Metacognition in Early Phase Psychosis: Toward Understanding Neural Substrates
Abstract
:1. Introduction
2. Results and Discussion
| Characteristic | M | SD | N (%) |
|---|---|---|---|
| Male | 20 (80%) | ||
| African American | 18 (72%) | ||
| Age (years) | 23.2 | 4.4 | |
| Education | |||
| Middle/junior high school | 2 (8%) | ||
| High school, no degree | 6 (24%) | ||
| High school, degree | 7 (28%) | ||
| Some university courses | 7 (28%) | ||
| Associate’s degree | 1 (4%) | ||
| Bachelor’s degree | 2 (8%) | ||
| SES a | 2.7 | 1.1 | |
| Age of onset of psychosis | 21.0 | 4.8 | |
| Duration of treatment (months) b | 15.2 | 11.7 | |
| Antipsychotic drug exposure c | 164.1 | 187.5 | |
| CGI-S | 3.0 | 0.8 | |
| PANSS Total | 51.4 | 13.3 | |
| Positive | 12.0 | 4.9 | |
| Negative | 14.7 | 5.2 | |
| Disorganized | 15.1 | 5.1 | |
| MAS-A Total | 11.4 | 5.0 | |
| Self Reflectivity | 4.5 | 1.8 | |
| Other Reflectivity | 2.4 | 1.2 | |
| Decentration | 0.6 | 0.7 | |
| Mastery | 4.0 | 1.7 |
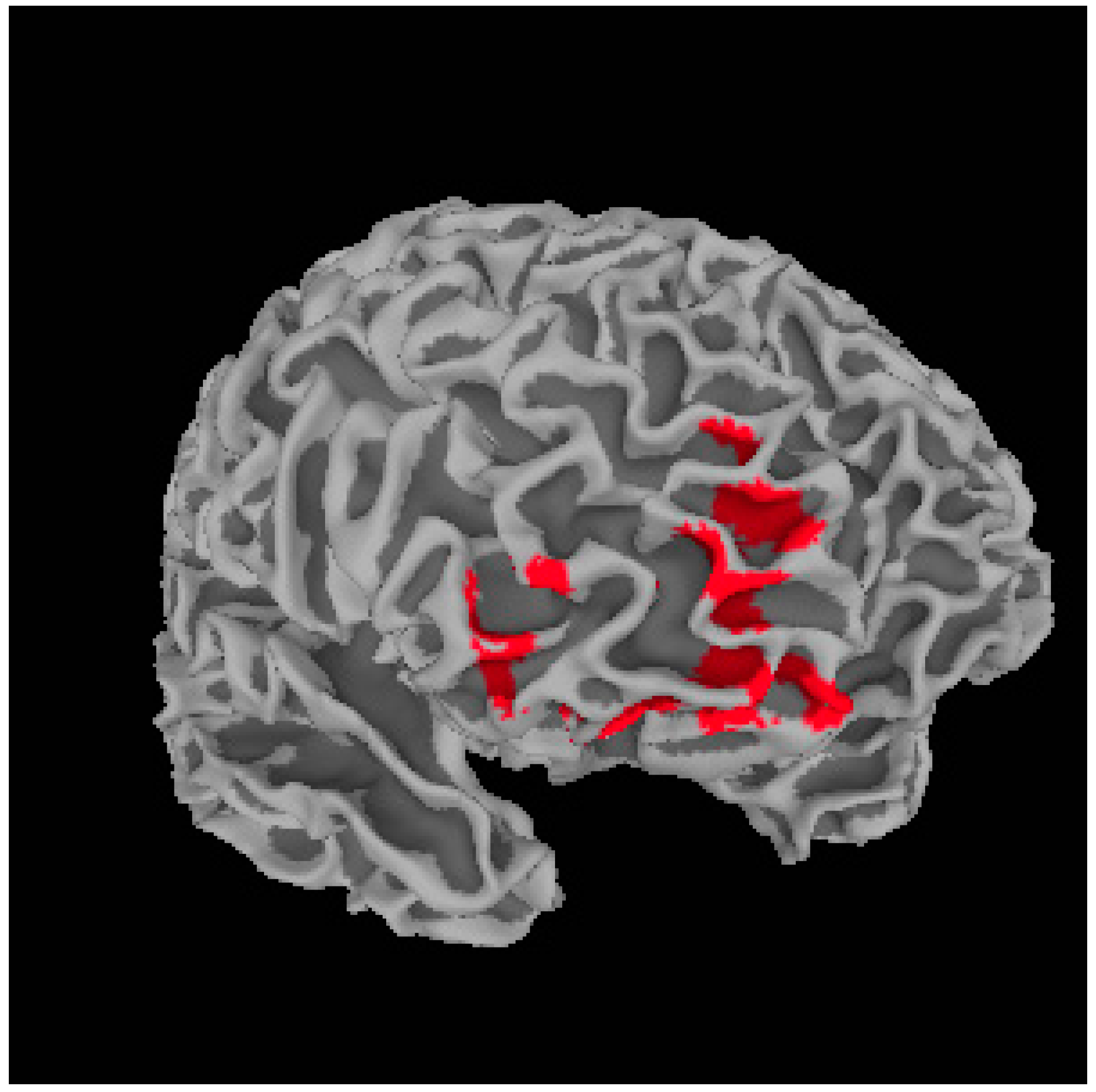
