Area-Specific Cell Stimulation via Surface-Mediated Gene Transfer Using Apatite-Based Composite Layers
Abstract
:1. Introduction
| Type of Gene | Gene | Plasmid DNA | Sample Name | Transfected Cell |
|---|---|---|---|---|
| Reporter gene | Firefly luciferase (FL) | pGL3 (1) | DF-FL | CHO-K1 cell |
| Renilla luciferase (RL) | pRL-TK (2) | DF-RL | ||
| Differentiation factor gene | Vascular endothelial growth factor (VEGF) | pCI-VEGF (3) | DF-V | C3H10T1/2 embryonic cell |
| Bone morphogenetic protein-2 (BMP-2) | pCI-BMP (4) | DF-B |
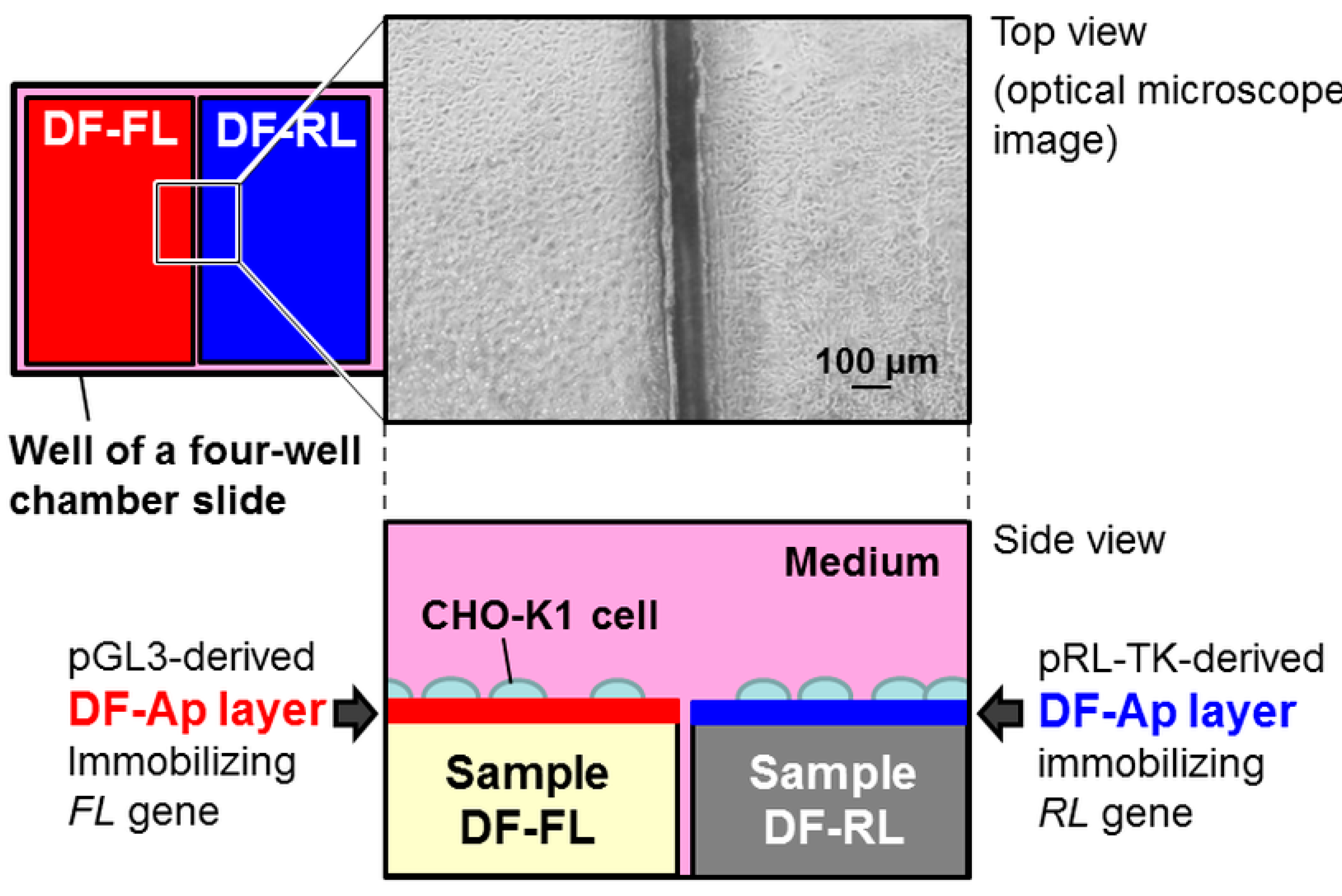
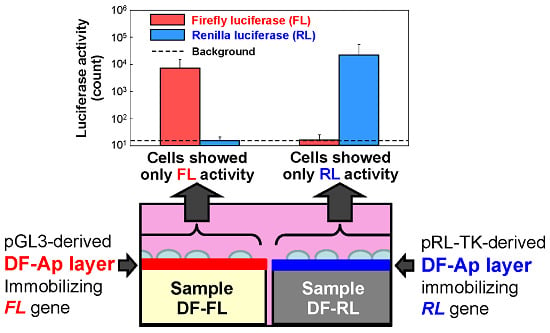
2. Results
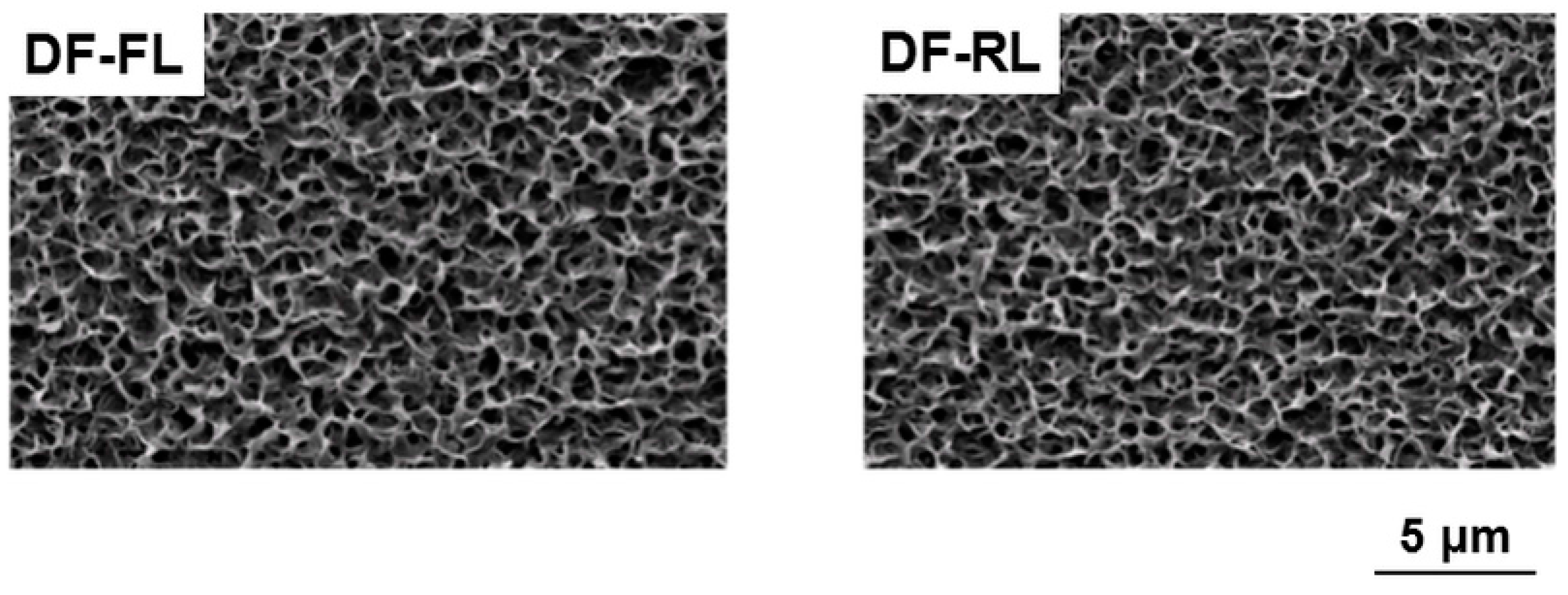
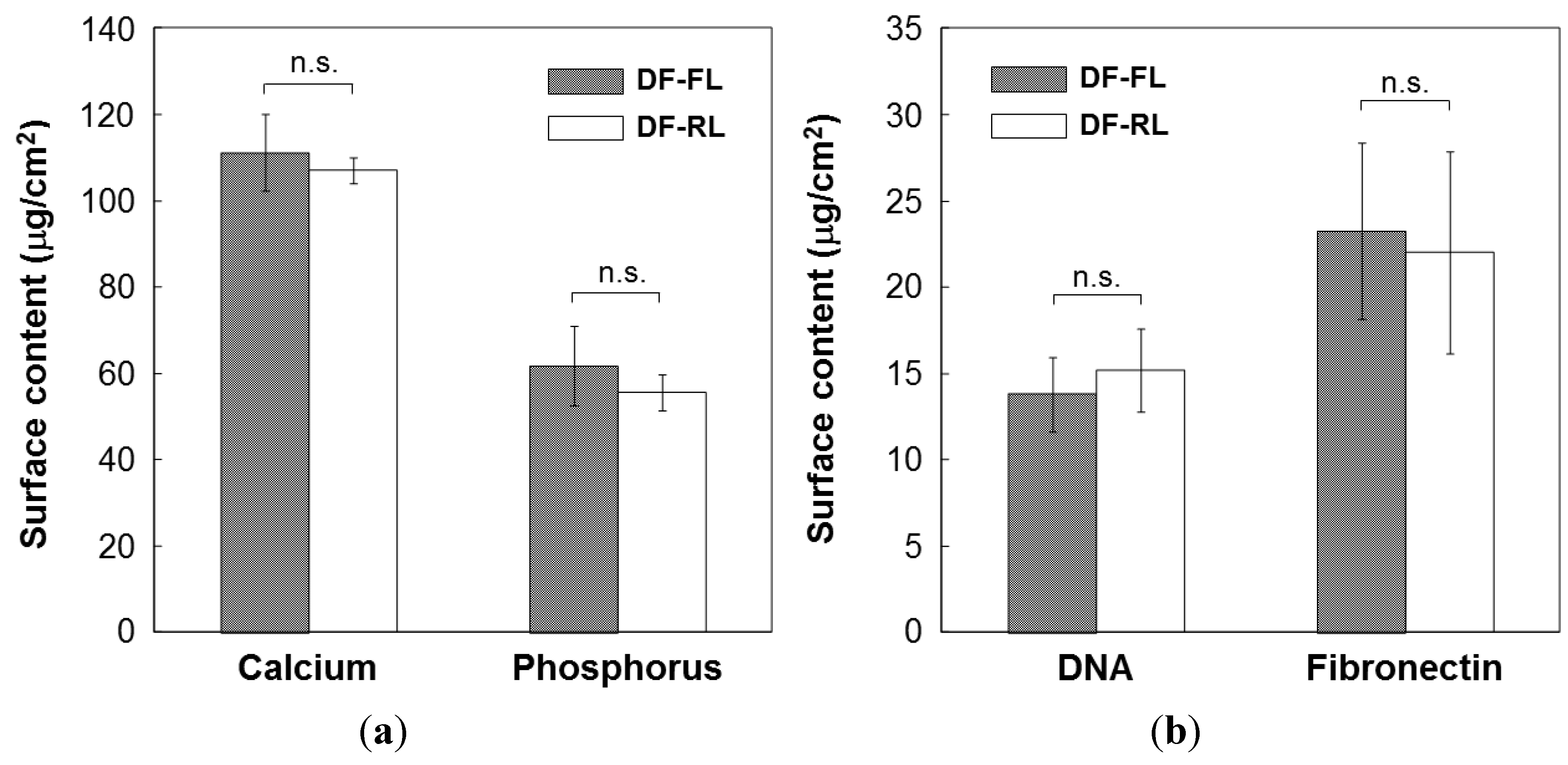
2.1. Surface Structures of Samples

2.2. Cotransfer of Reporter Genes by Conventional Lipofection
2.3. Dual Reporter Gene Transfer Using a Pair of DF-Ap Layers
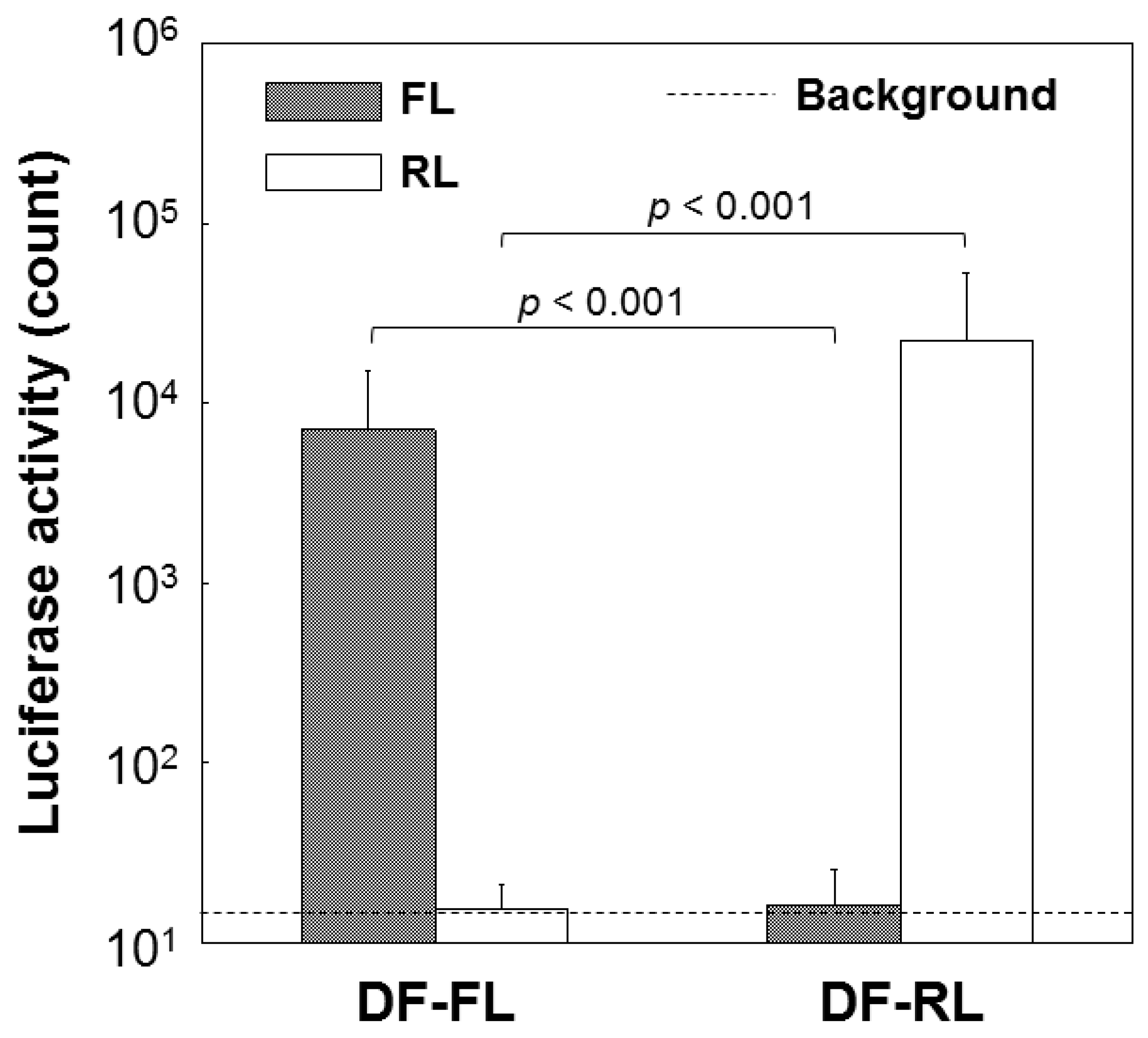
2.4. Dual Differentiation Factor Gene Transfer Using a Pair of DF-Ap Layers
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Alternate Dipping Treatment for Precoating with the Apatite Precursor
4.3. Biomimetic Process for Coating with DF-Ap Layers
4.4. Analysis of Sample Surfaces and Coating Solutions
4.5. Cotransfer of Reporter Genes by Conventional Lipofection
4.6. Observation of Cells on DF-Ap Layer
4.7. Dual Reporter Gene Transfer Using a Pair of DF-Ap Layers
4.8. Dual Differentiation Factor Gene Transfer Using a Pair of DF-Ap Layers
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bennett, M.J.; Aberle, A.M.; Balasubramaniam, R.P.; Malone, J.G.; Malone, R.W.; Nantz, M.H. Cationic lipid-mediated gene delivery to murine lung: Correlation of lipid hydration with in vivo transfection activity. J. Med. Chem. 1997, 40, 4069–4078. [Google Scholar] [CrossRef] [PubMed]
