Effect of Hepatitis C Virus Genotype 1b Core and NS5A Mutations on Response to Peginterferon Plus Ribavirin Combination Therapy
Abstract
:1. Introduction
2. Results
2.1. Response to Therapy
2.2. Specific Amino Acid Patterns in the Core Region According to Virological Response
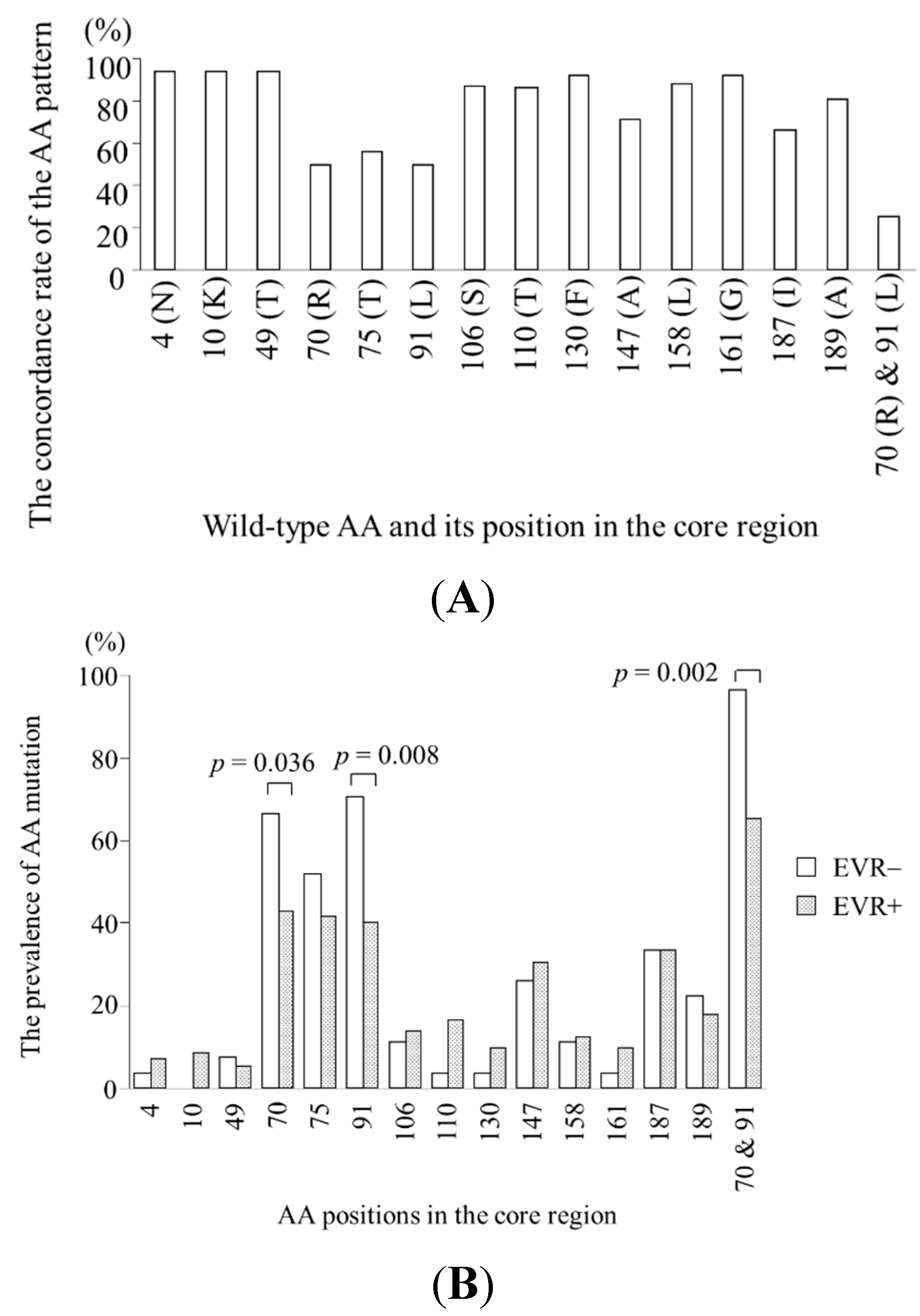

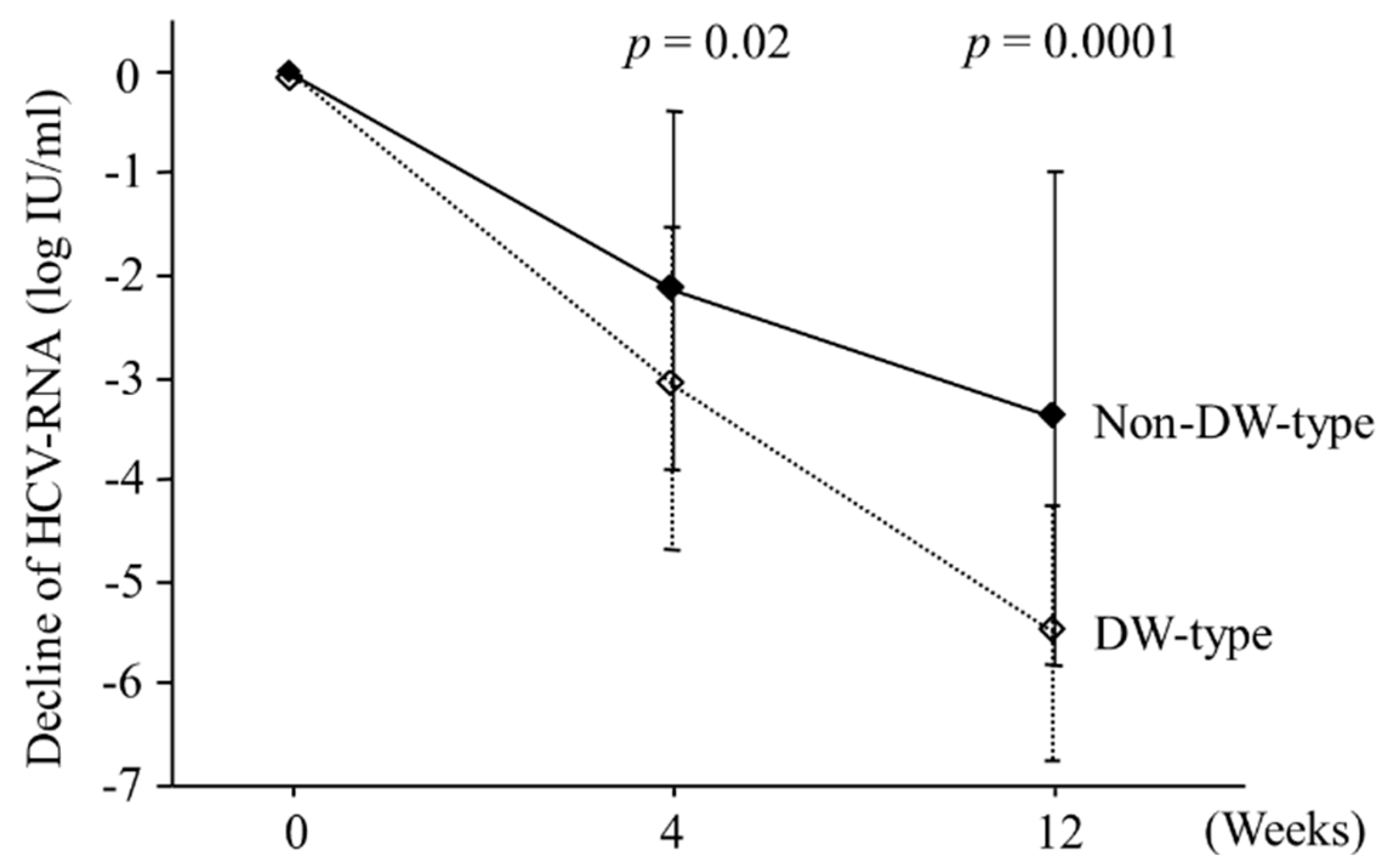
2.3. Viral Kinetics According to Amino Acid Patterns in the Core Region
2.4. Predictors of EVR as Determined by Univariate and Multivariate Analysis
| Factor | Category | Odds Ratio (95% CI) | p Value |
|---|---|---|---|
| Platelet (/mm3) | ≥14 × 104 | 3.17 (1.17–8.60) | 0.02 |
| <14 × 104 | 1 | ||
| Core positions 70 and 91 | DW-type | 11.7 (1.46–94.5) | 0.02 |
| Non–DW-type | 1 |
2.5. Predictors of SVR as Determined by Univariate and Multivariate Analysis
| Factor | Category | Odds Ratio (95% CI) | p Value |
|---|---|---|---|
| Hemoglobin (g/dL) | ≥14 | 6.21 (1.07–35.9) | 0.04 |
| <14 | 1 | ||
| Platelet (/mm3) | ≥14 × 104 | 5.15 (1.11–23.8) | 0.04 |
| <14 × 104 | 1 | ||
| Core positions 70 and 91 | DW-type | 6.76 (1.37–33.3) | 0.02 |
| Non-DW-type | 1 | ||
| Number of mutations in the ISDR | ≥2 | 12.3 (1.41–107) | 0.02 |
| <2 | 1 | ||
| Core position 110 | Non-wild | 14.5 (1.31–167) | 0.03 |
| Wild | 1 | ||
| AST/ALT | <0.9 | 6.17 (1.58–23.8) | 0.009 |
| ≥0.9 | 1 |
2.6. Predictors of NR as Determined by Univariate and Multivariate Analysis
| Factor | Category | Odds Ratio (95% CI) | p Value |
|---|---|---|---|
| Platelet (/mm3) | ≥14 × 104 | 0.32 (0.12–0.86) | 0.02 |
| <14 × 104 | 1 | ||
| Core positions 70 and 91 | DW-type | 0.10 (0.012–0.79) | 0.03 |
| Non-DW-type | 1 |
2.7. Analysis of Factors Associated with DW-Type at Positions 70 and 91
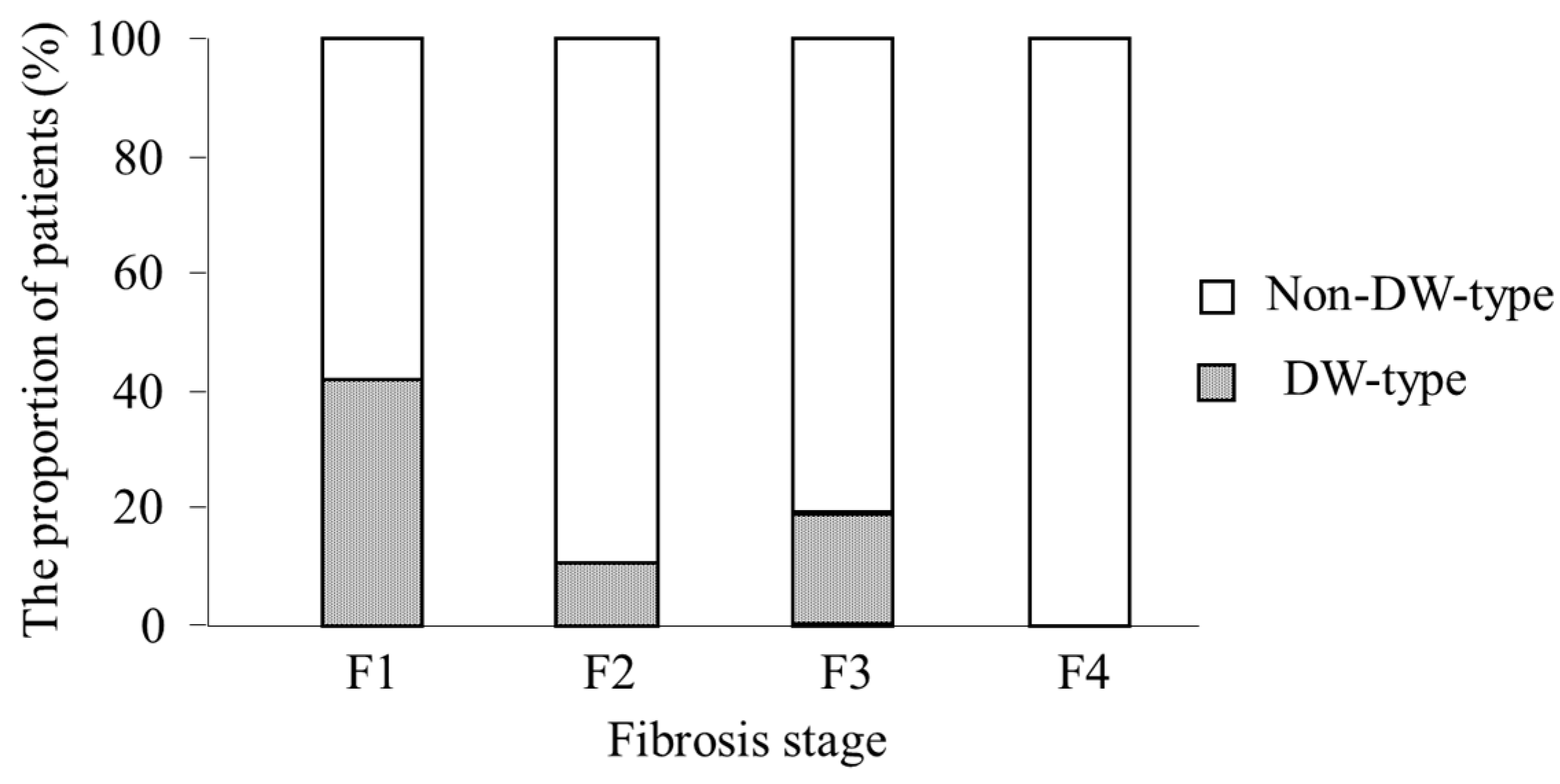
3. Discussion
4. Patients and Methods
4.1. Study Population
| Characteristics | Number (%) or Mean ± SD |
|---|---|
| Number of Patients | 103 |
| Gender (male/female) | 56/47 |
| Age (years) | 53.6 ± 11.4 |
| Previous IFN treatment | 38 (37%) |
| BMI (kg/m2) | 23.3 ± 2.8 |