Metacognition and Whole Brain Gray Matter Density
| Region | R/L | BA | MNI Coordinates (x, y, z) | t-Value (Peak Voxel) | Cluster Size (Voxels) |
|---|---|---|---|---|---|
| Dorsal Lateral Prefrontal Cortex | L | 10 | −32, 47, 7 | 6.22 | 692 |
| Dorsal Lateral Prefrontal Cortex | R | 10/11 | 27, 34, 24 | 6.00 | 589 |
| Medial Prefrontal Cortex | R | 10/11 | 18, 62, 7 | 5.75 | 1684 |
| Ventral Striatum | R | – | 10, 16, −6 | 4.77 | 611 |
| Precentral Gyrus | R | 4 | 63, −1, 45 | 4.44 | 747 |
| Anterior Cingulate Cortex | L | 32 | −3, 36, 27 | 4.39 | 860 |
| Region | MAS-A | Self-Reflect | Other-Reflect | Mastery |
|---|---|---|---|---|
| L.DLPFC | 0.455 * | 0.472 * | 0.274 | 0.522 ** |
| R.DLPFC | 0.650 ** | 0.686 ** | 0.462 * | 0.640 ** |
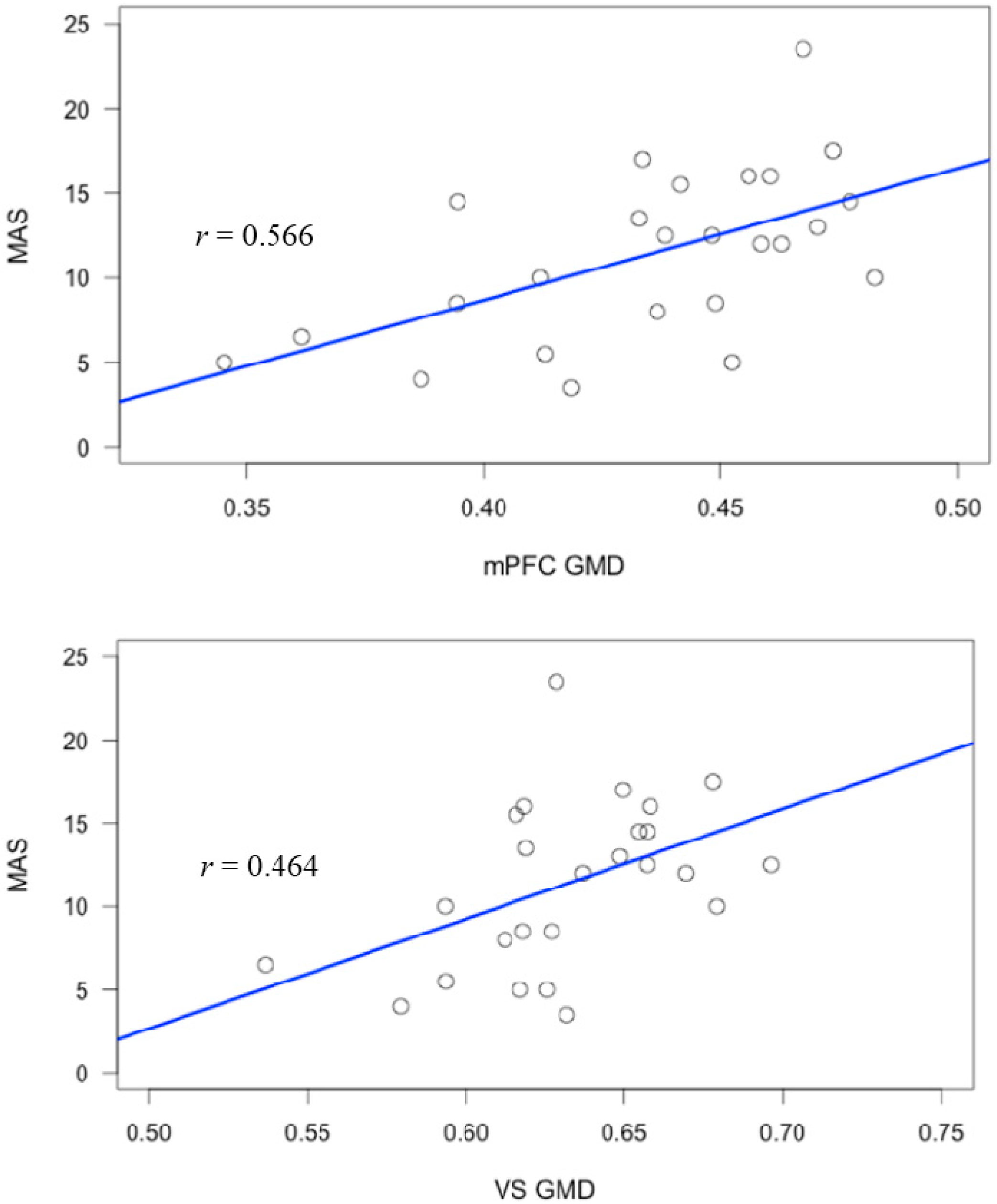
| mPFC | 0.565 ** | 0.606 ** | 0.411 * | 0.572 ** |
| VS | 0.506 ** | 0.405 * | 0.420 * | 0.524 ** |
| Precentral Gyrus | 0.260 | 0.275 | 0.087 | 0.312 |
| ACC | 0.568 ** | 0.599 ** | 0.455 * | 0.563 ** |
| Region | R/L | BA | MNI Coordinates (x, y, z) | t-Value (Peak Voxel) | Cluster Size (Voxels) |
|---|---|---|---|---|---|
| Medial prefrontal cortex | R | 9/10 | 18, 63, 7 | 4.07 | 2482 |
| Region | MAS-A | Self-Reflect | Other-Reflect | Mastery |
|---|---|---|---|---|
| mPFC | 0.566 ** | 0.605 ** | 0.424 * | 0.564 ** |
| VS | 0.464 * | 0.451 * | 0.367 | 0.513 ** |

3. Experimental Section
3.1. Study Participants
3.2. Metacognition and Symptom Assessment
3.2.1. Measurement of Metacognition
3.2.2. Measurement of Symptoms
3.3. Neuroimaging Acquisition and Analysis
Structural Magnetic Resonance Imaging (MRI) Data Acquisition and Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tandon, R.; Keshavan, M.S.; Nasrallah, H.A. Schizophrenia, “just the facts” what we know in 2008. 2. Epidemiology and etiology. Schizophr. Res. 2008, 102, 1–18. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization/Mental Disorders. Available online: http://www.who.int/mediacentre/factsheets/fs396/en/ (accessed on 14 April 2015).