- Templeton, N.S.; Lasic, D.D.; Frederik, P.M.; Strey, H.H.; Roberts, D.D.; Pavlakis, G.N. Improved DNA: Liposome complexes for increased systemic delivery and gene expression. Nat. Biotechnol. 1997, 15, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Uduehi, A.N.; Moss, S.H.; Nuttall, J.; Pouton, C.W. Cationic lipid-mediated transfection of differentiated Caco-2 cells: A filter culture model of gene delivery to a polarized epithelium. Pharm. Res. 1999, 16, 1805–1811. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, B.; Sachs, L.; Demeneix, B.A. Non-viral gene transfer: Applications in developmental biology and gene therapy. Biol. Cell 1995, 85, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Boussif, O.; Lezoualc’h, F.; Zanta, M.A.; Mergny, M.D.; Scherman, D.; Demeneix, B.; Behr, J.P. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: Polyethylenimine. Proc. Natl. Acad. Sci. USA 1995, 92, 7297–7301. [Google Scholar] [CrossRef] [PubMed]
- Felgner, P.L.; Barenholz, Y.; Behr, J.P.; Cheng, S.H.; Cullis, P.; Huang, L.; Jessee, J.A.; Seymour, L.; Szoka, F.; Thierry, A.R.; et al. Nomenclature for synthetic gene delivery systems. Hum. Gene Ther. 1997, 8, 511–512. [Google Scholar]
- Gebhart, C.L.; Kabanov, A.V. Evaluation of polyplexes as gene transfer agents. J. Control. Release 2001, 73, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Graham, F.L.; van der Eb, A.J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology 1973, 52, 456–467. [Google Scholar]