| Fibrosis stage (F1/F2/F3/F4) | 38/27/25/3 |
| Laboratory Data | |
| AST (IU/L) | 65 ± 61 |
| ALT (IU/L) | 79 ± 64 |
| AST/ALT ratio | 0.87 ± 0.25 |
| Hemoglobin (g/dL) | 14.5 ± 1.1 |
| Leucocyte (/mm3) | 5300 ± 1500 |
| Platelet (× 104/mm3) | 16.2 ± 6.3 |
| Total bilirubin (mg/dL) | 0.9 ± 0.4 |
| Serum creatinine (mg/dL) | 0.7 ± 0.2 |
| HCV RNA (log10 IU/mL) | 6.3 ± 0.6 |
| Amino Acid Patterns in the Core Region | |
| 70 wild/70 non-wild | 53/50 |
| 91 wild/91 non-wild | 53/50 |
| DW/Non-DW | 26/77 |
| 110 wild/110 non-wild | 14/89 |
| Number of Mutations in the ISDR | |
| ≥2/<2 | 21/82 |
4.2. Definition of Treatment Response
4.3. Laboratory Tests
4.4. Histopathological Examination
4.5. Nucleotide Sequences of the Core and NS5A Regions
4.6. Statistical Analysis
Acknowledgements
Author Contributions
Conflicts of Interest
Abbreviations
References
- Seeff, L.B. Natural history of chronic hepatitis C. Hepatology 2002, 36 (5 Suppl. 1), S35–S46. [Google Scholar] [CrossRef] [PubMed]
- Di Bisceglie, A.M.; Hoofnagle, J.H. Optimal therapy of hepatitis C. Hepatology 2002, 36 (5 Suppl. 1), S121–S127. [Google Scholar] [CrossRef] [PubMed]
- Poynard, T.; Yuen, M.F.; Ratziu, V.; Lai, C.L. Viral hepatitis C. Lancet 2003, 362, 2095–2100. [Google Scholar] [CrossRef]
- Yamada, G.; Iino, S.; Okuno, T.; Omata, M.; Kiyosawa, K.; Kumada, H.; Hayashi, N.; Sakai, T. Virological response in patients with hepatitis C virus genotype 1b and a high viral load: Impact of peginterferon-α-2a plus ribavirin dose reductions and host-related factors. Clin. Drug Investig. 2008, 28, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Manns, M.P.; McHutchison, J.G.; Gordon, S.C; Rustgi, V.K.; Shiffman, R.; Reindollar, V.K.; Goodman, Z.D.; Koury, K.; Ling, M.H.; Albrecht, J.K.; et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet 2001, 358, 958–965. [Google Scholar] [CrossRef]
- Fried, M.W.; Shiffman, M.L.; Reddy, K.R.; Smith, C.; Marinos, G.; Goncales, F.L., Jr.; Häussinger, D.; Diago, M.; Carosi, G.; Dhumeaux, D.; et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 2002, 347, 975–982. [Google Scholar] [CrossRef] [PubMed]
