Differential Antioxidant Responses and Perturbed Porphyrin Biosynthesis after Exposure to Oxyfluorfen and Methyl Viologen in Oryza sativa
Abstract
:1. Introduction
2. Results and Discussion
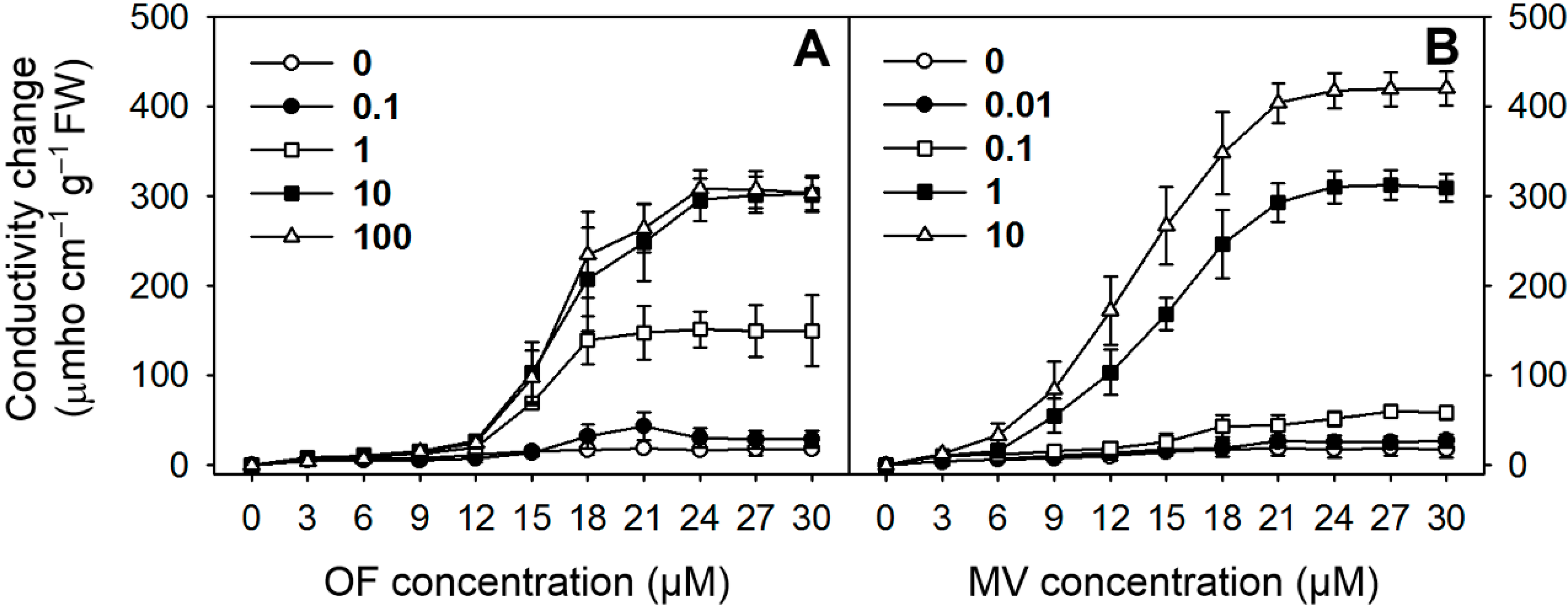
2.1. Differential Photooxidative Stress Responses in Rice Plants Treated with Oxyfluorfen (OF) and Methyl Viologen (MV)

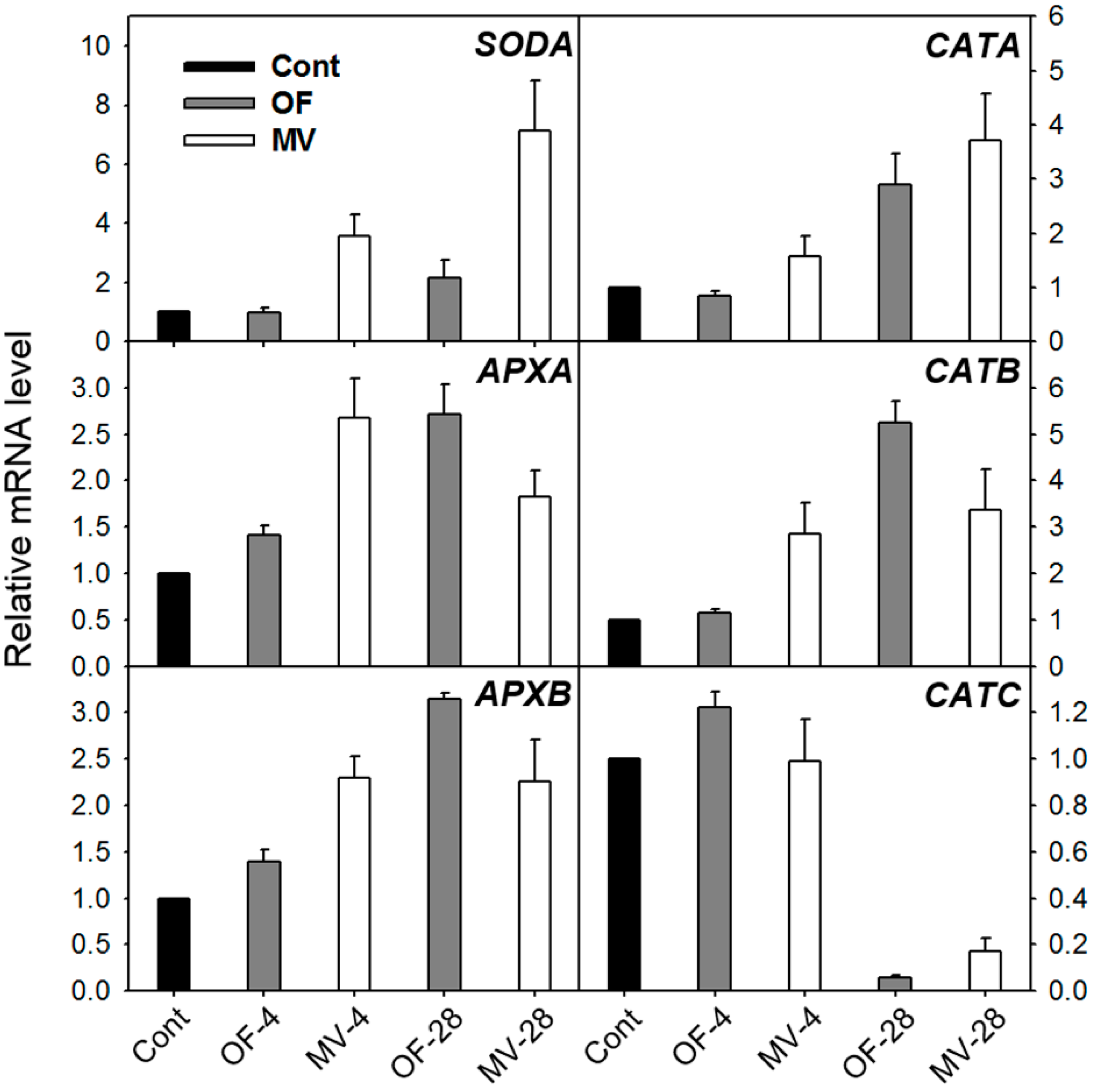
2.2. Effect of OF and MV on Activities and Expression Levels of Reactive Oxygen Species (ROS)-Scavenging Enzymes

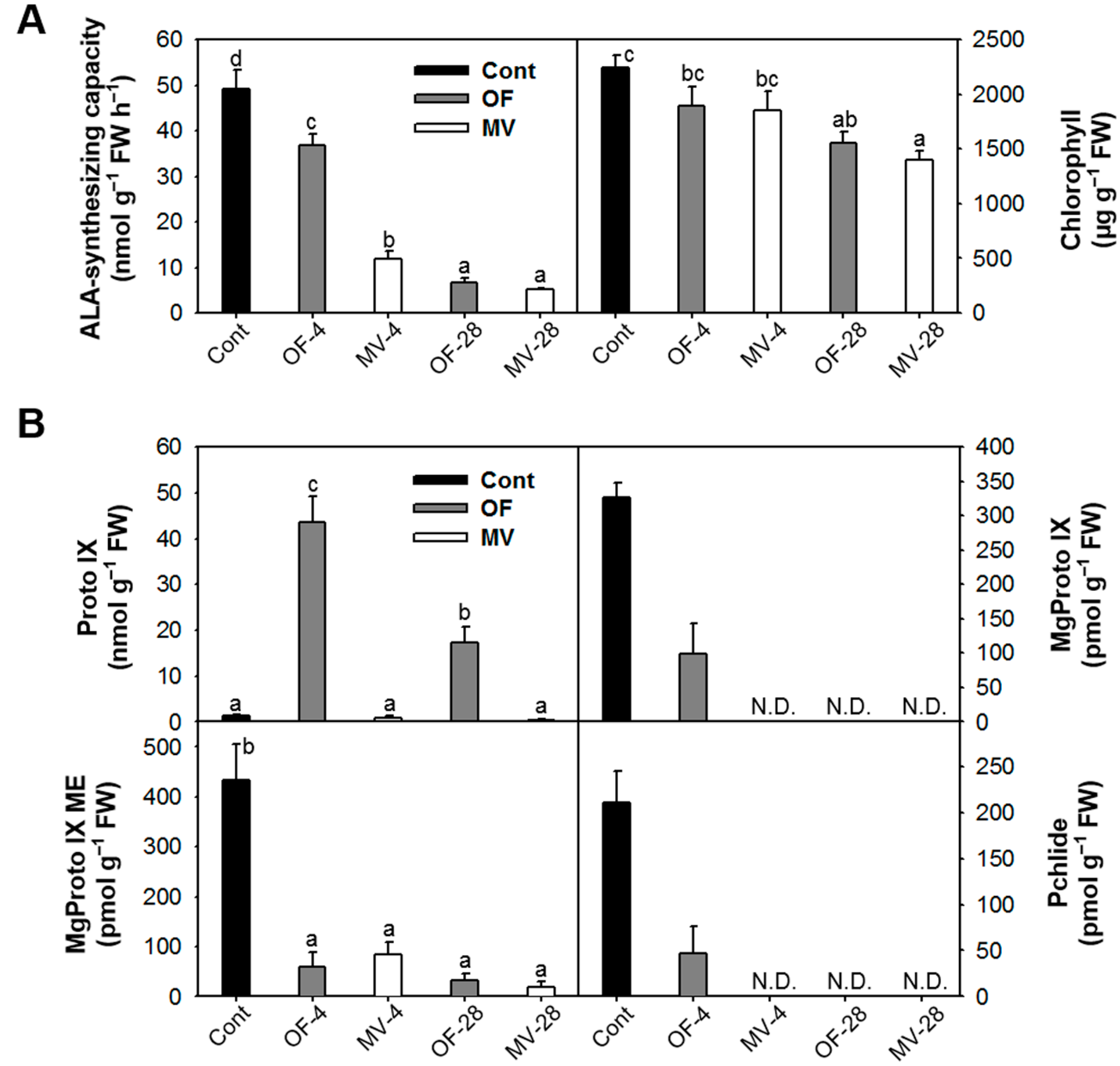
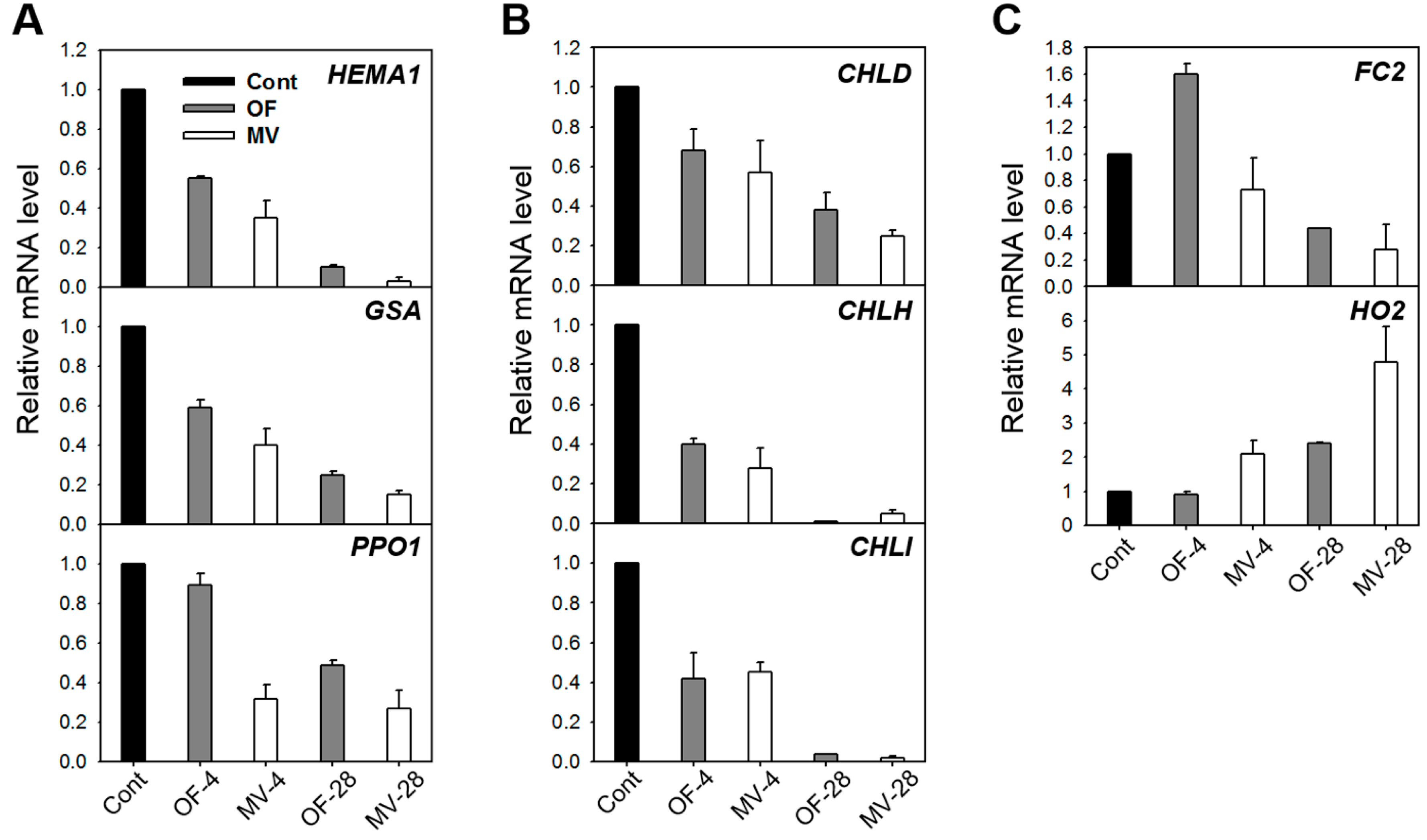
2.3. Photooxidative Stress-Induced Changes in Porphyrin Intermediates and Their Biosynthetic Genes
3. Experimental Section
3.1. Plant Growth and Pro-Oxidant Treatment
3.2. Cellular Leakage
3.3. Determination of MDA Content
3.4. In Vivo Detection of H2O2
3.5. Measurement of Photosynthetic Activity
3.6. Assays for Antioxidant Enzymes
3.7. ALA-Synthesizing Capacity
3.8. Porphyrin Extraction and Analysis
3.9. RNA Extraction and qRT-PCR
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Beale, S.I.; Weinstein, J.D. Biochemistry and regulation of photosynthetic pigment formation in plants and algae. In Biosynthesis of Tetrapyrroles; Jordan, P.M., Ed.; Elsevier: Amsterdam, The Netherlands, 1991; pp. 155–235. [Google Scholar]
- Tanaka, R.; Tanaka, A. Tetrapyrrole biosynthesis in higher plants. Annu. Rev. Plant Biol. 2007, 58, 321–346. [Google Scholar] [CrossRef] [PubMed]
- Reinbothe, S.; Reinbothe, C. The regulation of enzymes involved in chlorophyll biosynthesis. Eur. J. Biochem. 1996, 237, 323–343. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, B.C.; Oemüller, R. Reactive oxygen species generation and signaling in plants. Plant Signal. Behav. 2012, 7, 1621–1633. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, J.M.; Jacobs, N.J.; Sherman, T.D.; Duke, S.O. Effect of diphenyl ether herbicides on oxidation of protoporphyrinogen to protoporphyrin in organellar and plasma membrane enriched fractions of barley. Plant Physiol. 1991, 97, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Duke, M.V.; Duke, S.O. Cellular localization of protoporphyrinogen-oxidizing activities of etiolated barley (Hordeum vulgare L.) leaves (Relationship to mechanism of action of protoporphyrinogen oxidase-inhibiting herbicides). Plant Physiol. 1993, 102, 881–889. [Google Scholar] [PubMed]
- Fujii, T.; Yokoyama, E.; Inoue, K.; Sakurai, H. The sites of electron donation of Photosystem I to methyl viologen. Biochim. Biophys. Acta 1990, 1015, 41–48. [Google Scholar] [CrossRef]
- Suntres, Z.E. Role of antioxidants in paraquat toxicity. Toxicology 2002, 180, 65–77. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C. Lipid peroxidation initiated by superoxide-dependent hydroxyl radicals using complexed iron and hydrogen peroxide. FEBS Lett. 1984, 172, 245–249. [Google Scholar] [CrossRef]
- Phung, T.-H.; Jung, H.-I.; Park, J.-H.; Kim, J.-G.; Back, K.; Jung, S. Porphyrin biosynthesis control under water stress: Sustained porphyrin status correlates with drought tolerance in transgenic rice. Plant Physiol. 2011, 157, 1746–1764. [Google Scholar] [CrossRef] [PubMed]
- Tripathy, B.C.; Dalal, V. Modulation of chlorophyll biosynthesis by environmental cues. In Plastid Development in Leaves During Growth and Senescence, Advances in Photosynthesis and Respiration; Biswal, B., Krupinska, K., Biswal, U.C., Eds.; Springer Science + Buisiness Media: Dordrecht, The Netherlands, 2013; Volume 36, pp. 601–639. [Google Scholar]