- Batard, P.; Jordan, M.; Wurm, F. Transfer of high copy number plasmid into mammalian cells by calcium phosphate transfection. Gene 2001, 270, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Roy, I.; Mitra, S.; Maitra, A.; Mozumdar, S. Calcium phosphate nanoparticles as novel non-viral vectors for targeted gene delivery. Int. J. Pharm. 2003, 250, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, E.H.; Kunou, M.; Harada, I.; Kundu, A.K.; Akaike, T. Dramatic effect of Mg2+ on transfecting mammalian cells by DNA/calcium phosphate precipitates. Anal. Biochem. 2004, 328, 96–97. [Google Scholar] [CrossRef] [PubMed]
- Kakizawa, Y.; Furukawa, S.; Kataoka, K. Block copolymer-coated calcium phosphate nanoparticles sensing intracellular environment for oligodeoxynucleotide and siRNA delivery. J. Control. Release 2004, 97, 345–356. [Google Scholar] [CrossRef] [PubMed]
- Kutsuzawa, K.; Chowdhury, E.H.; Nagaoka, M.; Maruyama, K.; Akiyama, Y.; Akaike, T. Surface functionalization of inorganic nano-crystals with fibronectin and E-cadherin chimera synergistically accelerates trans-gene delivery into embryonic stem cells. Biochem. Biophys. Res. Commun. 2006, 350, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Shea, L.D.; Smiley, E.; Bonadio, J.; Mooney, D.J. DNA delivery from polymer matrices for tissue engineering. Nat. Biotechnol. 1999, 17, 551–554. [Google Scholar] [CrossRef] [PubMed]
- Ziauddin, J.; Sabatini, D.M. Microarrays of cells expressing defined cDNAs. Nature 2001, 411, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Ono, I.; Yamashita, T.; Jin, H.Y.; Ito, Y.; Hamada, H.; Akasaka, Y.; Nakasu, M.; Ogawa, T.; Jimbow, K. Combination of porous hydroxyapatite and cationic lipids as a vector for BMP-2 gene therapy. Biomaterials 2004, 25, 4709–4718. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, T.; Uchimura, E.; Kishi, M.; Funeriu, D.P.; Miyake, M.; Miyake, J. Transfection microarray of human mesenchymal stem cells and on-chip siRNA gene knockdown. J. Control. Release 2004, 96, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Tan, J.; Saltzman, W.M. Surface-mediated gene transfer from nanocomposites of controlled texture. Nat. Mater. 