- Lysaker, P.H.; Erickson, M.A.; Buck, B.; Buck, K.D.; Olesek, K.; Grant, M.L.; Salvatore, G.; Popolo, R.; Dimaggio, G. Metacognition and social function in schizophrenia: Associations over a period of five months. Cogn. Neuropsychiatry 2011, 16, 241–255. [Google Scholar] [CrossRef] [PubMed]
- Dimaggio, G.; Vanheule, V.; Lysaker, P.H.; Carcione, A.; Nicolo, G. Impaired self-reflection in psychiatric disorders among adults: A proposal for the existence of a network of semi independent functions. Conscious Cogn. 2009, 18, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Lysaker, P.H.; Vohs, J.L.; Ballard, R.; Fogley, R.; Salvatore, G.; Popolo, R.; Dimaggio, G. Metacognition, self reflection and recovery in schizophrenia: Review of the literature. Future Neurol. 2013, 8, 103–115. [Google Scholar] [CrossRef]
- Abdel-Hamid, M.; Lehmkämper, C.; Sonntag, C.; Juckel, G.; Daum, I.; Brüne, M. Theory of mind in schizophrenia: The role of clinical symptomatology and neurocognition in understanding other people’s thoughts and intentions. Psychiatry Res. 2009, 30, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Brüno, N.; Sachs, N.; Demily, C.; Franck, N.; Pacherie, E. Delusions and metacognition in patients with schizophrenia. Cogn. Neuropsychiatry 2012, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Buchy, L.; Malla, A.; Joober, R.; Lepage, M. Delusions are associated with low self-reflectiveness in first-episode psychosis. Schizophr. Res. 2009, 112, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Hamm, J.A.; Renard, S.B.; Fogley, R.L.; Leonhardt, B.L.; Dimaggio, G.; Buck, K.D.; Lysaker, P.H. Metacognition and social cognition in schizophrenia: Stability and relationship to concurrent and prospective symptom assessments. J. Clin. Psychol. 2012, 68, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- Lysaker, P.H.; Carcione, A.; Dimaggio, G.; Johannesen, J.K.; Nicolò, G.; Procacci, M.; Semerari, A. Metacognition amidst narratives of self and illness in schizophrenia: Associations with neurocognition, symptoms, insight and quality of life. Acta Psychiatr. Scand. 2005, 112, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Lysaker, P.H.; Dimaggio, G.; Buck, K.D.; Carcione, A.; Nicolò, G. Metacognition within narratives of schizophrenia: Associations with multiple domains of neurocognition. Schizophr. Res. 2007, 93, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Shean, G.; Meyers, J. Social cognition and symptoms in schizophrenia. Psychiatry Res. 2009, 170, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Tas, C.; Brown, E.C.; Esen-Danaci, A.; Lysaker, P.H.; Brüne, M. Intrinsic motivation and metacognition as predictors of learning potential in patients with remitted schizophrenia. J. Psychiatr. Res. 2012, 46, 1086–1092. [Google Scholar] [CrossRef] [PubMed]
- Vohs, J.L.; Lysaker, P.H.; Francis, M.; Hamm, J.; Buck, K.D.; Olesek, K.; Outcalt, J.; Dimaggio, G.; Leonhardt, B.; Liffick, E.; et al. Metacognition, social cognition, and symptoms in patients with first episode and prolonged psychosis. Schizophr. Res. 2014, 153, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Koren, D.; Seidman, L.J.; Goldsmith, M.; Harvey, P.D. Real-world cognitive- and metacognitive-dysfunction in schizophrenia: A new approach for measuring (and remediating) more “right stuff.”. Schizophr. Bull. 2006, 32, 310–326. [Google Scholar] [CrossRef] [PubMed]
- Langdon, R.; Coltheart, M.; Ward, P.B.; Catts, S.V. Mentalizing, executive planning and disengagement in schizophrenia. Cogn. Neuropsychiatry 2001, 6, 81–108. [Google Scholar] [CrossRef]
- Lysaker, P.H.; Dimaggio, G.; Buck, K.D.; Callaway, S.S.; Salvatore, G; Carcione, A.; Nicolò, G.; Stanghellini, G. Poor insight in schizophrenia: Links between different forms of metacognition with awareness of symptoms, treatment need, and consequences of illness. Compr. Psychiatry 2011, 52, 253–260. [Google Scholar] [CrossRef] [PubMed]