- Kim, J.-G.; Back, K.; Lee, H.Y.; Lee, H.-J.; Phung, T.-H.; Grimm, B.; Jung, S. Increased expression of Fe-chelatase leads to increased metabolic flux into heme and confers protection against photodynamically induced oxidative stress. Plant Mol. Biol. 2014, 86, 271–287. [Google Scholar] [CrossRef] [PubMed]
- Phung, T.-H.; Jung, S. Alterations in the porphyrin biosynthesis and antioxidant responses to chilling and heat stresses in Oryza sativa. Biol. Plant. 2015, 59, 341–349. [Google Scholar] [CrossRef]
- Mittler, R.; Vanderauwera, S.; Gollery, M.; van Breusegem, F. Reactive oxygen gene network of plants. Trends Plant Sci. 2004, 9, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Noctor, G.; Mhamdi, A.; Foyer, C.H. The roles of reactive oxygen metabolism in drought: Not so cut and dried. Plant Physiol. 2014, 164, 1636–1648. [Google Scholar] [CrossRef] [PubMed]
- Chagas, R.M.; Silveira, J.A.G.; Ribeiro, R.V.; Vitorello, V.A.; Carrer, H. Photochemical damage and comparative performance of superoxide dismutase and ascorbate peroxidase in sugarcane leaves exposed to paraquat-induced oxidative stress. Pestic. Biochem. Physiol. 2008, 90, 181–188. [Google Scholar] [CrossRef]
- Hawkes, T.R. Mechanisms of resistance to paraquat in plants. Pest Manag. Sci. 2014, 70, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Moustaka, J.; Moustaka, M. Photoprotective mechanism of the non-target organism Arabidopsis thaliana to paraquat exposure. Pestic. Biochem. Physiol. 2014, 111, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Moustaka, J.; Tanou, G.; Adamakis, I.-D.; Eleftheriou, E.P.; Moustakas, M. Leaf age-dependent photoprotective and antioxidative response mechanisms to paraquat-induced oxidative stress in Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 13989–14006. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Fujita, Y.; Luchi, S.; Yamada, K.; Kobayashi, Y.; Urano, K.; Kobayashi, M.