2004, 3, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Oyane, A.; Tsurushima, H.; Ito, A. Novel gene-transferring scaffolds having a cell adhesion molecule–DNA–apatite nanocomposite surface. Gene Ther. 2007, 14, 1750–1753. [Google Scholar] [CrossRef] [PubMed]
- Oyane, A.; Tsurushima, H.; Ito, A. Highly efficient gene transfer system using laminin–DNA–apatite composite layer. J. Gene Med. 2010, 12, 194–206. [Google Scholar] [CrossRef] [PubMed]
- Oyane, A.; Murayama, M.; Yamazaki, A.; Sogo, Y.; Ito, A.; Tsurushima, H. Fibronectin–DNA–apatite composite layer for highly efficient and area-specific gene transfer. J. Biomed. Mater. Res. A 2010, 92A, 1038–1047. [Google Scholar]
- Yazaki, Y.; Oyane, A.; Tsurushima, H.; Sogo, Y.; Ito, A.; Yamazaki, A. Control of gene transfer on a DNA-fibronectin-apatite composite layer by the incorporation of carbonate and fluoride ions. Biomaterials 2011, 32, 4896–4902. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.B.; Tran, K.K.; Shen, H. Enabling customization of non-viral gene delivery systems for individual cell types by surface-induced mineralization. Biomaterials 2009, 30, 6386–6393. [Google Scholar] [CrossRef] [PubMed]
- Luong, L.N.; McFalls, K.M.; Kohn, D.H. Gene delivery via DNA incorporation within a biomimetic apatite coating. Biomaterials 2009, 30, 6996–7004. [Google Scholar] [CrossRef] [PubMed]
- Oyane, A.; Wang, X.P.; Sogo, Y.; Ito, A.; Tsurushima, H. Calcium phosphate composite layers for surface-mediated gene transfer. Acta Biomater. 2012, 8, 2034–2046. [Google Scholar] [CrossRef] [PubMed]
