Conformational Motions and Functionally Key Residues for Vitamin B12 Transporter BtuCD–BtuF Revealed by Elastic Network Model with a Function-Related Internal Coordinate
Abstract
:1. Introduction
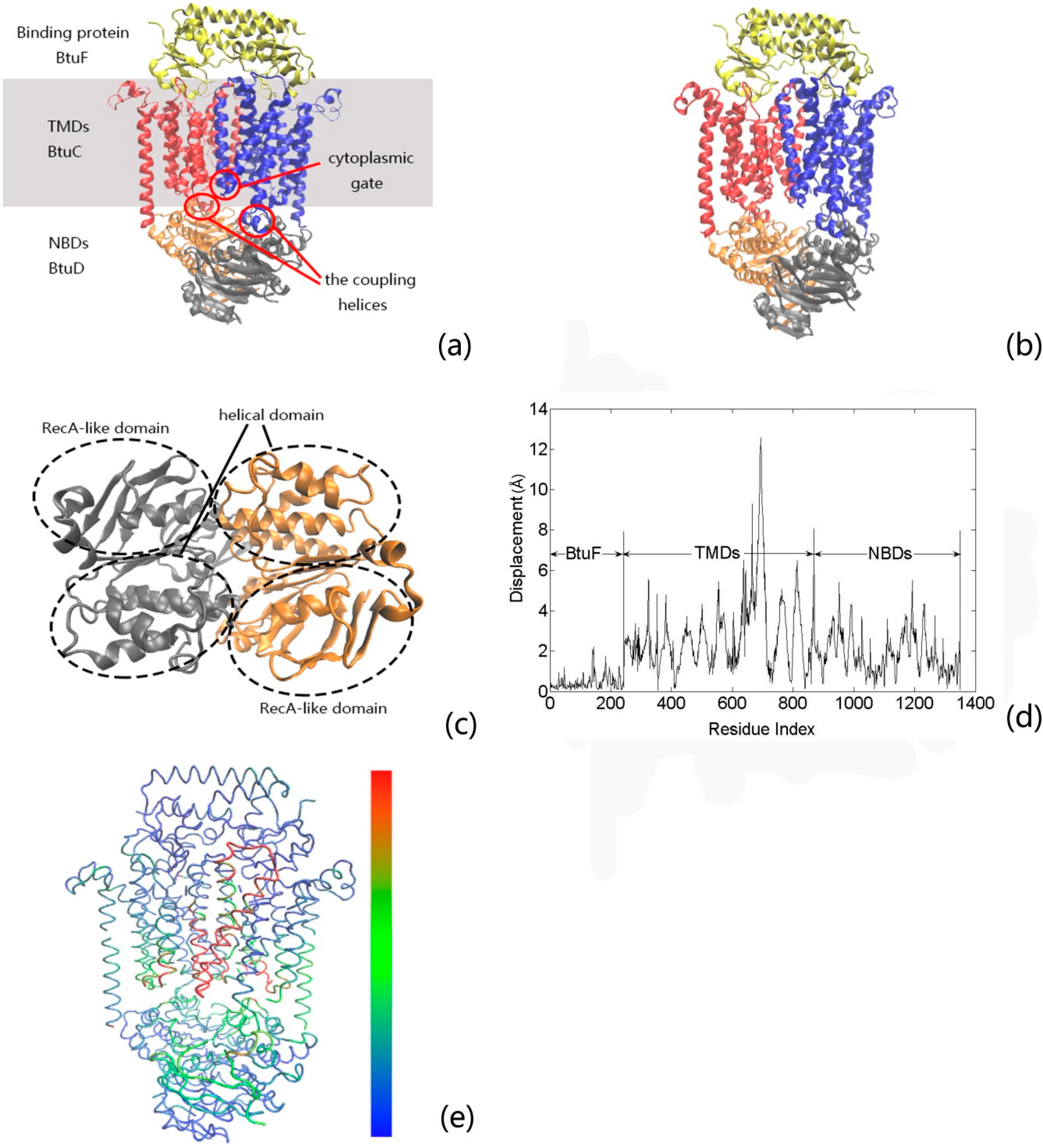
2. Results and Discussion
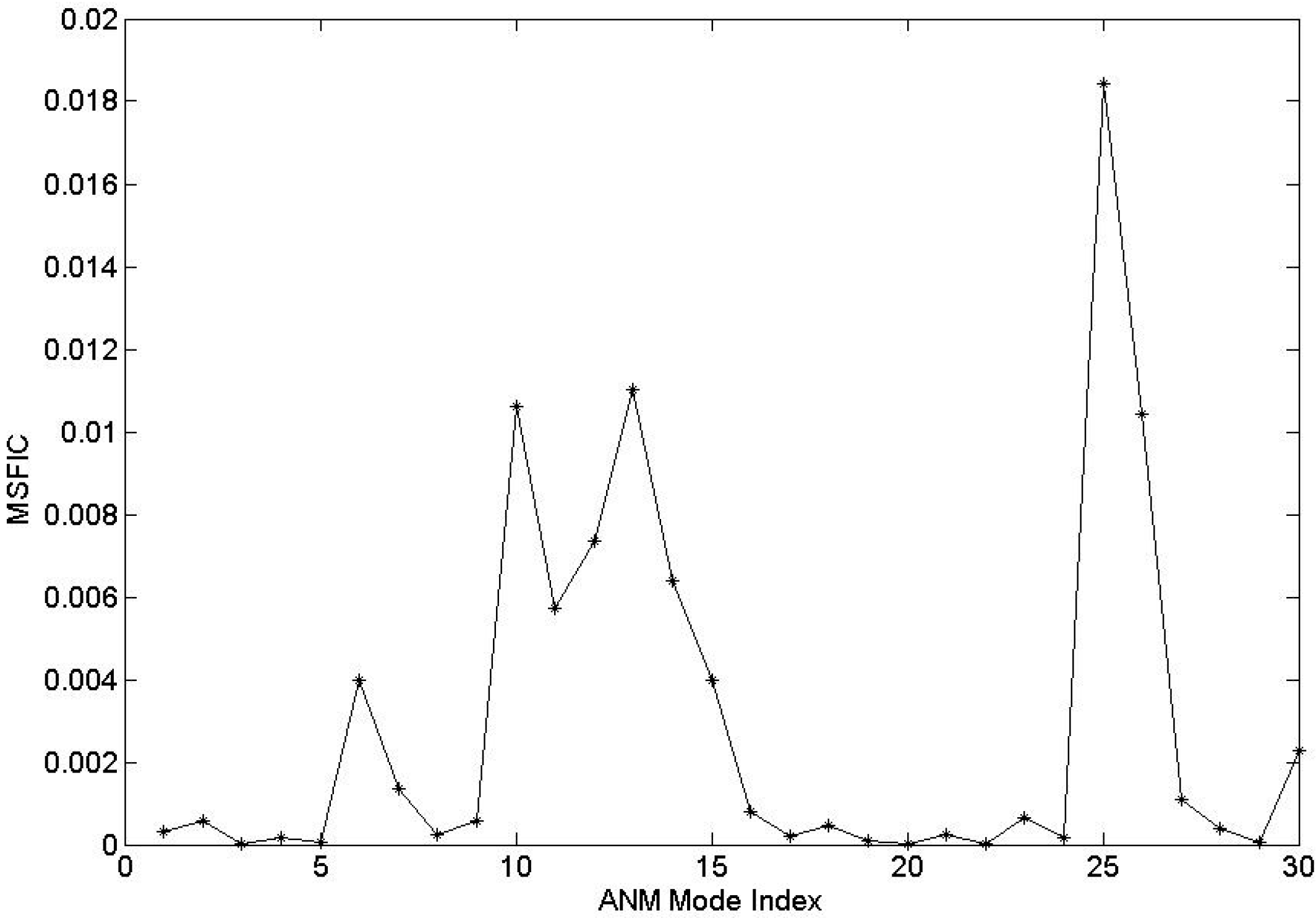
2.1. The Contribution of Each Motion Modes to the Channel-Gating Function of BtuCD–BtuF
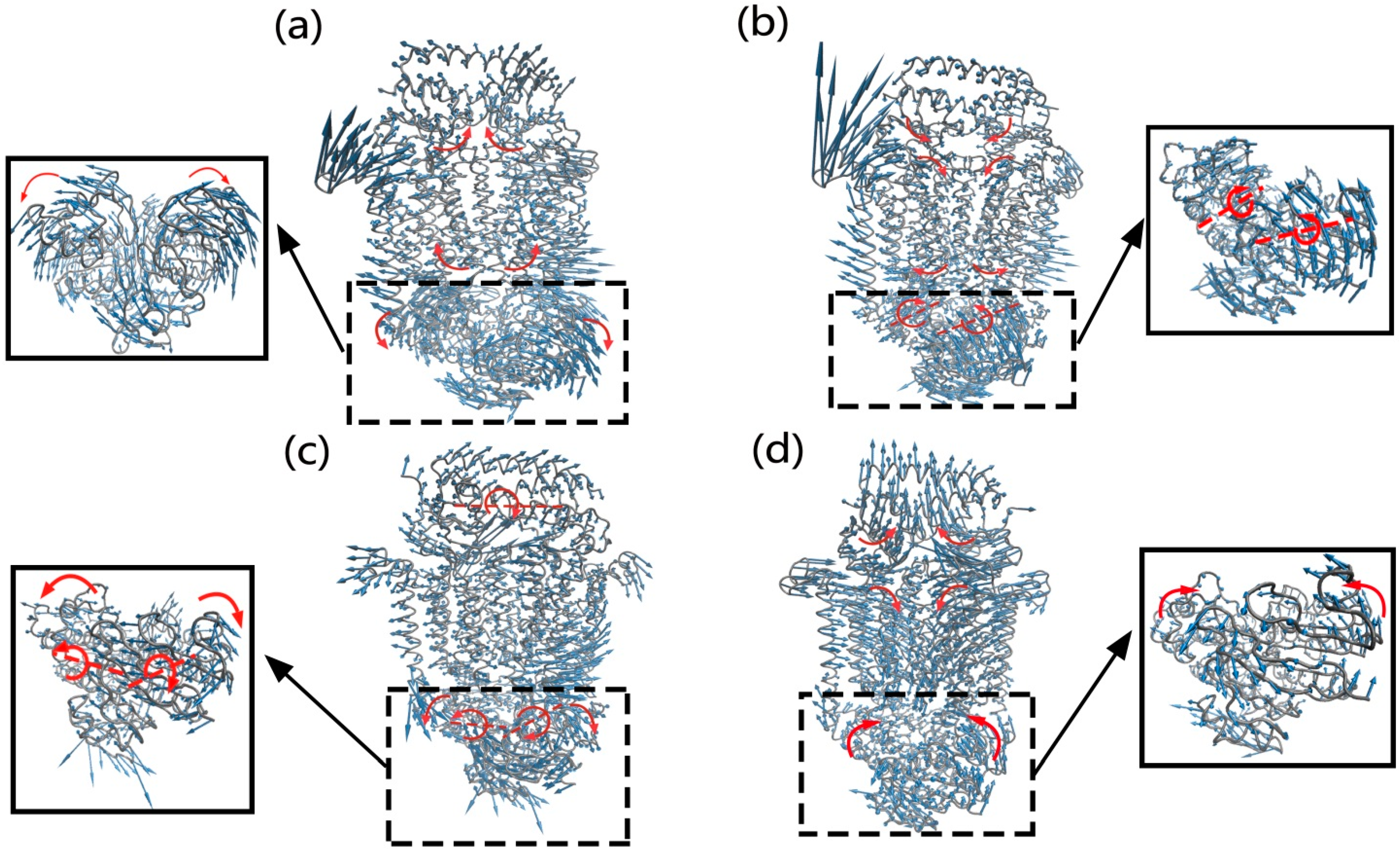
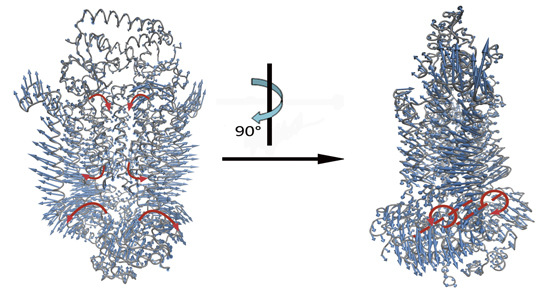
2.2. The Motions of BtuCD–BtuF Relevant to the Channel-Gating Function
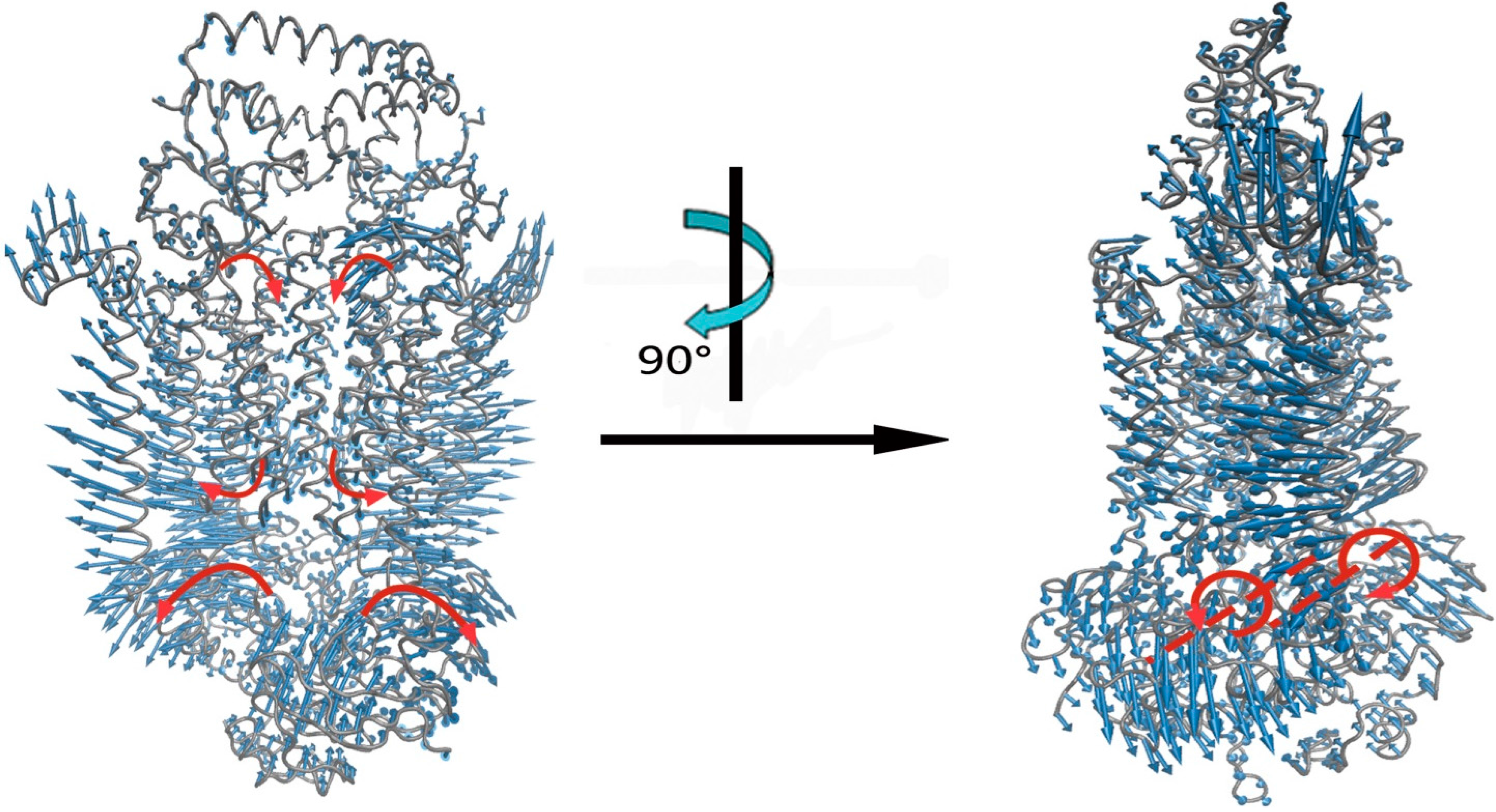
2.3. The Key Residue Interactions that Control the Channel-Gating Motion of BtuCD–BtuF

3. Experimental Section
3.1. Anisotropic Network Model (ANM)
3.2. The Internal Coordinate Related to the Channel-Gating Function of BtuCD-F
3.3. Methods to Analyze the Function-Related Motions and Identify the Functionally Important Residue Interactions
3.3.1. The Mean-Square Fluctuation of the Internal Coordinate for Each Normal Mode
3.3.2. The Cross-Correlation between the Displacement of the Inter-Distance and the Movement of Each Residue in the Transporter
3.3.3. The Perturbation Method to Identify Functionally Key Residue Interactions
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dassa, E.; Bouige, P. The ABC of ABCs: A phylogenetic and functional classification of ABC systems in living organisms. Res. Microbiol. 2001, 152, 211–229. [Google Scholar] [CrossRef]
- Davidson, A.L.; Chan, J. ATP-binding cassette transporters in bacteria. Annu. Rev. Biochem. 2004, 73, 241–268. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.C.; Johnson, E.; Lewinson, O. ABC transporters: The power to change. Nat. Rev. Mol. Cell Biol. 2009, 10, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Locher, K.P.; Borths, E. ABC transporter architecture and mechanism: Implications from the crystal structures of BtuCD and BtuF. FEBS Lett. 2004, 564, 264–268. [Google Scholar] [CrossRef]