- MacBeth, A.; Gumley, A.; Schwannauer, M.; Carcione, A.; Fisher, R.; McLeod, H.; Dimaggio, G. Associations between metacognition, symptoms and functioning in a first episode psychosis sample. Compr. Psychiatry 2014, 55, 268–273. [Google Scholar] [CrossRef] [PubMed]
- McCleod, H.J.; Gumley, A.I.; MacBeth, A.; Schwannauer, M.; Lysaker, P.H. Metacognitive functioning predicts positive and negative symptoms over 12 months of first episode psychosis. J. Psychiatr. Rehabil. 2014, 54, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Vohs, J.L.; Lysaker, P.H. Metacognitive mastery and intrinsic motivation in schizophrenia. J. Nerv. Ment. Dis. 2014, 202, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Vohs, J.L.; Lysaker, P.H.; Liffick, E.; Francis, M.M.; Leonhardt, B.L.; James, A.; Buck, K.D.; Hamm, J.; Minor, K.S.; Mehdiyoun, N.; et al. Metacognitive capacity as a predictor of insight in First Episode Psychosis. J. Nerv. Ment. Dis. 2015, 203, 372–378. [Google Scholar]
- Harrison, G.; Hopper, K.; Craig, T.; Laska, E.; Siegel, C.; Wanderling, J. Recovery from psychotic illness: A 15- and 25-year international follow-up study. Br. J. Psychiatry 2001, 178, 506–517. [Google Scholar] [CrossRef] [PubMed]
- McGorry, P.D.; Killackey, E.; Yung, A. Early intervention in psychosis: Concepts, evidence, and future directions. World Psychiatry 2008, 7, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Barsaglinia, A.; Sartorib, G.; Benettia, S.; Pettersson-Yeoa, W.; Mechellia, A. The effects of psychotherapy on brain function: A systematic and critical review. Prog. Neurobiol. 2014, 114, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Shenton, M.E.; Dickey, C.C.; Frumin, M.; McCarley, R.W. A review of MRI findings in schizophrenia. Schizophr. Res. 2001, 49, 1–52. [Google Scholar] [CrossRef]
- Brown, G.C.; Thompson, W.K. Functional brain imaging in schizophrenia: Selected results and methods. Curr. Top. Behav. Neurosci. 2010, 4, 181–214. [Google Scholar] [PubMed]
- Ashburner, J.; Friston, K.J. Voxel-based morphometry—The methods. NeuroImage 2000, 11, 805–821. [Google Scholar] [CrossRef] [PubMed]
- Good, C.D.; Johnsrude, I.S.; Ashburner, J.; Henson, R.N.; Friston, K.J.; Frackowiak, R.S. A voxel-based morphometric study of ageing in 465 normal adult human brains. NeuroImage 2001, 14, 21–36. [Google Scholar] [CrossRef] [PubMed]
- Bora, E.; Fornito, A.; Radua, J.; Walterfang, M.; Seal, M.; Wood, S.J.; Yücel, M.; Velakoulis, D.; Pantelis, C. Neuroanatomical abnormalities in schizophrenia: A multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr. Res. 2011, 127, 346–357. [Google Scholar] [CrossRef] [PubMed]
- Ellison-Wright, I.; Glahn, D.C.; Laird, A.R.; Thelen, S.M.; Bullmore, E. The anatomy of first-episode and chronic schizophrenia: An anatomical likelihood estimation meta-analysis. Am. J. Psychiatry 2008, 165, 1015–1023. [Google Scholar] [CrossRef] [PubMed]
- Fusar-Poli, P.; Bonoldi, I.; Yung, A.R.; Borgwardt, S.; Kempton, M.J.; Valmaggia, L.; Barale, F.; Caverzasi, E.; McGuire, P. Predicting psychosis: Meta-analysis of transition outcomes in individuals at high clinical risk. Arch. Gen. Psychiatry 2012, 69, 220–229. [Google Scholar] [CrossRef] [PubMed]
- Radua, J.; Borgwardt, S; Crescini, A.; Mataix-Cols, D.; Meyer-Lindenberg, A.; McGuire, P.K.; Fusar-Poli, P. Multimodal meta-analysis of structural and functional brain changes in first episode psychosis and the effects of antipsychotic medication. Neurosci. Biobehav. Rev. 2012, 36, 2325–2333. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.M.; Dolan, R.J. The neural basis of metacognitive ability. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 1338–1349. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.M.; Ryu, J.; Golfinos, J.G.; Blackmon, K.E. Domain-specific impairment in metacognitive accuracy following anterior prefrontal lesions. Brain 2014, 137, 2811–2822. [Google Scholar] [CrossRef] [PubMed]