- Oyane, A.; Yazaki, Y.; Araki, H.; Sogo, Y.; Ito, A.; Yamazaki, A.; Tsurushima, H. Fabrication of a DNA-lipid-apatite composite layer for efficient and area-specific gene transfer. J. Mater. Sci. Mater. Med. 2012, 23, 1011–1019. [Google Scholar] [CrossRef]
- Yazaki, Y.; Oyane, A.; Tsurushima, H.; Araki, H.; Sogo, Y.; Ito, A.; Yamazaki, A. Coprecipitation of DNA-lipid complexes with apatite and comparison with superficial adsorption for gene transfer applications. J. Biomater. Appl. 2014, 28, 937–945. [Google Scholar] [CrossRef] [PubMed]
- He, C.X.; Tabata, Y.; Gao, J.Q. Non-viral gene delivery carrier and its three-dimensional transfection system. Int. J. Pharm. 2010, 386, 232–242. [Google Scholar] [PubMed]
- Yamauchi, F.; Kato, K.; Iwata, H. Micropatterned, self-assembled monolayers for fabrication of transfected cell microarrays. Biochim. Biophys. Acta 2004, 1672, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Chang, F.H.; Lee, C.H.; Chen, M.T.; Kuo, C.C.; Chiang, Y.L.; Hang, C.Y.; Roffler, S. Surfection: A new platform for transfected cell arrays. Nucleic Acids Res. 2004, 32, e33. [Google Scholar] [CrossRef] [PubMed]
- Baghdoyan, S.; Roupioz, Y.; Pitaval, A.; Castel, D.; Khomyakova, E.; Papine, A.; Soussaline, F.; Gidrol, X. Quantitative analysis of highly parallel transfection in cell microarrays. Nucleic Acids Res. 2004, 32, e77. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.; Muthuswamy, J. Bio-chip for spatially controlled transfection of nucleic acid payloads into cells in a culture. Lab. Chip. 2007, 7, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, F.; Kato, K.; Iwata, H. Layer-by-layer assembly of poly(ethyleneimine) and plasmid DNA onto transparent indium-tin oxide electrodes for temporally and spatially specific gene transfer. Langmuir 2005, 21, 8360–8367. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, F.; Kato, K.; Iwata, H. Spatially and temporally controlled gene transfer by electroporation into adherent cells on plasmid DNA-loaded electrodes. Nucleic Acids Res. 2004, 32, e187. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Fujimoto, H.; Ogino, T.; Iwata, H. Site-specific gene transfer with high efficiency onto a carbon nanotube-loaded electrode. J. R. Soc. Interface 2008, 5, 909–918. [Google Scholar] [CrossRef] [PubMed]
- Nomikou, N.; McHale, A.P.; McHale, A.P. Exploiting ultrasound-mediated effects in delivering targeted; site-specific cancer therapy. Cancer Lett. 2010, 296, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Thein, M.; Cheng, A.; Khanna, P.; Zhang, C.; Park, E.-J.; Ahmed, D.; Goodrich, C.J.; Asphahani, F.; Wu, F.; Smith, N.B.; et al. Site-specific sonoporation of human melanoma cells at the cellular level using high lateral-resolution ultrasonic micro-transducer arrays. Biosens. Bioelectron. 2011, 27, 25–33. [Google Scholar]
- Suzuki, R.; Takizawa, T.; Negishi, Y.; Utoguchi, N.; Maruyama, K. Effective gene delivery with liposomal bubbles and ultrasound as novel non-viral system. J. Drug Target. 2007, 15, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, Y.; Morikawa, N.; Ohkubo-Suzuki, A.; Miyoshi, S.; Arakawa, H.; Kita, Y.; Nishimura, S. An epoch-making application of discharge plasma phenomenon to gene-transfer. Biotechnol. Bioeng. 2005, 92, 865–870. [Google Scholar] [CrossRef] [PubMed]
- Sakai, Y.; Khajoee, V.; Ogawa, Y.; Kusuhara, K.; Katayama, Y.; Hara, T. A novel transfection method for mammalian cells using gas plasma. J. Biotechnol. 2006, 121, 299–308. [Google Scholar] [CrossRef] [PubMed]
