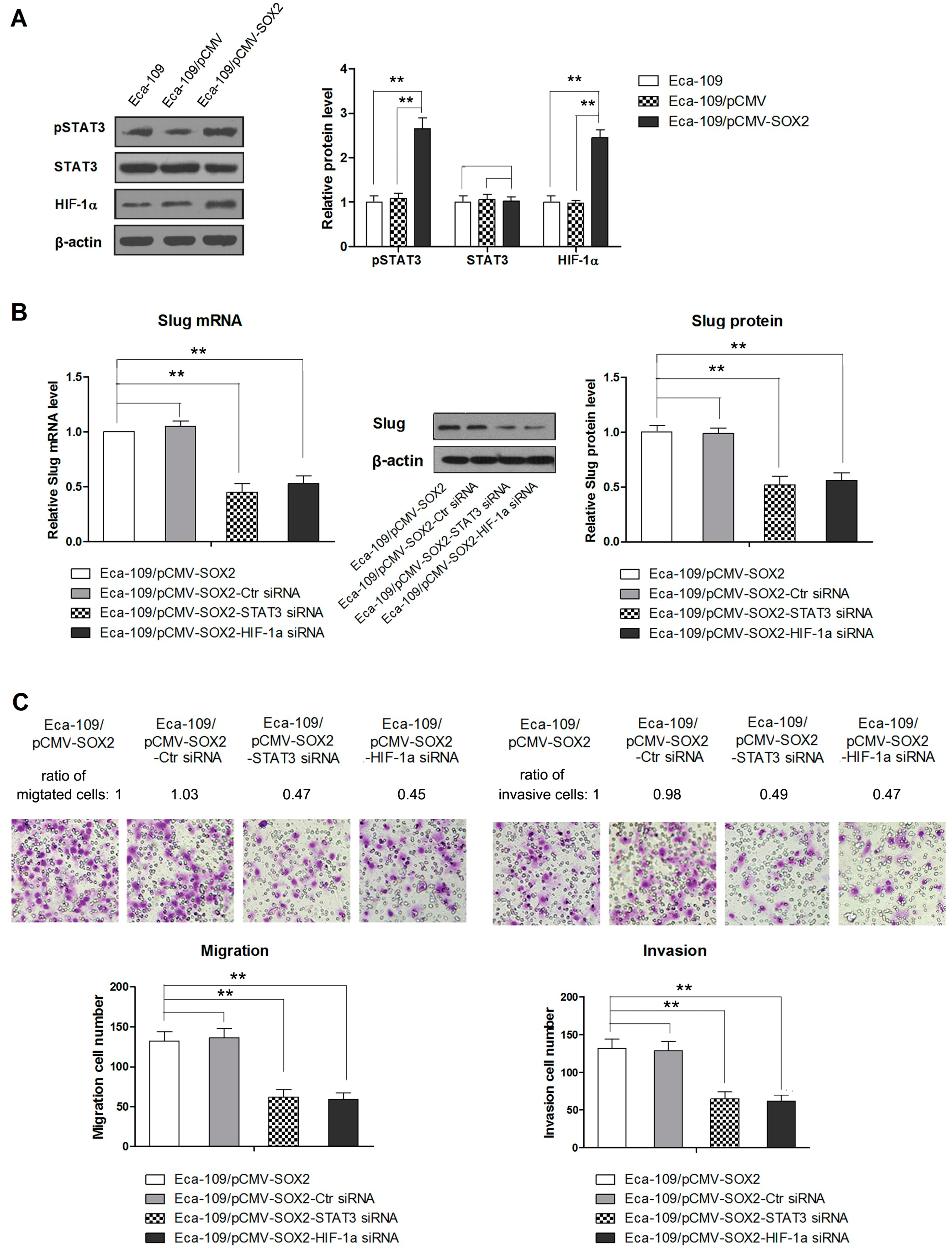
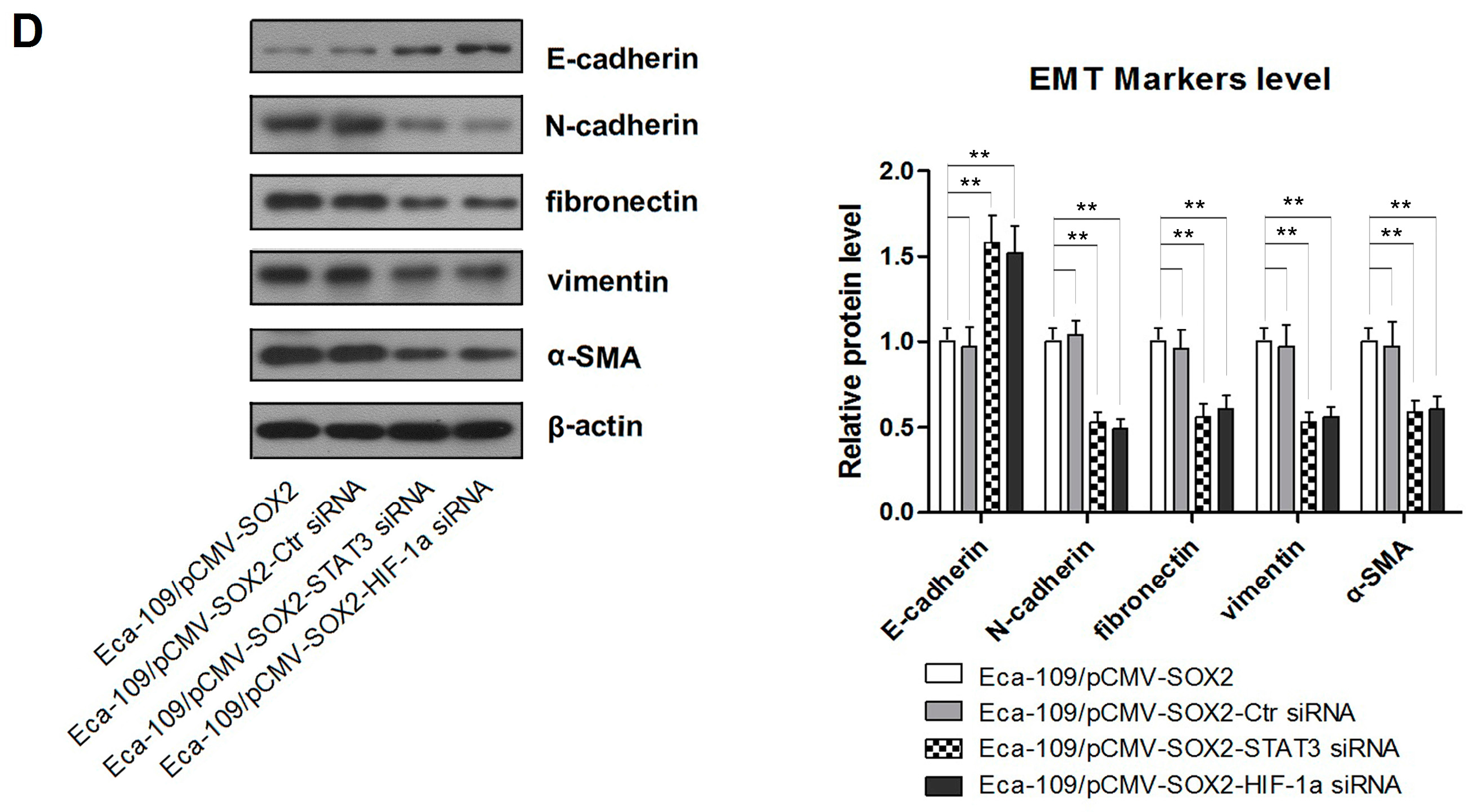
3. Discussions
4. Experimental Section
4.1. Cell Culture
4.2. Generation of Plasmid Constructs and Establishment of SOX2-Ovexpressing Cell Line
4.3. Quantitative RT-PCR (qRT-PCR)
4.4. Western Blotting
4.5. Transwell Migration Assay
4.6. Invasion Assay
4.7. Knockdown of STAT3, HIF-1α and Slug
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sakai, N.S.; Samia-Aly, E.; Barbera, M.; Fitzgerald, R.C. A review of the current understanding and clinical utility of miRNAs in esophageal cancer. Semin. Cancer Biol. 2013, 23, 512–521. [Google Scholar] [CrossRef] [PubMed]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [PubMed]
- Sagar, P.M.; Gauperaa, T.; Sue-Ling, H.; McMahon, M.J.; Johnston, D. An audit of the treatment of cancer of the oesophagus. Gut 1994, 35, 941–945. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; Grobmyer, S.R.; Smith, R.; Ben-David, K.; Ang, D.; Vogel, S.B.; Hochwald, S.N. Esophageal cancer—The five year survivors. J. Surg. Oncol. 2011, 103, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Pevny, L.H.; Lovell-Badge, R. SOX genes find their feet. Curr. Opin. Genet. Dev. 1997, 7, 338–344. [Google Scholar] [CrossRef]
- Stevanovic, M.; Zuffardi, O.; Collignon, J.; Lovell-Badge, R.; Goodfellow, P. The cDNA sequence and chromosomal location of the human SOX2 gene. Off. J. Int. Mamm. Genome Soc. 1994, 5, 640–642. [Google Scholar] [CrossRef]
- Masui, S.; Nakatake, Y.; Toyooka, Y.; Shimosato, D.; Yagi, R.; Takahashi, K.; Okochi, H.; Okuda, A.; Matoba, R.; Sharov, A.A.; et al. Pluripotency governed by SOX2 via regulation of OCT3/4 expression in mouse embryonic stem cells. Nat. Cell Biol. 2007, 9, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Fong, H.; Hohenstein, K.A.; Donovan, P.J. Regulation of self-renewal and pluripotency by SOX2 in human embryonic stem cells. Stem Cells 2008, 26, 1931–1938. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Tanabe, K.; Ohnuki, M.; Narita, M.; Ichisaka, T.; Tomoda, K.; Yamanaka, S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007, 131, 861–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williamson, K.A.; Hever, A.M.; Rainger, J.; Rogers, R.C.; Magee, A.; Fiedler, Z.; Keng, W.T.; Sharkey, F.H.; McGill, N.; Hill, C.J.; et al. Mutations in SOX2 cause anophthalmia-esophageal-genital (AEG) syndrome. Hum. Mol. Genet. 