- Philippi, C.L.; Duff, M.C.; Denburg, N.L.; Tranel, D.; Rudrauf, D. Medial PFC damage abolishes the self-reference effect. J. Cogn. Neurosci. 2011, 24, 475–481. [Google Scholar] [CrossRef] [PubMed]
- Bzdok, D.; Langner, R.; Schilbach, L.; Engemann, A.D.; Laird, A.R.; Fox, P.T.; Eickhoff, S.B. Segregation of the human medial prefrontal cortex in social cognition. Front. Hum. Neurosci. 2013, 7, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Kalenzaga, S.; Sperduti, M.; Anssens, A.; Martinelli, P.; Devauchell, A-D.; Gallarda, T.; Delhommeau, M.; Lion, S.; Amado, I.; Krebs, M.O.; et al. Episodic memory and self-reference via semantic autobiographical memory: Insights from an fMRI study in younger and older adults. Front. Behav. Neurosci. 2015, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.M.; Lee, S.M.; Gabrieli, J.D.E. Dissociable neural system supporting knowledge about human character and appearance in ourselves and others. J. Cogn. Neurosci. 2010, 29, 2222–2230. [Google Scholar] [CrossRef] [PubMed]
- Ubeda-Bañon, I.; Novejarque, A.; Mohedano-Moriano, A.; Pro-Sistiaga, P.; de la Rosa-Prieto, D.; Insausti, R.; Martinez-Garcia, F.; Lanuza, E.; Martinez-Marcos, A. Projections from the posterolateral olfactory amygdala to the ventral striatum: Neural basis for reinforcing properties of chemical stimuli. BMC Neurosci. 2007, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Villalba, R.M.; Smith, Y. Differential striatal spine pathology in Parkinson’s disease and cocaine addiction: A key role of dopamine? Neuroscience 2013, 251, 2–20. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, W.; Furuta, T.; Nakamura, K.C.; Hioki, H.; Fujiyama, F.; Arai, R.; Kaneko, T. Single nigrostriatal dopaminergic neurons form widely spread and highly dense axonal arborizations in the neostriatum. J. Neurosci. 2009, 29, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Pissadaki, E.K.; Bolam, J.P. The energy cost of action potential propagation in dopamine neurons: Clues to susceptibility in Parkinson’s disease. Front. Comput. Neurosci. 2013, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, P.A.; Monchi, O. Differential effects of dopaminergic therapies on dorsal and ventral striatum in Parkinson’s disease: Implications for cognitive functions. Parkinsons Dis. 2011, 2011, 572743. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.C.; Knutson, B. Valence and salience contribute to nucleus accumbens activation. NeuroImage 2008, 39, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Clithero, J.A.; Smith, D.V.; Carter, R.M.; Huettel, S.A. Within- and cross-participant classifiers reveal different neural coding of information. NeuroImage 2011, 56, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Monk, C.S.; Klein, R.G.; Tezler, E.H.; Schroth, E.A.; Mannuzza, S.; Moulton, J.L.; Guardino, M.; Masten, C.L.; McClure-Tone, E.B.; Fromm, S.; et al. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am. J. Psychiatry 2008, 165, 90–98. [Google Scholar] [CrossRef] [PubMed]
- Mühlberger, M.J.; Wieser, M.J.; Gerdes, A.B.M.; Frey, M.C.; Weyers, P.; Pauli, P. Stop looking angry and smile, please: Start and stop of the very same facial expression differentially activate threat- and reward-related brain networks. Soc. Cogn. Affect. Neurosci. 2011, 6, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Lysaker, P.H.; Shea, A.M.; Buck, K.D.; Dimaggio, G.; Nicolò, G.; Procacci, M.; Salvatore, G.; Rand, K.L. Metacognition as a mediator of the effects of impairments in neurocognition on social function in schizophrenia spectrum disorders. Acta Psychiatr. Scand. 2010, 122, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Van Donkersgoed, R.J.M.; de Jong, S.; van der Gaag, M.; Alema, A.; Lysaker, P.H.; Wunderink, L.; Pijnenborg, G.H.M. A manual-based individual therapy to improve metacognition in schizophreani: Protocol of a multi-center RCT. BMC Psychiatry 2014, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Bleuler, E. Dementia Praecox or the Group of Schizophrenias; International Universities Press: New York, NY, USA, 1950. [Google Scholar]