- Rice, A.J.; Park, A.; Pinkett, H.W. Diversity in ABC transporters: Type I, II and III importers. Crit. Rev. Biochem. Mol. Biol. 2014, 49, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Gottesman, M.M.; Ambudkar, S.V. Overview: ABC transporters and human disease. J. Bioenerg. Biomembr. 2001, 33, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Borst, P.; Elferink, R.O. Mamalian ABC transporters in health and disease. Annu. Rev. Biochem. 2002, 1, 537–592. [Google Scholar] [CrossRef] [PubMed]
- Štefková, J.; Poledne, R.; Hubáček, J.A. ATP-binding cassette (ABC) transporters in human metabolism and diseases. Physiol. Res. 2004, 53, 235–243. [Google Scholar] [PubMed]
- Abuznait, A.H.; Kaddoumi, A. Role of ABC transporters in the pathogenesis of Alzheimer’s disease. ACS Chem. Neurosci. 2012, 3, 820–831. [Google Scholar] [CrossRef] [PubMed]
- Tarling, E.J.; de Aguiar Vallim, T.Q.; Edwards, P.A. Role of ABC transporters in lipid transport and human disease. Trends Endocrinol. Metab. 2013, 24, 342–350. [Google Scholar] [CrossRef] [PubMed]
- Borths, E.L.; Poolman, B.; Hvorup, R.N.; Locher, K.P.; Rees, D.C. In vitro functional characterization of BtuCD-F, the Escherichia coli ABC transporter for vitamin B12 uptake. Biochemistry 2005, 44, 16301–16309. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Jeschke, G.; Goetz, B.A.; Locher, K.P.; Bordignon, E. Transmembrane gate movements in the type II ATP-binding cassette (ABC) importer BtuCD-F during nucleotide cycle. J. Biol. Chem. 2011, 286, 41008–41017. [Google Scholar] [CrossRef] [PubMed]