2006, 15, 1413–1422. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.M.; Diez-Valle, R.; Manterola, L.; Rubio, A.; Liu, D.; Cortes-Santiago, N.; Urquiza, L.; Jauregi, P.; Lopez de Munain, A.; Sampron, N.; et al. Genetic and epigenetic modifications of SOX2 contribute to the invasive phenotype of malignant gliomas. PLoS ONE 2011, 6, e26740. [Google Scholar] [CrossRef] [PubMed]
- Gen, Y.; Yasui, K.; Zen, Y.; Zen, K.; Dohi, O.; Endo, M.; Tsuji, K.; Wakabayashi, N.; Itoh, Y.; Naito, Y.; et al. SOX2 identified as a target gene for the amplification at 3q26 that is frequently detected in esophageal squamous cell carcinoma. Cancer Genet. Cytogenet. 2010, 202, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Gen, Y.; Yasui, K.; Nishikawa, T.; Yoshikawa, T. SOX2 promotes tumor growth of esophageal squamous cell carcinoma through the Akt/mammalian target of rapamycin complex 1 signaling pathway. Cancer Sci. 2013, 104, 810–816. [Google Scholar] [CrossRef] [PubMed]
- Steeg, P.S. Tumor metastasis: Mechanistic insights and clinical challenges. Nat. Med. 2006, 12, 895–904. [Google Scholar] [CrossRef] [PubMed]
- De Craene, B.; Berx, G. Regulatory networks defining EMT during cancer initiation and progression. Nat. Rev. Cancer 2013, 13, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Li, H.; Wang, S.; Liu, M.; Feng, Y.; Wang, X. Icariin protects rat cardiac H9c2 cells from apoptosis by inhibiting endoplasmic reticulum stress. Int. J. Mol. Sci. 2013, 14, 17845–17860. [Google Scholar] [CrossRef] [PubMed]
- Guarino, M. Epithelial-mesenchymal transition and tumour invasion. Int. J. Biochem. Cell Biol. 2007, 39, 2153–2160. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Lv, G.; Chu, Z.; Piao, L.; Liu, X.; Wang, T.; Jiang, Y.; Zhang, P. Identification of natural splice variants of SAMHD1 in virus-infected HCC. Oncol. Rep. 2014, 31, 687–692. [Google Scholar] [PubMed]
- Stolzenburg, S.; Rots, M.G.; Beltran, A.S.; Rivenbark, A.G.; Yuan, X.; Qian, H.; Strahl, B.D.; Blancafort, P. Targeted silencing of the oncogenic transcription factor SOX2 in breast cancer. Nucleic Acids Res. 2012, 40, 6725–6740. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.C.; Wang, C.H.; Wu, L.C.; Hsia, C.Y.; Chi, C.W.; Yin, P.H.; Chang, C.J.; Sung, M.T.; Wei, Y.H.; Lu, S.H.; et al. Mitochondrial dysfunction represses HIF-1α protein synthesis through ampk activation in human hepatoma HEPG2 cells. Biochim. Biophys. Acta 2013, 1830, 4743–4751. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Liu, W.; Yuan, Q.; Liu, X.; Ou, Y.; He, S.; Yuan, S.; Qin, L.; Chen, Q.; Nong, K.; et al. Silencing of DLGAP5 by siRNA significantly inhibits the proliferation and invasion of hepatocellular carcinoma cells. PLoS ONE 2013, 8, e80789. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zheng, C.; Zhang, L.; Chen, Y.; Ye, Y.; Zhao, M. Knockdown of STAT3 expression in SKOV3 cells by biodegradable siRNA-PLGA/CSO conjugate micelles. Collods Surf. B Biointerfaces 2015, 127C, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.R.; Sharma, R.; Saraya, A.; Chattopadhyay, T.K.; DattaGupta, S.; Walfish, P.G.; Chauhan, S.S.; Ralhan, R. Slug is a predictor of poor prognosis in esophageal squamous cell carcinoma patients. PLoS ONE 2013, 8, e82846. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Jeong, K.J.; Shin, S.C.; Kang, J.; Park, C.G.; Lee, H.Y. STAT3 mediates TGF-β1-induced TWIST1 expression and prostate cancer invasion. Cancer Lett. 2013, 336, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.H.; Choi, M.J.; Jeong, K.J.; Kim, J.J.; Hwang, M.H.; Shin, S.C.; Park, C.G.; Lee, H.Y. A ROS/STAT3/HIF-1α signaling cascade mediates EGF-induced TWIST1 expression and prostate cancer cell invasion. Prostate 2014, 74, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Sun, B.; Zhao, X.; Gu, Q.; Dong, X.; Mo, J.; Sun, T.; Wang, J.; Sun, R.; Liu, Y. Hypoxia promotes vasculogenic mimicry formation by inducing epithelial-mesenchymal transition in ovarian carcinoma. Gynecol. Oncol. 2014, 133, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Cheng, Q.; Zhou, Y.; Wang, Y.; Chen, X. Slug is a key mediator of hypoxia induced cadherin switch in hnscc: Correlations with poor prognosis. Oral Oncol. 2013, 49, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.R.; Zhang, H.Q.; Qu, Y.; Mu, J.; Zhao, D.; Zhou, L.J.; Yan, H.; Ye, J.W.; Liu, Y. Expression of MET and SOX2 genes in non-small cell lung carcinoma with EGFR mutation. Oncol. Rep. 2011, 26, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Lengerke, C.; Fehm, T.; Kurth, R.; Neubauer, H.; Scheble, V.; Muller, F.; Schneider, F.; Petersen, K.; Wallwiener, D.; Kanz, L.; et al. Expression of the embryonic stem cell marker SOX2 in early-stage breast carcinoma. BMC Cancer 2011, 11. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Sun, L.; Li, Y.; Kang, X.; Zhang, S.; Liu, Y. SOX2 expression predicts poor survival of hepatocellular carcinoma patients and it promotes liver cancer cell invasion by activating Slug. Med. Oncol. 2013, 30. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Pan, X.; Gao, A.; Zhu, W. Expression of SOX2 in cervical squamous cell carcinoma. Off. J. Balk. Union Oncol. 2014, 19, 203–206. [Google Scholar]
- Wang, S.; Tie, J.; Wang, R.; Hu, F.; Gao, L.; Wang, W.; Wang, L.; Li, Z.; Hu, S.; Tang, S.; et al. SOX2, a predictor of survival in gastric cancer, inhibits cell proliferation and metastasis by regulating pten. Cancer Lett. 2015, 358, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, C.; Fan, J.; Xiao, L.; Yin, B.; Zhou, L.; Xia, S. Comprehensive analysis of clinical significance of stem-cell related factors in renal cell cancer. World J. Surg. Oncol. 2011, 9, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Toschi, L.; Finocchiaro, G.; Nguyen, T.T.; Skokan, M.C.; Giordano, L.; Gianoncelli, L.; Perrino, M.; Siracusano, L.; di Tommaso, L.; Infante, M.; et al. Increased SOX2 gene copy number is associated with FGFR1 and PIK3CA gene gain in non-small cell lung cancer and predicts improved survival in early stage disease. PLoS ONE 2014, 9, e95303. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Marquez, R.; Llorente, J.L.; Rodrigo, J.P.; Garcia-Pedrero, J.M.; Alvarez-Marcos, C.; Suarez, C.; Hermsen, M.A. SOX2 expression in hypopharyngeal, laryngeal, and sinonasal squamous cell carcinoma. Hum. Pathol. 2014, 45, 851–857. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.B.; Shen, X.H.; Li, L.; Zhang, Y.F.; Chen, G.Q. SOX2 overexpression correlates with poor prognosis in laryngeal squamous cell carcinoma. Auris Nasus Larynx 2013, 40, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chang, D.Y.; Mercado-Uribe, I.; Liu, J. Sex-determining region Y-box 2 expression predicts poor prognosis in human ovarian carcinoma. Hum. Pathol. 2012, 43, 1405–1412. [Google Scholar] [CrossRef] [PubMed]