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Natural variation in a polyamine transporter determines paraquat tolerance in Arabidopsis. Proc. Natl. Acad. Sci. USA 2012, 109, 6343–6347. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Mu, J.; Bai, J.; Fu, F.; Zou, T.; An, F.; Zhang, J.; Jing, H.; Wang, Q.; Li, Z.; et al. PARAQUAT RESISTANT1, a Golgi-localized putative transporter protein, is involved in intracellular transport of paraquat. Plant Physiol. 2013, 162, 470–483. [Google Scholar] [CrossRef] [PubMed]
- Mock, H.-P.; Heller, W.; Molina, A.; Neubohn, B.; Sandermann, H., Jr.; Grimm, B. Expression of uroporphyrinogen decarboxylase or coproporphyrinogen oxidase antisense RNA in tobacco induces pathogen defense responses conferring increased resistance to tobacco mosaic virus. J. Biol. Chem. 1999, 274, 4231–4238. [Google Scholar] [CrossRef] [PubMed]
- Thordal-Christensen, H.; Zhang, Z.; Wei, Y.; Collinge, D.B. Subcellular localization of H2O2 in plants. H2O2 accumulation in papillae and hypersensitive response during the barley—powdery mildew interaction. Plant J. 1997, 11, 1187–1194. [Google Scholar] [CrossRef]
- Varadi, G.; Darko, E.; Lehoczki, E. Changes in the xanthophyll cycle and fluorescence quenching indicate light-dependent early events in the action of paraquat and the mechanism of resistance to paraquat in Erigeron Canadensis (L.) Cronq. Plant Physiol. 2000, 123, 1459–1470. [Google Scholar] [CrossRef] [PubMed]
- Krause, G.H.; Weis, E. Chlorophyll fluorescence as a tool in plant physiology. II. Interpretation of fluorescence signals. Photosynth. Res. 1984, 5, 139–157. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Koussevitzky, S.; Mittler, R.; Miller, G. ROS and redox signaling in the response of plants to abiotic stress. Plant Cell Environ. 2012, 35, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Reinbothe, S.; Reinbothe, C.; Apel, K.; Lebedev, N. Evolution of chlorophyll biosynthesis—The challenge to survive photooxidation. Cell 1996, 86, 703–705. [Google Scholar] [CrossRef]