- Green, M.J.; Cairns, M.J.; Wu, J.; Dragovic, M.; Jablensky, A.; Tooney, P.A.; Scott, R.J.; Carr, V.J. Genome-wide supported variant MIR137 and severe negative symptoms predict membership of an impaired cognitive subtype of schizophrenia. Mol. Psychiatry 2013, 18, 774–780. [Google Scholar] [CrossRef] [PubMed]
- Hori, H.; Yamamoto, N.; Fuji, T.; Teraishi, T.; Sasayama, D.; Matsuo, J.; Kawamoto, Y.; Kinoshita, Y.; Ota, M.; Hattori, K.; et al. Effects of the CACNA1C risk allele on neurocognition in patients with schizophrenia and health individuals. Sci. Rep. 2012, 2, 634. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, M.; Kulle, B.; Sundet, K.; Agartz, I.; Melle, I.; Djurovic, S.; Frigessi, A.; Andreassen, O.A. Genome-wide study identifies PTPRO and WDR72 and FOXQ1-SUMO1P1 interaction associated with neurocognitive function. J. Psychiatr. Res. 2012, 46, 271–278. [Google Scholar] [CrossRef] [PubMed]
- First, M.B.; Spitzer, R.L.; Gibbon, M.; Williams, J.B.W. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition with Psychotic Screen (SCID-I/P W/PSY SCREEN); Biometrics Research, New York State Psychiatric Institute: New York, NY, USA, 2002. [Google Scholar]
- Lysaker, P.H.; Clements, C.A.; Plascak-Hallberg, C.D.; Knipscheer, S.J.; Wright, D.E. Insight and personal narratives of illness in schizophrenia. Psychiatry 2002, 65, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Semerari, A.; Carcione, A.; Dimaggio, G.; Falcone, M.; Nicolò, G.; Procacci, M.; Alleva, G. How to evaluate metacognitive function in psychotherapy? The metacognition assessment scale and its applications. Clin. Psychol. Psychother. 2003, 10, 238–261. [Google Scholar] [CrossRef]
- Kay, S.R.; Fiszbein, A.; Opler, L.A. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr. Bull. 1987, 13, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.D.; Lysaker, P.H.; Beam-Goulet, J.L.; Milstein, R.M.; Lindenmayer, J-P. Five-component model of schizophrenia: Assessing the factorial invariance of the positive and negative syndrome scale. Psychiatry Res. 1994, 52, 295–303. [Google Scholar] [CrossRef]
- Structural Brain Mapping Group/VBM. Available online: http://dbm.neuro.uni-jena.de/vbm/ (accessed on 19 March 2015).
- Trust Centre for Neuroimaging/SPM (Statistical Parametric Mapping). Available online: http://www.fil.ion.ucl.ac.uk (accessed on 19 March 2015).
- Brainweb: Simulated Brain Database/Montreal Neurological Institute. Available online: http://www.bic.mni.mcgill.ca/brainweb (accessed on 19 March 2015).
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vohs, J.L.; Hummer, T.A.; Yung, M.G.; Francis, M.M.; Lysaker, P.H.; Breier, A. Metacognition in Early Phase Psychosis: Toward Understanding Neural Substrates. Int. J. Mol. Sci. 2015, 16, 14640-14654. https://doi.org/10.3390/ijms160714640
Vohs JL, Hummer TA, Yung MG, Francis MM, Lysaker PH, Breier A. Metacognition in Early Phase Psychosis: Toward Understanding Neural Substrates. International Journal of Molecular Sciences. 2015; 16(7):14640-14654. https://doi.org/10.3390/ijms160714640
Chicago/Turabian StyleVohs, Jenifer L., Tom A. Hummer, Matthew G. Yung, Michael M. Francis, Paul H. Lysaker, and Alan Breier. 2015. "Metacognition in Early Phase Psychosis: Toward Understanding Neural Substrates" International Journal of Molecular Sciences 16, no. 7: 14640-14654. https://doi.org/10.3390/ijms160714640