- Korkhov, V.M.; Mireku, S.A.; Locher, K.P. Structure of vitamin B12 transporter BtuCD-F in a nucleotide-bound state. Nature 2012, 490, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Hvorup, R.N.; Goetz, B.A.; Niederer, M.; Hollenstein, K.; Perozo, E.; Locher, K.P. Asymmetry in the structure of the ABC transporter-binding protein complex BtuCD–BtuF. Science 2007, 317, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Cadicux, N.; Bradbeer, C.; Reeger-Schneider, E.; Mohanty, A.K.; Wiener, M.C.; Kadner, R.J. Identification of the periplasmic cobalamin-binding protein BtuF of Escherichia coli. J. Bacteriol. 2002, 184, 706–717. [Google Scholar] [CrossRef]
- Chimento, D.P.; Mohanty, A.K.; Kadner, R.J.; Wiener, M.C. Substrate-induced transmembrane signaling in the cobalamin transporter BtuB. Nat. Struct. Biol. 2003, 10, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Goetz, B.A.; Perozo, E.; Locher, K.P. Distinct gate conformations of the ABC transporter BtuCD revealed by electron spin resonance spectroscopy and chemical cross-linking. FEBS Lett. 2009, 583, 266–270. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Brooks, B.R.; Doniach, S.; Thirumaial, D. Network of dynamically important residues in the open/closed transition in polymerases is strongly conserved. Structure 2005, 13, 567–577. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Tekpinar, M. Large-scale evaluation of dynamically important residues in proteins predicted by the perturbation analysis of a coarse-grained elastic model. BMC Struct. Biol. 2009, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Haliloglu, E.; Bahar, I.; Erman, B. Gaussian dynamics of folded proteins. Phys. Rev. Lett. 1997, 79, 3090–3093. [Google Scholar] [CrossRef]
- Atilgan, A.R.; Durrell, S.R.; Jernigan, R.L.; Demirel, M.C.; Keskin, O.; Bahar, I. Anisotropy of fluctuation dynamics of proteins with an elastic network model. Biophys. J. 2001, 80, 505–515. [Google Scholar] [CrossRef]