- Poynard, T.; Colombo, M.; Bruix, J.; Schiff, E.; Terg, R.; Flamm, S.; Moreno-Otero, R.; Carrilho, F.; Schmidt, W.; Berg, T.; et al. Peginterferon alfa-2b and ribavirin: Effective in patients with hepatitis C who failed interferon alfa/ribavirin therapy. Gastroenterology 2009, 136, 1618–1628.e2. [Google Scholar] [CrossRef] [PubMed]
- Hadziyannis, S.J.; Sette, H., Jr.; Morgan, T.R.; Balan, V.; Diago, M.; Marcellin, P.; Ramadori, G.; Bodenheimer, H., Jr.; Bernstein, D.; Rizzetto, M.; et al. Peginterferon-α2a and ribavirin combination therapy in chronic hepatitis C: A randomized study of treatment duration and ribavirin dose. Ann. Intern. Med. 2004, 140, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Feld, J.J.; Hoofnagle, J.H. Mechanism of action of interferon and ribavirin in treatment of hepatitis C. Nature 2005, 436, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Missiha, S.; Heathcote, J.; Arenovich, T.; Khan, K. Impact of asian race on response to combination therapy with peginterferon alfa-2a and ribavirin in chronic hepatitis C. Am. J. Gastroenterol. 2007, 102, 2181–2188. [Google Scholar] [CrossRef] [PubMed]
- Kau, A.; Vermehren, J.; Sarrazin, C. Treatment predictors of a sustained virologic response in hepatitis B and C. J. Hepatol. 2008, 49, 634–651. [Google Scholar] [CrossRef] [PubMed]
- Meissner, E.G.; Wu, D.; Osinusi, A.; Bon, D.; Virtaneva, K.; Sturdevant, D.; Porcella, S.; Wang, H.; Herrmann, E.; McHutchison, J.; et al. Endogenous intrahepatic IFNs and association with IFN-free HCV treatment outcome. J. Clin. Investig. 2014, 124, 3352–3363. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, N.; Sakuma, I.; Asahina, Y.; Kurosaki, M.; Murakami, T.; Yamamoto, C.; Ogura, Y.; Izumi, N.; Marumo, F.; Sato, C. Mutations in the nonstructural protein 5A gene and response to interferon in patients with chronic hepatitis C virus 1b infection. N. Engl. J. Med. 1996, 334, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Pascu, M.; Martus, P.; Hohne, M.; Wiedenmann, B.; Hopf, U.; Schreier, E.; Berg, T. Sustained virological response in hepatitis C virus type 1b infected patients is predicted by the number of mutations within the NS5A-ISDR: A meta-analysis focused on geographical differences. Gut 2004, 53, 1345–1351. [Google Scholar] [CrossRef] [PubMed]
- Akuta, N.; Suzuki, F.; Kawamura, Y.; Yatsuji, H.; Sezaki, H.; Suzuki, Y.; Hosaka, T.; Kobayashi, M.; Kobayashi, M.; Arase, Y.; et al. Predictive factors of early and sustained responses to peginterferon plus ribavirin combination therapy in Japanese patients infected with hepatitis C virus genotype 1b: Amino acid substitutions in the core region and low-density lipoprotein cholesterol levels. J. Hepatol. 2007, 46, 403–410. [Google Scholar] [PubMed]
- Donlin, M.J.; Cannon, N.A.; Yao, E.; Li, J.; Wahed, A.; Taylor, M.W.; Belle, S.H.; di Bisceglie, A.M.; Aurora, R.; Tavis, J.E.; et al. Pretreatment sequence diversity differences in the full-length hepatitis C virus open reading frame correlate with early response to therapy. J. Virol. 2007, 81, 8211–8224. [Google Scholar] [CrossRef] [PubMed]
- Okanoue, T.; Itoh, Y.; Hashimoto, H.; Yasui, K.; Minami, M.; Takehara, T.; Tanaka, E.; Onji, M.; Toyota, J.; Chayama, K.; et al. Predictive values of amino acid sequences of the core and NS5A regions in antiviral therapy for hepatitis C: A Japanese multi-center study. J. Gastroenterol. 2009, 44, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Blindenbacher, A.; Duong, F.H.; Hunziker, L.; Stutvoet, S.T.; Wang, X.; Terracciano, L.; Moradpour, D.; Blum, H.E.; Alonzi, T.; Tripodi, M.; et al. Expression of hepatitis C virus proteins inhibits interferon α signaling in the liver of transgenic mice. Gastroenterology 2003, 124, 1465–1475. [Google Scholar] [CrossRef]
- Heim, M.H.; Moradpour, D.; Blum, H.E. Expression of hepatitis C virus proteins inhibits signal transduction through the Jak-STAT pathway. J. Virol. 1999, 73, 8469–8475. [Google Scholar] [PubMed]