- Barajas-Lόpez, J.D.; Blanco, N.E.; Strand, A. Plastid-to-nucleus communication, signals controlling the running of the plant cell. Biochim. Biophys. Acta. 2013, 1833, 425–437. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Lee, Y.; Yang, K.; Lee, S.B.; Jang, S.M.; Ha, S.B.; Back, K. Dual targeting of Myxococcus xanthus protoporphyrinogen oxidase into chloroplasts and mitochondria and high level oxyfluorfen resistance. Plant Cell Environ. 2004, 27, 1436–1446. [Google Scholar] [CrossRef]
- Phung, T.-H.; Jung, S. Differential antioxidant defense and detoxification mechanisms in photodynamically stressed rice plants treated with the deregulators of porphyrin biosynthesis, 5-aminolevulinic acid and oxyfluorfen. Biochem. Biophys. Res. Commun. 2015, 459, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.; Lee, H.-J.; Lee, Y.; Kang, K.; Kim, Y.S.; Grimm, B.; Back, K. Toxic tetrapyrrole accumulation in protoporphyrinogen IX oxidase-overexpressing transgenic rice plants. Plant Mol. Biol. 2008, 67, 535–546. [Google Scholar] [CrossRef] [PubMed]
- Peter, E.; Rothbart, M.; Oelze, M.-L.; Shalygo, N.; Dietz, K.-J.; Grimm, B. Mg protoporphyrin monomethylester cyclase deficiency and effects on tetrapyrrole metabolism in different light conditions. Plant Cell Physiol. 2010, 51, 1229–1241. [Google Scholar] [CrossRef] [PubMed]
- Nagai, S.; Koide, M.; Takahashi, S.; Kikuta, A.; Mitsuko, A.; Sasaki-Sekimoto, Y.; Ohta, H.; Takamiya, K.-I.; Masuda, T. Induction of isoforms of tetrapyrrole biosynthetic enzymes, AtHEMA2 and AtFC1, under stress conditions and their physiological functions in Arabidopsis. Plant Physiol. 2007, 144, 1039–1051. [Google Scholar] [CrossRef] [PubMed]