- Bahar, I.; Lezon, T.R.; Yang, L.; Eyal, E. Global dynamics of proteins: Bridging between structure and function. Annu. Rev. Biophys. 2010, 39, 23–42. [Google Scholar] [CrossRef] [PubMed]
- Hinsen, K. Analysis of domain motions by approximate normal mode calculations. Proteins 1998, 33, 417–429. [Google Scholar] [CrossRef]
- Zimmermann, M.T.; Kloczkowski, A.; Jemigan, R.L. MAVENs: Motion analysis and visualization of elastic networks and structural ensembles. BMC Bioinform. 2011, 12, 264. [Google Scholar] [CrossRef] [PubMed]
- Atilgan, C.; Atilgan, A.R. Perturbation-response scanning reveals ligand entry-exit mechanisms of ferric binding protein. PLoS Comout. Biol. 2009, 5, e1000544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ming, D.; Cohn, J.D.; Wall, M.E. Fast dynamics perturbation analysis for prediction of protein functional sites. BMC Struct. Biol. 2008, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Brooks, B. Identification of dynamics correlations within the myosin motor domain by the normal mode analysis of an elastic network model. J. Mol. Biol. 2005, 346, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Haliloglu, T.; Gul, A.; Erman, B. Predicting important residues and interaction proteins. PLoS Comput. Biol. 2010, 6, e1000845. [Google Scholar] [CrossRef] [PubMed]
- Su, J.G.; Li, C.H.; Chen, W.Z.; Wang, C.X. Identification of key residues for protein conformational transition using elastic network model. J. Chem. Phys. 2011, 135, 174101. [Google Scholar] [CrossRef] [PubMed]
- Su, J.G.; Qi, L.S.; Zhu, Y.Y.; Du, H.J.; Hou, Y.X.; Hao, R.; Wang, J.H. Prediction of allosteric sites on protein surface with an elastic-network-model-based thermodynamic method. Phys. Rev. E 2014, 90, 022719. [Google Scholar] [CrossRef]