- Bode, J.G.; Ludwig, S.; Ehrhardt, C.; Albrecht, U.; Erhardt, A.; Schaper, F.; Heinrich, P.C.; Häussinger, D. IFN-α antagonistic activity of HCV core protein involves induction of suppressor of cytokine signaling-3. FASEB J. 2003, 17, 488–490. [Google Scholar] [CrossRef] [PubMed]
- Wohnsland, A.; Hofmann, W.P.; Sarrazin, C. Viral determinants of resistance to treatment in patients with hepatitis C. Clin. Microbiol. Rev. 2007, 20, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, S.; Imazeki, F.; Fukai, K.; Fujiwara, K.; Arai, M.; Kanda, T.; Yonemitsu, Y.; Yokosuka, O. Association between mutations in the core region of hepatitis C virus genotype 1 and hepatocellular carcinoma development. J. Hepatol. 2010, 52, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Hwang, S.B.; Jeng, K.S.; Zhu, N.; Lai, M.M. Homotypic interaction and multimerization of hepatitis C virus core protein. Virology 1996, 218, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Akuta, N.; Suzuki, F.; Hirakawa, M.; Kawamura, Y.; Yatsuji, H.; Sezaki, H.; Suzuki, Y.; Hosaka, T.; Kobayashi, M.; Kobayashi, M.; et al. Amino acid substitution in hepatitis C virus core region and genetic variation near the interleukin 28B gene predict viral response to telaprevir with peginterferon and ribavirin. Hepatology 2010, 52, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Eng, F.J.; Walewski, J.L.; Klepper, A.L.; Fishman, S.L.; Desai, S.M.; McMullan, L.K.; Evans, M.J.; Rice, C.M.; Branch, A.D. Internal initiation stimulates production of p8 minicore, a member of a newly discovered family of hepatitis C virus core protein isoforms. J. Virol. 2009, 83, 3104–3114. [Google Scholar] [CrossRef] [PubMed]
- Tachi, Y.; Katano, Y.; Honda, T.; Hayashi, K.; Ishigami, M.; Itoh, A.; Hirooka, Y.; Nakano, I.; Samejima, Y.; Goto, H. Impact of amino acid substitutions in the hepatitis C virus genotype 1b core region on liver steatosis and hepatic oxidative stress in patients with chronic hepatitis C. Liver Int. 2010, 30, 554–559. [Google Scholar] [CrossRef] [PubMed]