- Vanhee, C.; Zapotoczny, G.; Masquelier, D.; Ghislain, M.; Batoko, H. The Arabidopsis multistress regulator TSPO is a heme binding membrane protein and a potential scavenger of porphyrins via an autophagy-dependent degradation mechanism. Plant Cell 2011, 23, 785–805. [Google Scholar] [CrossRef] [PubMed]
- Avin-Wittenberg, T.; Tzin, V.; Angelovici, R.; Less, H.; Galili, G. Deciphering energy-associated gene networks operating in the response of Arabidopsis plants to stress and nutritional cues. Plant J. 2012, 70, 954–966. [Google Scholar] [CrossRef] [PubMed]
- Muramoto, T.; Tsurui, N.; Terry, M.J.; Yokota, A.; Kohchi, T. Expression and biochemical properties of a ferredoxin-dependent heme oxygenase required for phytochrome chromophore synthesis. Plant Physiol. 2002, 130, 1958–1966. [Google Scholar] [CrossRef] [PubMed]
- Terry, M.J.; Linley, P.J.; Kohchi, T. Making light of it: the role of plant heme oxygenases in phytochrome chromophore synthesis. Biochem. Soc. Trans. 2002, 30, 604–609. [Google Scholar] [CrossRef] [PubMed]
- Otterbein, L.E.; Soares, M.P.; Yamashita, K.; Bach, F.H. Heme oxygenase-1: Unleashing the protective properties of heme. Trends Immunol. 2003, 24, 449–455. [Google Scholar] [CrossRef]
- Yannarelli, G.G.; Noriega, G.O.; Batlle, A.; Tomaro, M.L. Heme oxygenase up-regulation in ultraviolet-B irradiated soybean plants involves reactive oxygen species. Planta 2006, 224, 1154–1162. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Duke, M.V.; Birk, J.H.; Yamamoto, M.; Duke, S.O. Biochemical and physiological effects of benzheterocycles and related compounds. J. Agric. Food Chem. 1995, 43, 2722–2727. [Google Scholar] [CrossRef]