- Su, J.G.; Du, H.H.; Hao, R.; Xu, X.J.; Li, C.H.; Chen, W.Z.; Wang, C.X. Identification of functionally key residues in AMPA receptor with a thermodynamic method. J. Phys. Chem. B 2013, 117, 8689–8696. [Google Scholar] [CrossRef] [PubMed]
- Hub, J.S.; de Groot, B.L. Detection of functional modes in protein dynanmics. PLoS Comput. Biol. 2009, 5, e1000480. [Google Scholar] [CrossRef] [PubMed]
- Cspozzi, F.; Luchinat, C.; Micheletti, C.; Pontiggia, F. Essential dynamics of helices provide a functional classification of EF-hand proteins. J. Prot. Res. 2007, 6, 4245–4255. [Google Scholar]
- Oloo, E.O.; Tieleman, D.P. Conformational transitions induced by the binding of MgATP to the vitamin B12 ATP-binding cassette (ABC) transporter BtuCD. J. Biol. Chem. 2004, 279, 45013–45019. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Fan, K.; Wang, W. The conformational transition pathways of ATP-binding cassette transporter BtuCD revealed by targeted molecular dynamics simulation. PLoS ONE 2012, 7, e30465. [Google Scholar] [CrossRef] [PubMed]
- Jacso, T.; Schneider, E.; Rupp, B.; Reif, B. Substrate transport activation is mediated through second periplasmic loop of transmembrane protein MalF in maltose transport complex of Escherichia coli. J. Biol. Chem. 2012, 287, 17040–17049. [Google Scholar] [CrossRef] [PubMed]
- Vashisth, H.; Brooks, C.L. Conformational sampling of maltose-transporter components in Cartesian collective variables is governed by the low-frequency normal modes. Structure 2012, 20, 1453–1462. [Google Scholar] [CrossRef] [PubMed]
- Damas, J.M.; Oliveira, A.S.F.; Baptista, A.M.; Soares, C.M. Structural consequences of ATP hydrolysis on the ABC transporter NBD dimer: Molecular dynamics studies of HlyB. Prot. Sci. 2011, 20, 1220–1230. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.M.; George, A.M. Nucleotide-dependent allostery within the ABC transporter ATP-binding cassette: A computational study of the MJ796 dimer. J. Biol. Chem. 2007, 282, 22793–22803. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.F.; Baptista, A.M.; Soares, C.M. Conformational changes induced by ATP-hydrolysis in an ABC transporter: A molecular dynamics study of the Sav1866 exporter. Proteins 2011, 79, 1977–1990. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Lu, G.; Lin, J.; Davison, A.L.; Quiocho, F.A. A tweezers-like motion of the ATP-binding cassette dimer in an ABC transport cycle. Mol. Cell 2003, 12, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Ma, J.; Fan, K.; Wang, W. The conformational coupling and translocation mechanism of vitamin B12 ATP-binding cassette transporter BtuCD. Biophys. J. 2008, 94, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Linton, K.J.; Higgins, C.F. Structure and function of ABC transporters: The ATP switch provides flexible control. Eur. J. Physiol. 2007, 453, 555–567. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.S.F.; Baptista, A.M.; Soares, C.M. Inter-domain communication mechanisms in an ABC importer: A molecular dynamics study of the MalFGK2E complex. PLoS Comput. Biol. 2011, 7, e1002128. [Google Scholar] [CrossRef] [PubMed]
- Oldham, M.L.; Chen, J. Crystal structure of the maltose transporter in a pretranslocation intermediate state. Science 2011, 332, 1202–1205. [Google Scholar] [CrossRef] [PubMed]
- Sonne, J.; Kandt, C.; Peters, G.H.; Hansen, F.Y.; Jensen, M.Ø.; Tieleman, D.P. Simulation of the coupling between nucleotide binding and transmembrane domains in the ATP binding cassette transporter BtuCD. Biophys. J. 2007, 92, 2727–2734. [Google Scholar] [CrossRef] [PubMed]
- Joseph, B.; Korkhov, V.M.; Yulikov, M.; Jeschke, G.; Bordignon, E. Conformational cycle of the vitamin B12 ABC importer in liposomes detected by double electron-electron resonance (DEER). J. Biol. Chem. 2014, 289, 3176–3185. [Google Scholar] [CrossRef] [PubMed]