- Akuta, N.; Suzuki, F.; Kawamura, Y.; Yatsuji, H.; Sezaki, H.; Suzuki, Y.; Hosaka, T.; Kobayashi, M.; Kobayashi, M.; Arase, Y.; et al. Amino acid substitutions in the hepatitis C virus core region are the important predictor of hepatocarcinogenesis. Hepatology 2007, 46, 1357–1364. [Google Scholar] [CrossRef] [PubMed]
- Fishman, S.L.; Factor, S.H.; Balestrieri, C.; Fan, X.; Dibisceglie, A.M.; Desai, S.M.; Benson, G.; Branch, A.D. Mutations in the hepatitis C virus core gene are associated with advanced liver disease and hepatocellular carcinoma. Clin. Cancer Res. 2009, 15, 3205–3213. [Google Scholar] [CrossRef] [PubMed]
- Ray, R.B.; Lagging, L.M.; Meyer, K.; Steele, R.; Ray, R. Transcriptional regulation of cellular and viral promoters by the hepatitis C virus core protein. Virus Res. 1995, 37, 209–220. [Google Scholar] [CrossRef]
- Sallberg, M.; Pumpen, P.; Zhang, Z.X.; Lundholm, P.; Gusars, I.; Ruden, U.; Wahren, B.; Magnius, L.O. Locations of antibody binding sites within conserved regions of the hepatitis C virus core protein. J. Med. Virol. 1994, 43, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Kadokura, M.; Maekawa, S.; Sueki, R.; Miura, M.; Komase, K.; Shindo, H.; Amemiya, F.; Uetake, T.; Inoue, T.; Sakamoto, M.; et al. Analysis of the complete open reading frame of hepatitis C virus in genotype 2a infection reveals critical sites influencing the response to peginterferon and ribavirin therapy. Hepatol. Int. 2011, 5, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Gale, M.J., Jr.; Korth, M.J.; Tang, N.M.; Tan, S.L.; Hopkins, D.A.; Dever, T.E.; Polyak, S.J.; Gretch, D.R.; Katze, M.G. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology 1997, 230, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Gale, M., Jr.; Blakely, C.M.; Kwieciszewski, B.; Tan, S.L.; Dossett, M.; Tang, N.M.; Korth, M.J.; Polyak, S.J.; Gretch, D.R.; Katze, M.G. Control of PKR protein kinase by hepatitis C virus nonstructural 5A protein: Molecular mechanisms of kinase regulation. Mol. Cell. Biol. 1998, 18, 5208–5218. [Google Scholar] [PubMed]