- Buege, J.A.; Aust, S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978, 52, 302–310. [Google Scholar] [PubMed]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B- and ozone-induced biochemical changes in antioxidant enzymes of Arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Woodbury, W.; Spencer, A.K.; Stahman, M.A. An improved procedure for using ferricyanide for detecting catalase isozymes. Anal. Biochem. 1971, 44, 301–305. [Google Scholar] [CrossRef]
- Olson, P.D.; Varner, J.E. Hydrogen peroxide and lignification. Plant J. 1993, 4, 887–892. [Google Scholar] [CrossRef]
- Papenbrock, J.; Mock, H.P.; Kruse, E.; Grimm, B. Expression studies in tetrapyrrole biosynthesis: Inverse maxima of magnesium chelatase and ferrochelatase activity during cyclic photoperiods. Planta 1999, 208, 264–273. [Google Scholar] [CrossRef]
- Lermontova, I.; Grimm, B. Overexpression of plastidic protoporphyrinogen IX oxidase leads to resistance to the diphenyl-ether herbicide acifluorfen. Plant Physiol. 2000, 122, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pham, N.-T.; Kim, J.-G.; Jung, S. Differential Antioxidant Responses and Perturbed Porphyrin Biosynthesis after Exposure to Oxyfluorfen and Methyl Viologen in Oryza sativa. Int. J. Mol. Sci. 2015, 16, 16529-16544. https://doi.org/10.3390/ijms160716529
Pham N-T, Kim J-G, Jung S. Differential Antioxidant Responses and Perturbed Porphyrin Biosynthesis after Exposure to Oxyfluorfen and Methyl Viologen in Oryza sativa. International Journal of Molecular Sciences. 2015; 16(7):16529-16544. https://doi.org/10.3390/ijms160716529
Chicago/Turabian StylePham, Nhi-Thi, Jin-Gil Kim, and Sunyo Jung. 2015. "Differential Antioxidant Responses and Perturbed Porphyrin Biosynthesis after Exposure to Oxyfluorfen and Methyl Viologen in Oryza sativa" International Journal of Molecular Sciences 16, no. 7: 16529-16544. https://doi.org/10.3390/ijms160716529