- Honing, J.; Pavlov, K.V.; Meijer, C.; Smit, J.K.; Boersma-van Ek, W.; Karrenbeld, A.; Burgerhof, J.G.; Kruyt, F.A.; Plukker, J.T. Loss of CD44 and SOX2 expression is correlated with a poor prognosis in esophageal adenocarcinoma patients. Ann. Surg. Oncol. 2014, 21, S657–S664. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Weinberg, R.A. Epithelial-mesenchymal transition: At the crossroads of development and tumor metastasis. Dev. Cell 2008, 14, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Fang, X.; Lou, X.; Hua, D.; Ding, W.; Foltz, G.; Hood, L.; Yuan, Y.; Lin, B. Silencing SOX2 induced mesenchymal-epithelial transition and its expression predicts liver and lymph node metastasis of crc patients. PLoS ONE 2012, 7, e41335. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, Y.; Chen, Y.; Chen, S.; Jia, X.; Sun, T.; Liu, Y.; Li, X.; Xiang, R.; Li, N. SOX2 promotes tumor metastasis by stimulating epithelial-to-mesenchymal transition via regulation of Wnt/β-catenin signal network. Cancer Lett. 2013, 336, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Hui, L.; Wang, Y.; Yang, H.; Jiang, X. SOX2 promotes the migration and invasion of laryngeal cancer cells by induction of MMP-2 via the PI3K/Akt/mTOR pathway. Oncol. Rep. 2014, 31, 2651–2659. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.C.; Carneiro, F.; Hoefler, H.; Becker, K.F. Role of the epithelial-mesenchymal transition regulator Slug in primary human cancers. Front. Biosci. 2009, 14, 3035–3050. [Google Scholar] [CrossRef]
- Shih, J.Y.; Yang, P.C. The EMT regulator slug and lung carcinogenesis. Carcinogenesis 2011, 32, 1299–1304. [Google Scholar] [CrossRef] [PubMed]
- Herreros-Villanueva, M.; Zhang, J.S.; Koenig, A.; Abel, E.V.; Smyrk, T.C.; Bamlet, W.R.; de Narvajas, A.A.; Gomez, T.S.; Simeone, D.M.; Bujanda, L.; et al. SOX2 promotes dedifferentiation and imparts stem cell-like features to pancreatic cancer cells. Oncogenesis 2013, 2. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, X.; Shao, S.; Zuo, X.; Ning, Q.; Luo, M.; Gu, S.; Zhao, X. Notch1 induces epithelial-mesenchymal transition and the cancer stem cell phenotype in breast cancer cells and STAT3 plays a key role. Int. J. Oncol. 2015, 46, 1141–1148. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.H.; Chen, M.T.; Wang, M.L.; Lee, Y.Y.; Chiou, G.Y.; Chien, C.S.; Huang, P.I.; Chen, Y.W.; Huang, M.C.; Chiou, S.H.; et al. Cisplatin-selected resistance is associated with increased motility and stem-like properties via activation of STAT3/Snail axis in atypical teratoid/rhabdoid tumor cells. Oncotarget 2015, 6, 1750–1768. [Google Scholar] [PubMed]