- Berg, T.; Mas Marques, A.; Hohne, M.; Wiedenmann, B.; Hopf, U.; Schreier, E. Mutations in the E2-PePHD and NS5A region of hepatitis C virus type 1 and the dynamics of hepatitis C viremia decline during interferon alfa treatment. Hepatology 2000, 32, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Sarrazin, C.; Herrmann, E.; Bruch, K.; Zeuzem, S. Hepatitis C virus nonstructural 5A protein and interferon resistance: A new model for testing the reliability of mutational analyses. J. Virol. 2002, 76, 11079–11090. [Google Scholar] [CrossRef] [PubMed]
- De Rueda, P.M.; Casado, J.; Paton, R.; Quintero, D.; Palacios, A.; Gila, A.; Quiles, R.; León, J.; Ruiz-Extremera, A.; Salmerón, J. Mutations in E2-PePHD, NS5A-PKRBD, NS5A-ISDR, and NS5A-V3 of hepatitis C virus genotype 1 and their relationships to pegylated interferon-ribavirin treatment responses. J. Virol. 2008, 82, 6644–6653. [Google Scholar] [CrossRef] [PubMed]
- Kato, N.; Hijikata, M.; Ootsuyama, Y.; Nakagawa, M.; Ohkoshi, S.; Sugimura, T.; Shimotohno, K. Molecular cloning of the human hepatitis C virus genome from Japanese patients with non-A, non-B hepatitis. Proc. Natl. Acad. Sci. USA 1990, 87, 9524–9528. [Google Scholar] [CrossRef] [PubMed]
- Pohl, A.; Behling, C.; Oliver, D.; Kilani, M.; Monson, P.; Hassanein, T. Serum aminotransferase levels and platelet counts as predictors of degree of fibrosis in chronic hepatitis C virus infection. Am. J. Gastroenterol. 2001, 96, 3142–3146. [Google Scholar] [CrossRef] [PubMed]
- Sulkowski, M.S. Anemia in the treatment of hepatitis C virus infection. Clin. Infect. Dis. 2003, 37 (Suppl 4), S315–S322. [Google Scholar] [CrossRef] [PubMed]
- Nakamoto, S.; Kanda, T.; Yonemitsu, Y.; Arai, M.; Fujiwara, K.; Fukai, K.; Kanai, F.; Imazeki, F.; Yokosuka, O. Quantification of hepatitis C amino acid substitutions 70 and 91 in the core coding region by real-time amplification refractory mutation system reverse transcription-polymerase chain reaction. Scand. J. Gastroenterol. 