- Tal, N.; Ovcharenko, E.; Lewinson, O. A single intact ATPase site of the ABC transporter BtuCD drives 5% transport activity yet supports full in vivo vitamin B12 utilization. Proc. Natl. Acad. Sci. USA 2013, 110, 5434–5439. [Google Scholar] [CrossRef] [PubMed]
- Saurin, W.; Koster, W.; Dassa, E. Bacterial binding protein-dependent permeases: Characterization of distinctive signatures for functionally related integral cytoplasmic membrane proteins. Mol. Microbiol. 1994, 12, 993–1004. [Google Scholar] [CrossRef] [PubMed]
- Davidson, A.L.; Dassa, E.; Orelle, C.; Chen, J. Structure, function, and evolution of bacterial ATP-binding cassette systems. Microbiol. Mol. Biol. Rev. 2008, 72, 317–364. [Google Scholar] [CrossRef] [PubMed]
- Hollenstein, K.; Dawson, R.J.; Locher, K.P. Structure and mechanism of ABC transporter proteins. Curr. Opin. Struct. Biol. 2008, 17, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Mourez, M.; Hofnung, N.; Dassa, E. Subunit interactions in ABC transporters: A conserved sequence in hydrophobic membrane proteins of periplasmic permeases defines an important site of interaction with the ATPase subunits. EMBO J. 1997, 16, 3066–3077. [Google Scholar] [CrossRef] [PubMed]
- Köster, W.; Böhm, B. Point mutations in two conserved glycine residues within the integral membrane protein FhuB affect iron (III) hydroxamate transport. Mol. Gen. Genet. 1992, 232, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Dawson, R.J.P.; Hollenstein, W.; Locher, K.P. Uptake or extrusion: Crystal structures of full ABC transporters suggest a common mechanism. Mol. Microbiol. 2007, 65, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Locher, K.P.; Lee, A.T.; Rees, D.C. The E. coli. BtuCD structure: A framework for ABC transporter architecture and mechanism. Science 2002, 296, 1091–1098. [Google Scholar] [CrossRef] [PubMed]