- Connolly, R.J.; Lopez, G.A.; Hoff, A.M.; Jaroszeski, M.J. Plasma facilitated delivery of DNA to skin. Biotechnol. Bioeng. 2009, 104, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Tsurushima, H.; Oyane, A.; Yazaki, Y.; Sogo, Y.; Ito, A.; Matsumura, A. BMP-2 gene–fibronectin–apatite composite layer enhances bone formation. J. Biomed. Sci. 2011, 18, 62. [Google Scholar] [PubMed]
- Wang, X.; Ito, A.; Li, X.; Sogo, Y.; Hirose, M.; Oyane, A.; Tsurushima, H. DNA-lipid-apatite composite layers enhance gene expression of mesenchymal stem cells. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 512–518. [Google Scholar] [PubMed]
- Leung, D.W.; Cachianes, G.; Kuang, W.J.; Goeddel, D.V.; Ferrara, N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 1989, 246, 1306–1309. [Google Scholar] [PubMed]
- Wozney, J.M.; Rosen, V.; Celeste, A.J.; Mitsock, L.M.; Whitters, M.J.; Kriz, R.W.; Hewick, R.M.; Wang, E.A. Novel regulators of bone formation: Molecular clones and activities. Science 1988, 242, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Oyane, A.; Araki, H.; Sogo, Y.; Ito, A.; Tsurushima, H. Spontaneous assembly of DNA–amorphous calcium phosphate nanocomposite spheres for surface-mediated gene transfer. CrystEngComm 2013, 15, 4994–4997. [Google Scholar] [CrossRef]
- Farr, A.; Roman, A. A pitfall of using a second plasmid to determine transfection efficiency. Nucleic Acids Res. 1992, 20, 920. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.H.; Oyane, A.; Tsurushima, H.; Shimizu, Y.; Sasaki, T.; Koshizaki, N. A new approach for hydroxyapatite coating on polymeric materials using laser-induced precursor formation and subsequent aging. Appl. Mater. Interfaces 2009, 1, 1520–1524. [Google Scholar] [CrossRef]