- Mou, W.; Xu, Y.; Ye, Y.; Chen, S.; Li, X.; Gong, K.; Liu, Y.; Chen, Y.; Li, X.; Tian, Y.; et al. Expression of SOX2 in breast cancer cells promotes the recruitment of M2 macrophages to tumor microenvironment. Cancer Lett. 2015, 358, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yi, L.; Ouyang, Q.; Xu, L.; Cui, H.; Xu, M. Neurotensin signaling regulates stem-like traits of glioblastoma stem cells through activation of IL-8/CXCR1/STAT3 pathway. Cell. Signal. 2014, 26, 2896–2902. [Google Scholar] [CrossRef] [PubMed]
- Mathieu, J.; Zhang, Z.; Zhou, W.; Wang, A.J.; Heddleston, J.M.; Pinna, C.M.; Hubaud, A.; Stadler, B.; Choi, M.; Bar, M.; et al. HIF induces human embryonic stem cell markers in cancer cells. Cancer Res. 2011, 71, 4640–4652. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Pan, C.; Sun, J.; Gilbert, C.; Drews-Elger, K.; Azzam, D.J.; Picon-Ruiz, M.; Kim, M.; Ullmer, W.; El-Ashry, D.; et al. VEGF drives cancer-initiating stem cells through VEGFR-2/STAT3 signaling to upregulate Myc and SOX2. Oncogene 2014, 34, 3107–3119. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, H.; Teng, C.; Huang, W.; Peng, J.; Wang, C. SOX2 Promotes the Epithelial to Mesenchymal Transition of Esophageal Squamous Cells by Modulating Slug Expression through the Activation of STAT3/HIF-α Signaling. Int. J. Mol. Sci. 2015, 16, 21643-21657. https://doi.org/10.3390/ijms160921643
Gao H, Teng C, Huang W, Peng J, Wang C. SOX2 Promotes the Epithelial to Mesenchymal Transition of Esophageal Squamous Cells by Modulating Slug Expression through the Activation of STAT3/HIF-α Signaling. International Journal of Molecular Sciences. 2015; 16(9):21643-21657. https://doi.org/10.3390/ijms160921643
Chicago/Turabian StyleGao, Hui, Chunyuan Teng, Wenjing Huang, Jianjun Peng, and Chunbo Wang. 2015. "SOX2 Promotes the Epithelial to Mesenchymal Transition of Esophageal Squamous Cells by Modulating Slug Expression through the Activation of STAT3/HIF-α Signaling" International Journal of Molecular Sciences 16, no. 9: 21643-21657. https://doi.org/10.3390/ijms160921643