- Lewinson, O.; Lee, A.T.; Locher, K.P.; Rees, D.C. A distinct mechanism for the ABC transporter BtuCD–BtuF revealed by the dynamics of complex formation. Nat. Struct. Mol. Biol. 2010, 17, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.C.; Sugiman-Marangos, S.; Junop, M.S.; Brown, E.D.; Li, Y. Exploring intermolecular interactions of a substrate binding protein using a riboswitch-based sensor. Chem. Biol. 2013, 20, 1502–1512. [Google Scholar] [CrossRef] [PubMed]
- Eyal, E.; Yang, L.; Bahar, I. Anisotropic network model: Systematic evaluation and a new web interface. Bioinformatics 2006, 22, 2619–2627. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, J.-G.; Zhang, X.; Zhao, S.-X.; Li, X.-Y.; Hou, Y.-X.; Wu, Y.-D.; Zhu, J.-Z.; An, H.-L. Conformational Motions and Functionally Key Residues for Vitamin B12 Transporter BtuCD–BtuF Revealed by Elastic Network Model with a Function-Related Internal Coordinate. Int. J. Mol. Sci. 2015, 16, 17933-17951. https://doi.org/10.3390/ijms160817933
Su J-G, Zhang X, Zhao S-X, Li X-Y, Hou Y-X, Wu Y-D, Zhu J-Z, An H-L. Conformational Motions and Functionally Key Residues for Vitamin B12 Transporter BtuCD–BtuF Revealed by Elastic Network Model with a Function-Related Internal Coordinate. International Journal of Molecular Sciences. 2015; 16(8):17933-17951. https://doi.org/10.3390/ijms160817933
Chicago/Turabian StyleSu, Ji-Guo, Xiao Zhang, Shu-Xin Zhao, Xing-Yuan Li, Yan-Xue Hou, Yi-Dong Wu, Jian-Zhuo Zhu, and Hai-Long An. 2015. "Conformational Motions and Functionally Key Residues for Vitamin B12 Transporter BtuCD–BtuF Revealed by Elastic Network Model with a Function-Related Internal Coordinate" International Journal of Molecular Sciences 16, no. 8: 17933-17951. https://doi.org/10.3390/ijms160817933