2009, 44, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Ohno, O.; Mizokami, M.; Wu, R.R.; Saleh, M.G.; Ohba, K.; Orito, E.; Mukaide, M.; Williams, R.; Lau, J.Y. New hepatitis C virus (HCV) genotyping system that allows for identification of HCV genotypes 1a, 1b, 2a, 2b, 3a, 3b, 4, 5a, and 6a. J. Clin. Microbiol. 1997, 35, 201–207. [Google Scholar] [PubMed]
- Desmet, V.J.; Gerber, M.; Hoofnagle, J.H.; Manns, M.; Scheuer, P.J. Classification of chronic hepatitis: diagnosis, grading and staging. Hepatology 1994, 19, 1513–1520. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, N.; Sakuma, I.; Asahina, Y.; Kurosaki, M.; Murakami, T.; Yamamoto, C.; Izumi, N.; Marumo, F.; Sato, C. Comparison of full-length sequences of interferon-sensitive and resistant hepatitis C virus 1b. Sensitivity to interferon is conferred by amino acid substitutions in the NS5A region. J. Clin. Investig. 1995, 96, 224–230. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakamoto, S.; Imazeki, F.; Arai, M.; Yasui, S.; Nakamura, M.; Haga, Y.; Sasaki, R.; Kanda, T.; Shirasawa, H.; Yokosuka, O. Effect of Hepatitis C Virus Genotype 1b Core and NS5A Mutations on Response to Peginterferon Plus Ribavirin Combination Therapy. Int. J. Mol. Sci. 2015, 16, 21177-21190. https://doi.org/10.3390/ijms160921177
Nakamoto S, Imazeki F, Arai M, Yasui S, Nakamura M, Haga Y, Sasaki R, Kanda T, Shirasawa H, Yokosuka O. Effect of Hepatitis C Virus Genotype 1b Core and NS5A Mutations on Response to Peginterferon Plus Ribavirin Combination Therapy. International Journal of Molecular Sciences. 2015; 16(9):21177-21190. https://doi.org/10.3390/ijms160921177
Chicago/Turabian StyleNakamoto, Shingo, Fumio Imazeki, Makoto Arai, Shin Yasui, Masato Nakamura, Yuki Haga, Reina Sasaki, Tatsuo Kanda, Hiroshi Shirasawa, and Osamu Yokosuka. 2015. "Effect of Hepatitis C Virus Genotype 1b Core and NS5A Mutations on Response to Peginterferon Plus Ribavirin Combination Therapy" International Journal of Molecular Sciences 16, no. 9: 21177-21190. https://doi.org/10.3390/ijms160921177