- Oyane, A.; Sakamaki, I.; Shimizu, Y.; Kawaguchi, K.; Koshizaki, N. Liquid-phase laser process for simple and area-specific calcium phosphate coating. J. Biomed. Mater. Res. A 2012, 100A, 2573–2580. [Google Scholar] [CrossRef]
- Oyane, A.; Tsurushima, H.; Ito, A. Simple surface modification process to produce a transparent apatite–polystyrene composite for in situ observation of cell behavior. Chem. Lett. 2006, 35, 1300–1301. [Google Scholar]
- Oyane, A.; Uchida, M.; Yokoyama, Y.; Choong, C.; Triffitt, J.; Ito, A. Simple surface modification of poly(ε-caprolactone) to induce its apatite-forming ability. J. Biomed. Mater. Res. A 2005, 75A, 138–145. [Google Scholar] [CrossRef]
- Uchida, M.; Oyane, A.; Kim, H.M.; Kokubo, T.; Ito, A. Biomimetic coating of laminin–apatite composite on titanium metal with excellent cell adhesive property. Adv. Mater. 2004, 16, 1071–1074. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yazaki, Y.; Oyane, A.; Sogo, Y.; Ito, A.; Yamazaki, A.; Tsurushima, H. Area-Specific Cell Stimulation via Surface-Mediated Gene Transfer Using Apatite-Based Composite Layers. Int. J. Mol. Sci. 2015, 16, 8294-8309. https://doi.org/10.3390/ijms16048294
Yazaki Y, Oyane A, Sogo Y, Ito A, Yamazaki A, Tsurushima H. Area-Specific Cell Stimulation via Surface-Mediated Gene Transfer Using Apatite-Based Composite Layers. International Journal of Molecular Sciences. 2015; 16(4):8294-8309. https://doi.org/10.3390/ijms16048294
Chicago/Turabian StyleYazaki, Yushin, Ayako Oyane, Yu Sogo, Atsuo Ito, Atsushi Yamazaki, and Hideo Tsurushima. 2015. "Area-Specific Cell Stimulation via Surface-Mediated Gene Transfer Using Apatite-Based Composite Layers" International Journal of Molecular Sciences 16, no. 4: 8294-8309. https://doi.org/10.3390/ijms16048294